Effects of Regioisomerism on the Antiproliferative Activity of Hydroxystearic Acids on Human Cancer Cell Lines
Abstract
1. Introduction
2. Results and Discussion
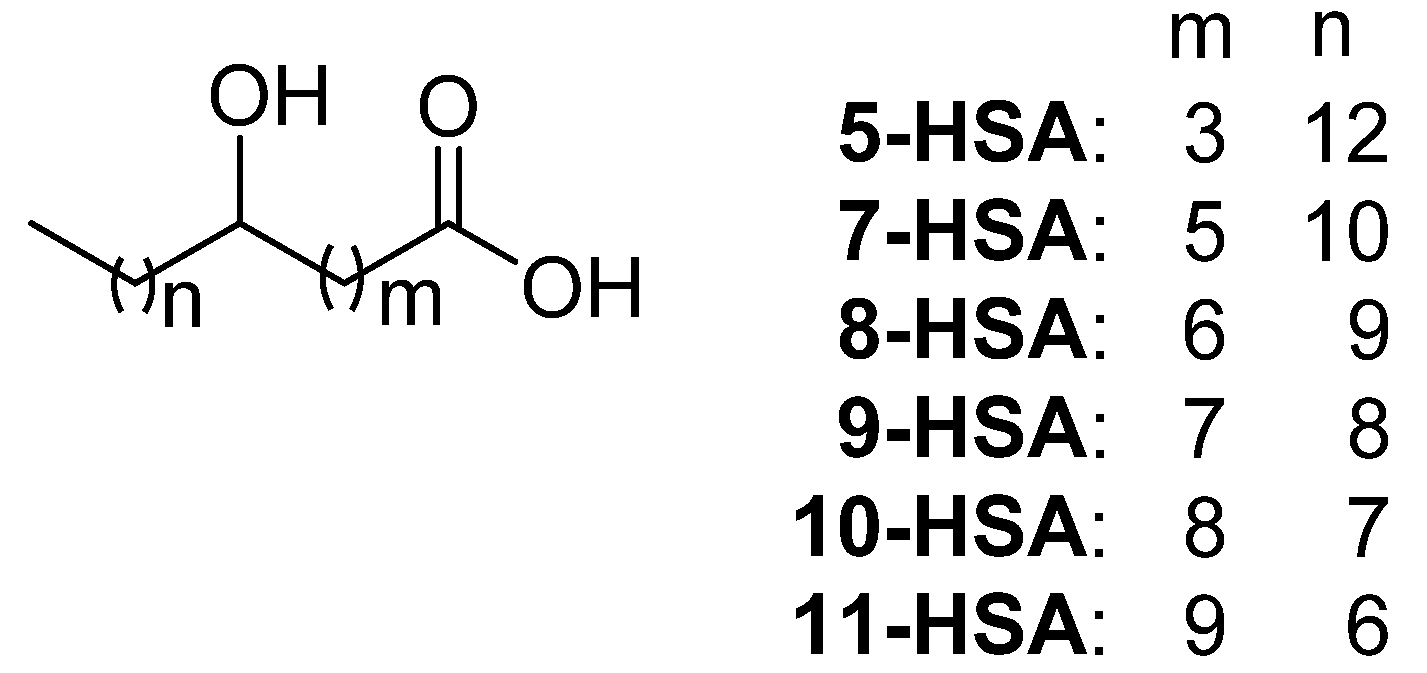
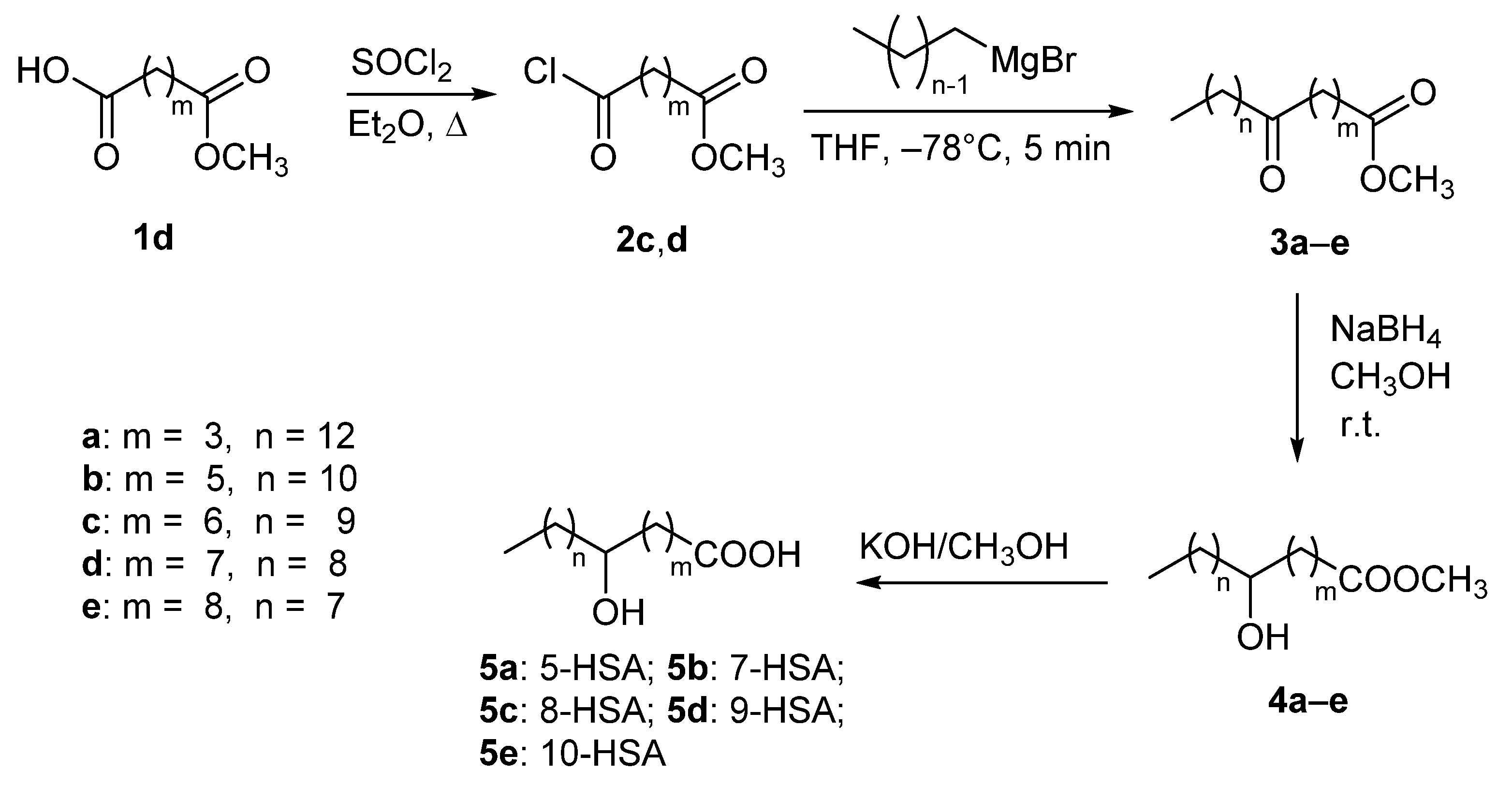
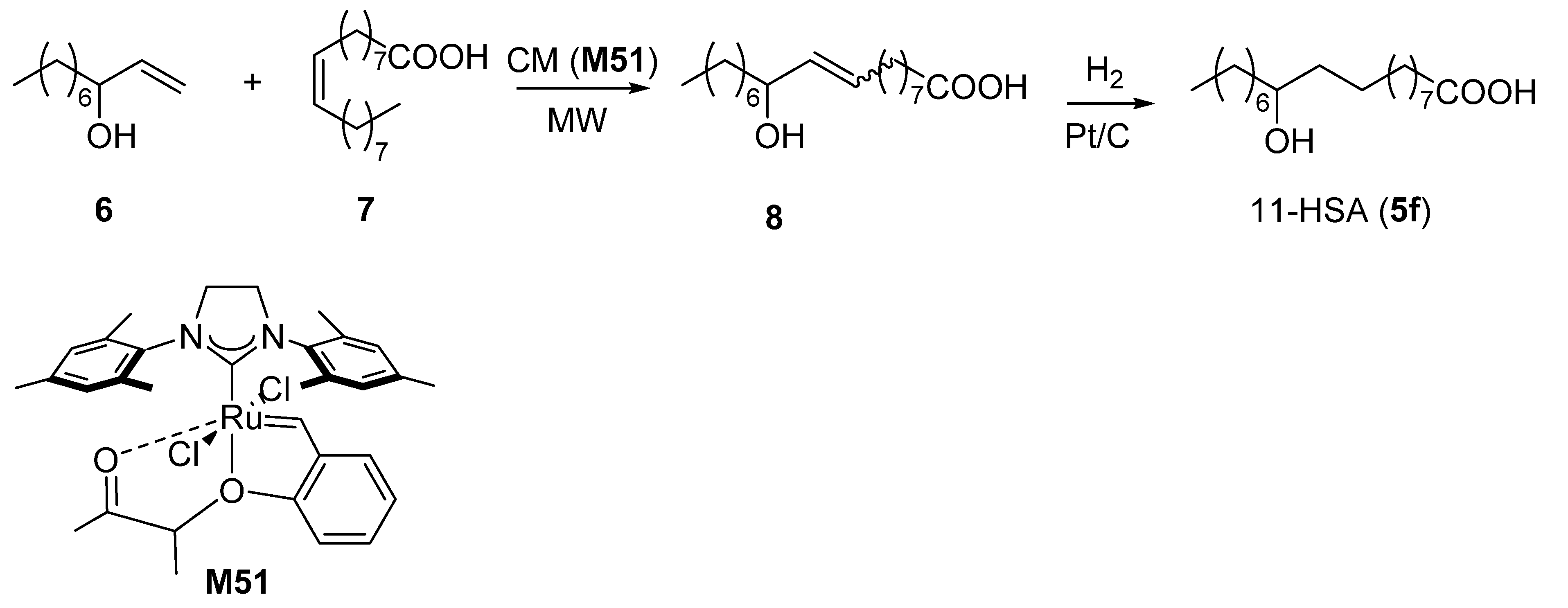
2.1. Synthesis of Regioisomeric Hydroxystearic Acids
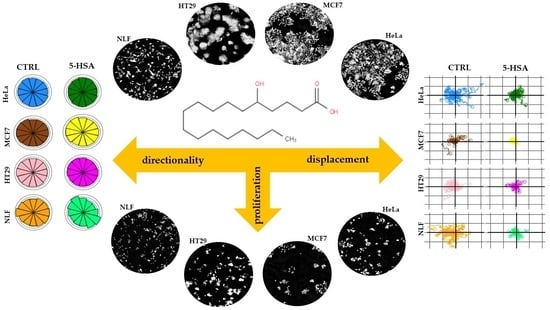
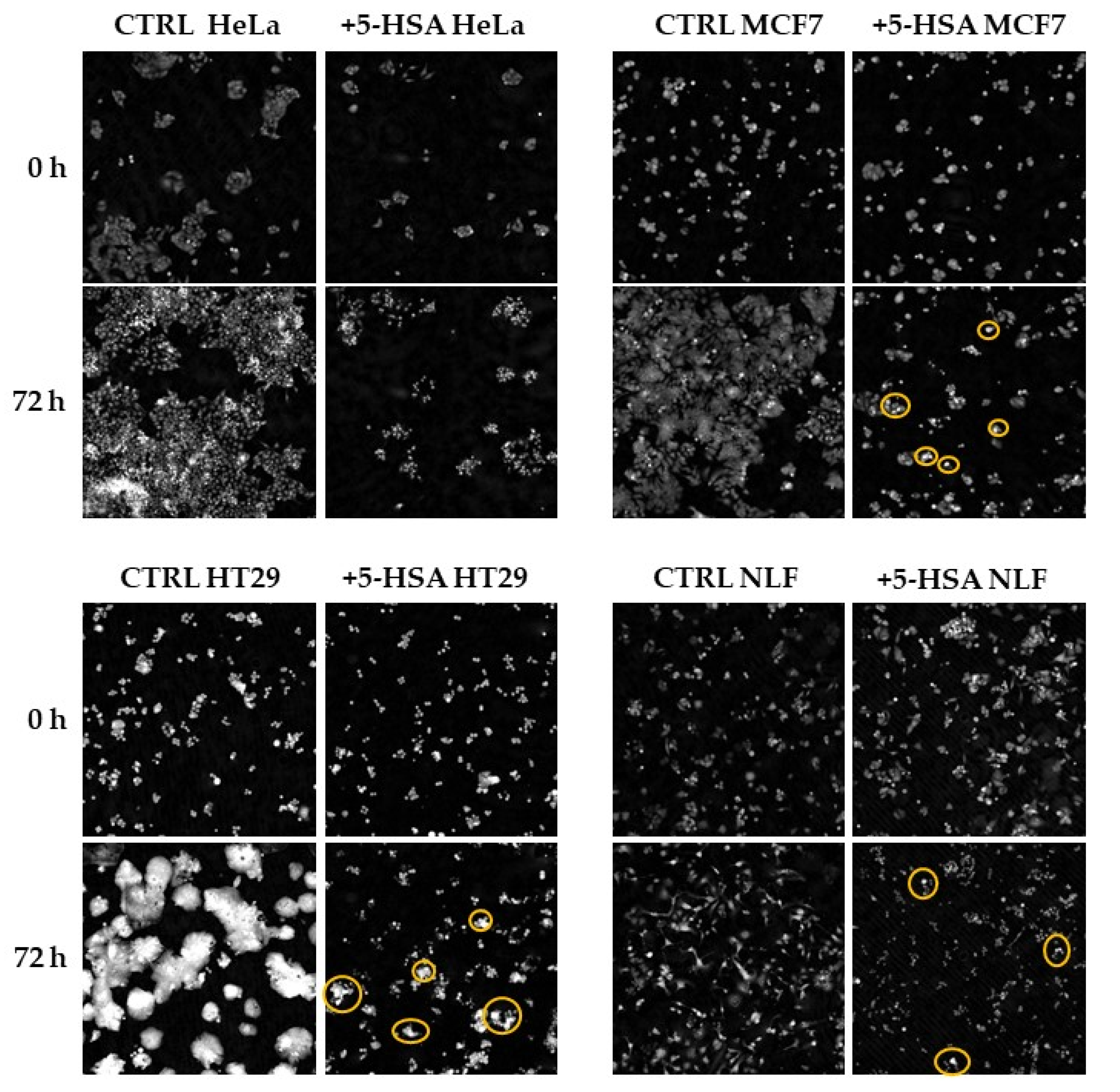
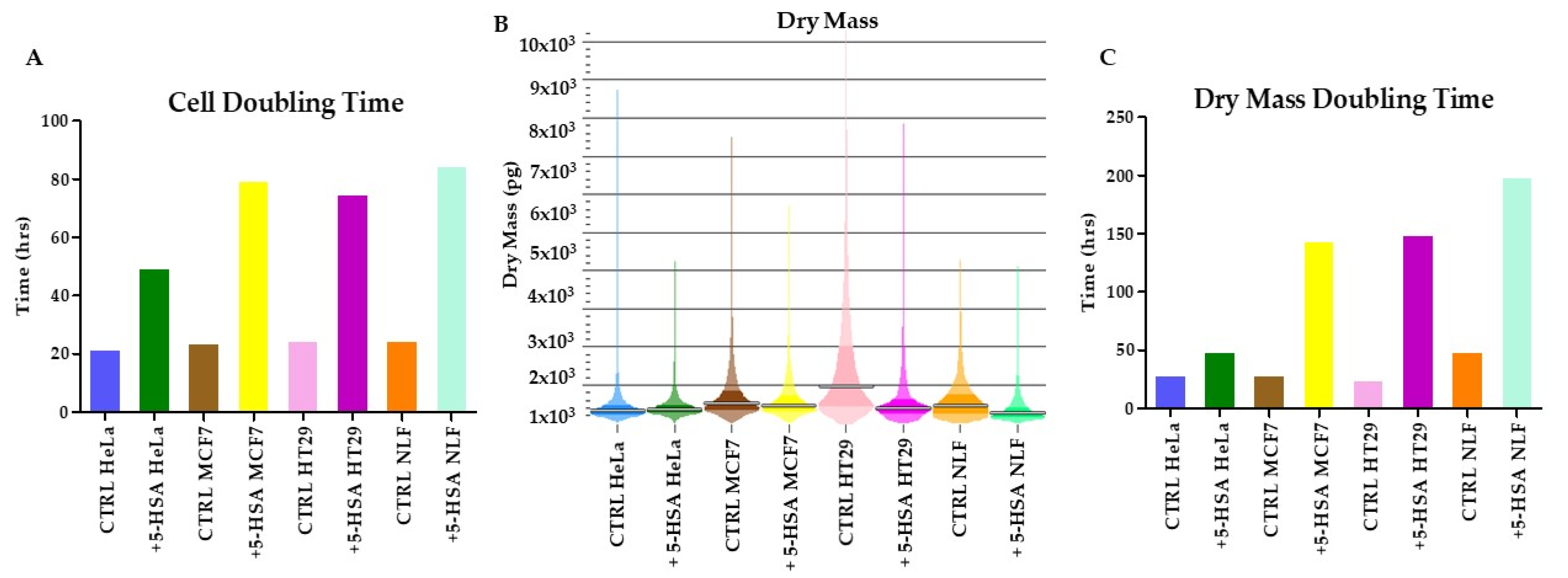
2.2. Biological Activity
Cell Growth Inhibitory Effects of HSAs on Human Cancer Cell Lines
3. Materials and Methods
3.1. Chemical Synthesis
3.1.1. General
3.1.2. Synthesis of Methyl 5-Hydroxyoctadecanoate (4a)
3.1.3. Synthesis of 5-Hydroxyoctadecanoic Acid (5-HSA, 5a)
3.1.4. Synthesis of 11-Hydroxyoctadecanoic Acid (11-HSA, 5f)
Synthesis of 11-Hydroxy-9-octadecenoic Acid (8)
Synthesis of 11-Hydroxyoctadecanoic Acid (11-HSA, 5f)
3.2. Biology
3.2.1. Cell Culture and Treatments
3.2.2. MTT Assay
3.2.3. Quantitative Phase Image (QPI) Microscopy
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- McDermott, G.N. Miscellaneous oil and fat products. In Bailey’s Industrial Oil and Fat Products; Swern, D., Ed.; John Wiley & Sons: New York, NY, USA, 1982. [Google Scholar]
- Teeter, H.M.; Gast, L.E.; Bell, E.W.; Cowan, J.C. Synthetic lubricants from hydroxystearic acids. Ind. Eng. Chem. 1953, 45, 1777–1779. [Google Scholar] [CrossRef]
- Farroq, M.; Ramli, A.; Gul, S.; Muhammad, N.J. The Study of Wear Behaviour of 12-hydroxystearic Acid in Vegetable Oils. Appl. Chem. 2011, 11, 1381–1385. [Google Scholar] [CrossRef]
- Pryde, E.H.; Princen, L.H.; Mukherjiee, K.D. New Sources of Fats and Oils; American oil Chemists Society: Champaign, IL, USA, 1981. [Google Scholar]
- Svensson, M. Surfactants Based on Natural Fatty Acids. In Surfactants from Renewable Resources; Kjellin, M., Johansson, I., Eds.; John Wiley & Sons: Chichester, UK, 2010. [Google Scholar]
- Petrović, Z.S.; Cvetković, I.; Hong, D.; Wan, X.; Zhang, W.; Abraham, T.; Malsam, J. Polyester Polyols and Polyurethanes from Ricinoleic Acid. J. Appl. Pol. Sci. 2008, 108, 1184–1190. [Google Scholar] [CrossRef]
