Chemical Diversity of Essential Oil of Valeriana jatamansi from Different Altitudes of Himalaya and Distillation Methods
Abstract
:1. Introduction
2. Results
2.1. Essential Oil Content
2.2. Composition of Essential Oil
2.3. Principal Component Analysis (PCA)
2.4. Physico-Chemical Properties of Soil of Naturally Distributed Locations of Valeriana jatamansi
3. Discussion
4. Materials and Methods
4.1. Study Material
4.2. Soil Collection and Analysis
4.3. Essential Oil Extraction
4.4. GC–MS Analysis
4.5. GC Analysis
4.6. Identification of Components
4.7. Experimental Design and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- The Angiosperm Phylogeny (APG). An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants, APG III. Bot. J. Linn. Soc. 2009, 161, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Reveal, J.L.; Chase, M.W. APG III: Bibliographical information and synonymy of Magnoliidae. Phytotaxa 2011, 19, 71. [Google Scholar] [CrossRef] [Green Version]
- Rather, A.M.; Nawchoo, I.A.; Ganie, A.H.; Singh, H.; Dutt, B.; Wani, A.A. Bioactive compounds & medicinal properties of Valeriana jatamansi Jones-a review. J. Life Sci. 2012, 9, 952–955. [Google Scholar]
- Jugran, A.K.; Bhatt, I.D.; Rawal, R.S.; Nandi, S.K.; Pande, V. Patterns of morphological and genetic diversity of Valeriana jatamansi Jones in different habitats and altitudinal range of West Himalaya, India. Flora-Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 13–21. [Google Scholar] [CrossRef]
- Mathela, C.S.; Chanotiya, C.S.; Sammal, S.S.; Pant, A.K.; Pandey, S. Compositional diversity of terpenoids in the himalayan valeriana genera. Chem. Biodivers. 2005, 2, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, B.; Sharma, P.; Shivani; Pal, P.K. Biology, chemical diversity, agronomy, conservation and industrial importance of Valeriana jatamansi: A natural sedative. J. Appl. Res. Med. Aromat. Plants 2020, 16, 100243. [Google Scholar] [CrossRef]
- Rivera, J.O.; Loya, A.M.; Ceballos, R. Use of herbal medicines and implications for conventional drug therapy medical sciences. Alter. Integr. Med. 2013, 2, 130. [Google Scholar] [CrossRef]
- Jugran, A.K.; Rawat, S.; Bhatt, I.D.; Rawal, R.S. Valeriana jatamansi: An herbaceous plant with multiple medicinal uses. Phytother. Res. 2019, 33, 482–503. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J. Valerian the Genus Valeriana; Harwood Academic Publishers: Reading, UK, 1997; pp. 155–178. [Google Scholar]
- Mishra, L.C. Scientific Basis for Ayurvedic Therapies; CRC Press: New York, NY, USA, 2004. [Google Scholar]
- Baby, R.; Cabezas, M.; Castro, E.; Filip, R.; Walsöe de Reca, N.E. Quality control of medicinal plants with an electronic nose. Sens. Actuators B Chem. 2005, 106, 24–28. [Google Scholar] [CrossRef]
- Ming, D.S.; Yu, D.Q.; Yang, Y.Y.; He, C.H. The structures of three novel sesquiterpenoids from Valeriana jatamansi Jones. Tetrahedron Lett. 1997, 38, 5205–5208. [Google Scholar] [CrossRef]
- Prakash, V. Indian Valerianaceae: A Monograph on Medicinally Important Family; Scientific Publishers: Jodhpur, India, 1999; pp. 1–2. [Google Scholar]
- Violon, C.; van Cauwenbergh, N.; Vercruysse, A. Valepotriate content in different in vitro cultures of Valerianaceae and characterization of Valeriana officinalis L. callus during a growth period. Pharm. Weekbl. 1983, 5, 205–209. [Google Scholar] [CrossRef]