- Marrion, A.; Marrion, A.R. Binders for conventional coatings. In The Chemistry and Physics of Coatings; Marrion, A., Ed.; RCS Publication: London, UK, 2004; Volume 1, pp. 96–150. [Google Scholar]
- Naughton, F.C. Production, chemistry, and commercial applications of various chemicals from castor oil. J. Am. Oil Chem. Soc. 1974, 51, 65–71. [Google Scholar] [CrossRef]
- Drovetskaya, T.V.; Yu, W.H.; Diantonio, E.F.; Jordan, S.L. Hair Styling and Conditioning Personal Care Films. US Patent No. US 2010/0209377 A1, 19 August 2010. [Google Scholar]
- Grissett, G.A.; Keenan, D.M.; Macedo, F.A.; Williams, D.R. Fibrous Toilette Article. US Patent No. US 2005/0277566A1, 12/15/2005, 22 January 2008. [Google Scholar]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Shahidi, F. (Ed.) Nutraceutical and Specialty Lipids and Their Co-Products; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Hughes, N.E.; Marangoni, A.G.; Wright, A.J.; Rogers, M.A.; Rush, J.W.E. Potential food applications of edible oil organogels. Trends Food Sci. Technol. 2009, 20, 470–480. [Google Scholar] [CrossRef]
- Toro-Vazquez, J.F.; Morales-Rueda, J. Relationship between molecular structure and thermo-mechanical properties of candelilla wax and amides derived from (R)-12-hydroxystearic acid as gelators of safflower oil. Food Biophys. 2010, 5, 193–202. [Google Scholar] [CrossRef]
- Gunstone, F.D. Fatty Acid and Lipid Chemistry; Springer: Berlin, Germany, 1996. [Google Scholar]
- Fulco, A.J. Fatty acid metabolism in bacteria. Prog. Lipid Res. 1983, 22, 133–160. [Google Scholar] [CrossRef]
- Harwood, J.L.; Russell, N.J. Lipids in Plants and Microbes; Allen, G. & Unwin: London, UK, 1984. [Google Scholar]
- Mubofu, E.B. Castor oil as a potential renewable resource for the production of functional materials. Sustain. Chem. Process. 2016, 4, 11. [Google Scholar] [CrossRef]
- Rawlings, A.V.; Wandeler, E.; Bendik, I.; Fuchs, P.; Monneuse, J.-M.; Imfeld, D.; Schütz, R. Effect of regioisomers of hydroxystearic acids as peroxisomal proliferator-activated receptor agonists to boost the anti-ageing potential of retinoids. Int. J. Cosmet. Sci. 2021, 43, 619–626. [Google Scholar] [CrossRef]
- Wood, P.L. Fatty Acyl Esters of Hydroxy Fatty Acid (FAHFA) Lipid Families. Metabolites 2020, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Brejchova, K.; Balas, L.; Paluchova, V.; Brezinova, M.; Durand, T.; Kuda, O. Understanding FAHFAs: From structure to metabolic regulation. Prog. Lipid Res. 2020, 79, 101053. [Google Scholar] [CrossRef] [PubMed]
- Riecan, M.; Paluchova, V.; Lopes, M.; Brejchova, K.; Kuda, O. Branched and Linear Fatty Acid Esters of Hydroxy Fatty Acids (FAHFA) Relevant to Human Health. Pharmacol. Ther. 2021, 231, 107972. [Google Scholar] [CrossRef] [PubMed]
- Kokotou, M.G.; Kokotos, A.C.; Gkikas, D.; Mountanea, O.G.; Mantzourani, C.; Almutairi, A.; Lei, X.; Ramanadham, S.; Politis, P.K.; Kokotos, G. Saturated Hydroxy Fatty Acids Exhibit a Cell Growth Inhibitory Activity and Suppress the Cytokine-Induced β-Cell Apoptosis. J. Med. Chem. 2020, 63, 12666–12681. [Google Scholar] [CrossRef] [PubMed]
- Calonghi, N.; Cappadone, C.; Pagnotta, E.; Farruggia, G.; Buontempo, F.; Boga, C.; Brusa, G.L.; Santucci, M.A.; Masotti, L. 9-Hydroxystearic acid upregulates p21WAF1 in HT29 cancer cells. Biochem. Biophys. Res. Commun. 2004, 314, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Calonghi, N.; Cappadone, C.; Pagnotta, E.; Boga, C.; Bertucci, C.; Fiori, J.; Tasco, G.; Casadio, R.; Masotti, L. Histone deacetylase 1: A target of 9-hydroxystearic acid in the inhibition of cell growth in human colon cancer. J. Lipid Res. 2005, 46, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Calonghi, N.; Pagnotta, E.; Parolin, C.; Tognoli, C.; Boga, C.; Masotti, L. 9-Hydroxystearic acid interferes with EGF signalling in a human colon adenocarcinoma. Biochem. Biophys. Res. Commun. 2006, 342, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Pagnotta, E.; Calonghi, N.; Boga, C.; Masotti, L. N-methylformamide and 9-hydroxystearic acid: Two anti-proliferative and differentiating agents with different modes of action in colon cancer cells. Anti-Cancer Drugs 2006, 17, 521–526. [Google Scholar] [CrossRef]
- Calonghi, N.; Pagnotta, E.; Parolin, C.; Molinari, C.; Boga, C.; Dal Piaz, F.; Brusa, G.L.; Santucci, M.A.; Masotti, L. Modulation of apoptotic signalling by 9-hydroxystearic acid in osteosarcoma cells. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2007, 1771, 139–146. [Google Scholar] [CrossRef]
- Parolin, C.; Calonghi, N.; Presta, E.; Boga, C.; Caruana, P.; Naldi, M.; Andrisano, V.; Masotti, L.; Sartor, G. Mechanism and stereoselectivity of HDAC I inhibition by (R)-9-hydroxystearic acid in colon cancer. Biochim. Biophys. Acta 2012, 1821, 1334–1340. [Google Scholar] [CrossRef]
- Albadri, S.; Naso, F.; Marion, T.; Gauron, C.; Parolin, C.; Vougny, J.; Duroure, K.; Fiori, J.; Boga, C.; Vriz, S.; et al. Redox signaling via lipid peroxidation regulates retinal progenitor cell differentiation. Developmental Cell 2019, 50, 73–89.e6. [Google Scholar] [CrossRef] [PubMed]
- Busi, A.; Aluigi, A.; Guerrini, A.; Boga, C.; Sartor, G.; Calonghi, N.; Sotgiu, G.; Posati, T.; Corticelli, F.; Fiori, J.; et al. Unprecedented behavior of (9R)-9-hydroxystearic acid loaded keratin nanoparticles on cancer cell cycle. Mol. Pharm. 2019, 16, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, G.; Boga, C.; Telese, D.; Cassani, M.C.; Boanini, E.; Nitti, P.; Ballarin, B.; Ghirri, A.; Barucca, G.; Rinaldi, D. Magnetic Nanoparticles Coated with (R)-9-Acetoxystearic Acid for Biomedical Applications. ACS Omega 2020, 5, 12707–12715. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Torricelli, P.; Boga, C.; Micheletti, G.; Cassani, M.C.; Fini, M.; Bigi, A. (9R)-9-Hydroxystearate-Functionalized Hydroxyapatite as Anti-Proliferative and Cytotoxic Agent towards Osteosarcoma Cells. Langmuir 2016, 32, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Cassani, M.C.; Rubini, K.; Boga, C.; Bigi, A. (9R)-9-Hydroxystearate-Functionalized Anticancer Ceramics Promote Loading of Silver Nanoparticles. Nanomaterials 2018, 8, 390. [Google Scholar] [CrossRef]
- Asaro, F.; Boga, C.; De Zorzi, R.; Geremia, S.; Gigli, L.; Nitti, P.; Semeraro, S. (R)-10-Hydroxystearic Acid: Crystals vs. Organogel. Int. J. Mol. Sci. 2020, 21, 8124. [Google Scholar] [CrossRef]
- Cristofolini, L.; Fontana, M.P.; Boga, C.; Konovalov, O. Microscopic Structure of Cristalline Langmuir Monolayers of Hydroxystearic Acids by X-ray Reflectivity and GID: OH Group Position and Dimensionality Effect. Langmuir 2005, 21, 11213–11219. [Google Scholar] [CrossRef]
- Asaro, F.; Boga, C.; Demitri, N.; De Zorzi, R.; Drioli, S.; Gigli, L.; Micheletti, G.; Nitti, P.; Zangrando, E. X-Ray Crystal Structures and Organogelator Properties of (R)-9-Hydroxystearic Acid. Molecules 2019, 24, 2854. [Google Scholar] [CrossRef]
- Ebert, C.; Felluga, F.; Forzato, C.; Foscato, M.; Gardossi, L.; Nitti, P.; Pitacco, G.; Boga, C.; Caruana, P.; Micheletti, G.; et al. Enzymatic kinetic resolution of hydroxystearic acids: A combined experimental and molecular modelling investigation. J. Molec. Catal. B Enzym. 2012, 83, 38–45. [Google Scholar] [CrossRef]
- Boga, C.; Drioli, S.; Forzato, C.; Micheletti, G.; Nitti, P.; Prati, F. An Easy Route to Enantiomerically Enriched 7- and 8-Hydroxystearic Acids by Olefin-Metathesis-Based Approach. Synlett 2016, 27, 1354–1358. [Google Scholar] [CrossRef][Green Version]
- Calonghi, N.; Boga, C.; Telese, D.; Bordoni, S.; Sartor, G.; Torsello, C.; Micheletti, G. Synthesis of 9-Hydroxystearic Acid Derivatives and Their Antiproliferative Activity on HT 29 Cancer Cells. Molecules 2019, 24, 3714. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, C.; Hudaib, M.; Boga, C.; Calonghi, N.; Cappadone, C.; Masotti, L. Gas chromatography/mass spectrometric assay of endogenous cellular lipid peroxidation products: Quantitative analysis of 9- and 10-hydroxystearic acids. Rapid Commun. Mass Spectr. 2002, 16, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Kasprowicz, R.; Suman, R.; O’Toole, P. Characterising live cell behaviour: Traditional label-free and quantitative phase imaging approaches. Int. J. Biochem. Cell Biol. 2017, 84, 89–95. [Google Scholar] [CrossRef]
- Marrison, J.; Räty, L.; Marriott, P.; O’Toole, P. Ptychography—A label free, high-contrast imaging technique for live cells using quantitative phase information. Sci. Rep. 2013, 3, 2369. [Google Scholar] [CrossRef]
- Nelson, A.T.; Kolar, M.J.; Chu, Q.; Syed, I.; Kahn, B.B.; Saghatelian, A.; Siegel, D. Stereochemistry of Endogenous Palmitic Acid Ester of 9-Hydroxystearic Acid and Relevance of Absolute Configuration to Regulation. J. Am. Chem. Soc. 2017, 139, 4943–4947. [Google Scholar] [CrossRef] [PubMed]
- Asaro, F.; Drioli, S.; Forzato, C.; Nitti, P. An Efficient Synthesis of Chiral Non-Racemic Hydroxyalkanoic Acids by Olefin Cross-Metathesis Reactions. ChemistrySelect 2018, 3, 13372–13376. [Google Scholar] [CrossRef]
- Satyanarayana, S.; Reddy, B.V.S.; Narender, R. A Concise Total Synthesis of Lyngbic Acid, Hermitamides A and B. Tetrahedron Lett. 2014, 55, 6027–6029. [Google Scholar] [CrossRef]
- Rybak, A.; Meier, M.A.R. Cross-Metathesis of Fatty Acid Derivatives with Methyl Acrylate: Renewable Raw Materials for the Chemical Industry. Green Chem. 2007, 9, 1356–1361. [Google Scholar] [CrossRef]
- Zha, S.; Kuwano, K.; Shibahara, T.; Ishibashi, F. Algicidal Hydroxylated C18 Unsaturated Fatty Acids from the Red Alga Tricleocarpa Jejuensis: Identification, Synthesis and Biological Activity. Fitoterapia 2020, 145, 104639. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Ötvös, S.B.; Wu, Y.-C.; Mándity, I.M.; Chang, F.-R.; Fülöp, F. Highly Selective Continuous-Flow Synthesis of Potentially Bioactive Deuterated Chalcone Derivatives. ChemPlusChem 2015, 80, 859–864. [Google Scholar] [CrossRef]
- Bergstrŏm, S.; Aulin Erdtman, G.; Rolander, B.; Stenhagen, E.; Östling, S. The Monoketo- and Monohydroxyoctadecanoic Acids. Preparation and characterization by Thermal and X-ray Methods. Acta Chem. Scand. 1952, 6, 1157–1174. [Google Scholar] [CrossRef]
- Tullock, A.P. Solvent effects on the nuclear magnetic resonance spectra of methyl hydroxystearates. J. Am. Oil Chem. Soc. 1966, 43, 670–674. [Google Scholar] [CrossRef]
- Tullock, A.P. Carbon-13 NMR spectra of all the isomeric methyl hydroxy- and acetoxyoctadecanoates. Determination of chemical shifts by deuterium isotope effects. Org. Magn. Res. 1978, 11, 109–115. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Beckwith, A.L.J.; Goosen, A. 28. Photochemical transformations. Part XVI. A novel synthesis of lactones. J. Chem. Soc. 1965, 181–190. [Google Scholar] [CrossRef]
- Knothe, G.; Bagby, M.O.; Weisleder, D.; Peterson, R.E. Allylic Mono- and Di-hydroxylation of Isolated Double Bonds with Selenium Dioxide-tert-Butyl Hydroperoxide. NMR Characterization of Long-chain Enols, Allylic and Saturated 1,4-Diols, and Enones. J. Chem. Soc. Perkin Trans. II 1994, 1661–1670. [Google Scholar] [CrossRef]
- Calonghi, N.; Farruggia, G.; Boga, C.; Micheletti, G.; Fini, E.; Romani, L.; Telese, D.; Faraci, E.; Bergamini, C.; Cerini, S.; et al. Root Extracts of Two Cultivars of Paeonia Species: Lipid Composition and Biological Effects on Different Cell Lines: Preliminary Results. Molecules 2021, 26, 655. [Google Scholar] [CrossRef]
- Barer, R. Interference microscopy and mass determination. Nature 1952, 169, 366–367. [Google Scholar] [CrossRef]
- Zangle, T.A.; Teitell, M.A. Live-cell mass profiling: An emerging approach in quantitative biophysics. Nat. Methods 2014, 11, 1221–1228. [Google Scholar] [CrossRef]
- Maiden, A.M.; Rodenburg, J.M.; Humphry, M.J. Optical ptychography: A practical implementation with useful resolution. Opt. Lett. 2010, 35, 2585–2587. [Google Scholar] [CrossRef]
- Suman, R.; Smith, G.; Hazel, K.E.; Kasprowicz, R.; Coles, M.; O’Toole, P.; Chawla, S. Label-free imaging to study phenotypic behavioural traits of cells in complex co-cultures. Sci. Rep. 2016, 6, 22032. [Google Scholar] [CrossRef]

| 5-HSA IC50 | 7-HSA IC50 | 8-HSA IC50 | 9-HSA IC50 | 10-HSA IC50 | 11HSA IC50 | |
|---|---|---|---|---|---|---|
| CaCo2 R square | 25.1 μM 0.8889 | 42.5 μM 0.9731 | > 100μM | 32.6 μM 0.9472 | 68.3 μM 0.9730 | 27.6 μM 0.9944 |
| HT29 R square | 51.3 μM 0.9575 | 14.7 μM 0.9255 | > 100μM | 30.7 μM 0.9635 | 77.2 μM 0.9048 | 56.9 μM 0.8918 |
| HeLa R square | 22.1 μM 0.9886 | 26.6 μM 0.9703 | > 100μM | 26.9 μM 0.9321 | 41.7 μM 0.8073 | 31.5 μM 0.8981 |
| MCF7 R square | 46.4 μM 0.9820 | 21.4 μM 0.9447 | > 100μM | 49.1 μM 0.9866 | 38.7 μM 0.9599 | 35.8 μM 0.9681 |
| PC3 R square | 31.6 μM 0.9516 | 24.3 μM 0.9183 | > 100μM | 23.4 μM 0.9499 | 34.0 μM 0.8826 | 61.4 μM 0.9610 |
| NLF R square | 38.5 μM 0.9971 | 24.9 μM 0.8366 | > 100μM | 33.1 μM 0.9960 | 74.6 μM 0.9887 | 29.7 μM 0.9841 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calonghi, N.; Boga, C.; Nitti, P.; Telese, D.; Bordoni, S.; Farruggia, G.; Asaro, F.; Grandi, M.; Zalambani, C.; Micheletti, G. Effects of Regioisomerism on the Antiproliferative Activity of Hydroxystearic Acids on Human Cancer Cell Lines. Molecules 2022, 27, 2396. https://doi.org/10.3390/molecules27082396
Calonghi N, Boga C, Nitti P, Telese D, Bordoni S, Farruggia G, Asaro F, Grandi M, Zalambani C, Micheletti G. Effects of Regioisomerism on the Antiproliferative Activity of Hydroxystearic Acids on Human Cancer Cell Lines. Molecules. 2022; 27(8):2396. https://doi.org/10.3390/molecules27082396
Chicago/Turabian StyleCalonghi, Natalia, Carla Boga, Patrizia Nitti, Dario Telese, Silvia Bordoni, Giovanna Farruggia, Fioretta Asaro, Martina Grandi, Chiara Zalambani, and Gabriele Micheletti. 2022. "Effects of Regioisomerism on the Antiproliferative Activity of Hydroxystearic Acids on Human Cancer Cell Lines" Molecules 27, no. 8: 2396. https://doi.org/10.3390/molecules27082396
APA StyleCalonghi, N., Boga, C., Nitti, P., Telese, D., Bordoni, S., Farruggia, G., Asaro, F., Grandi, M., Zalambani, C., & Micheletti, G. (2022). Effects of Regioisomerism on the Antiproliferative Activity of Hydroxystearic Acids on Human Cancer Cell Lines. Molecules, 27(8), 2396. https://doi.org/10.3390/molecules27082396