- Ernst, E. Scientific basis for ayurvedic therapies. Focus Altern. Complement Ther. 2010, 9, 243. [Google Scholar] [CrossRef]
- Kaur, R.; Sood, M.; Chander, M.; Mahajan, R.; Kumar, V.; Sharma, D.R. In vitro propagation of Valeriana jatamansi. Plant Cell Tissue Organ Cult. 1999, 59, 227–229. [Google Scholar] [CrossRef]
- Wagner, H.; Jurcic, K.; Schaette, R. Comparative studies on the sedative action of Valeriana extracts, valepotriates and their degradation products. Planta Med. 1980, 39, 358–365. [Google Scholar] [CrossRef]
- Diapher, A.; Hindwarch, I. A double blind placebo controlled investigation of the effects of two doses of valerian preparation on the sleep, cognitive and psychomotor function of sleep disturbed order adults. Phytother Res. 2004, 18, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Chopra, R.; Nayar, S.; Chopra, I. Glossary of Indian Medicinal Plants, 1st ed.; CSIR Publication: New Delhi, India, 1956; Volume 251, p. 14. [Google Scholar]
- Pande, A.; Shukla, Y.N. Naphthoic acid derivative from Valeriana wallichii. Phytochemistry 1993, 32, 1350–1351. [Google Scholar] [CrossRef]
- You, J.S.; Peng, M.; Shi, J.L.; Zheng, H.Z.; Liu, Y.; Zhao, B.S.; Guo, J.Y. Evaluation of anxiolytic activity of compound Valeriana jatamansi Jones in mice. BMC Complement Altern. Med. 2012, 12, 223. [Google Scholar] [CrossRef] [Green Version]
- Bagchi, P.; Hopper, W. Virtual screening of compounds with Valeriana jatamansi with α–synuclein. In Proceedings of the International Conference on Bioscience, Biochemistry, and Bioinformatics, IPCBEE, Singapore, 26–28 February 2011; pp. 1–4. [Google Scholar]
- Purohit, S.S.; Vyas, S.P. Medicinal Plant Cultivation-A Scientific Approach; Agrobios: Jodhpur, India, 2005; p. 228. [Google Scholar]
- Bos, R.; Woerdenbag, H.; van Putten, F.; Hendriks, H.; Scheffer, J. Seasonal variation of the essential oil, valerenic acid and derivatives, and valepotriates in Valeriana officinalis roots and rhizomes, and the selection of plants suitable for phytomedicines. Planta. Med. 1998, 64, 143–147. [Google Scholar] [CrossRef]
- Sundaresan, V.; Sahni, G.; Verma, R.S.; Padalia, R.C.; Mehrotra, S.; Thul, S.T. Impact of geographic range on genetic and chemical diversity of Indian valerian (Valeriana jatamansi) from North-Western Himalaya. Biochem. Genet. 2012, 50, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Zhou, L.; Liu, Z.L. Identification of insecticidal constituents from the essential oil of Valeriana jatamansi Jones against Liposcelis bostrychophila Badonnel. J. Chem. 2013, 853912. [Google Scholar]
- Singh, S.K.; Katoch, R.; Kapila, R.K. Chemotypic variation for essential oils in Valeriana jatamansi Jones populations from Himachal Pradesh. J. Essent. Oil Res. 2013, 25, 154–159. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chauhan, A. Chemical differentiation of rhizome and root essential oils of Indian Valerian (Valeriana jatamansi Jones). J. Essent. Oil-Bear. Plants 2013, 16, 835–840. [Google Scholar] [CrossRef]
- Raina, A.P.; Negi, K.S. Essential oil composition of Valeriana jatamansi Jones from Himalayan regions of India. Indian J. Pharm. Sci. 2015, 77, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.D.; Gopichand; Meena, R.L.; Sharma, B.; Singh, B.; Kaul, V.K.; Ahuja, P.S. Seasonal variation of bioactive components in Valeriana jatamansi from Himachal Pradesh, India. Ind. Crops Prod. 2010, 32, 292–296. [Google Scholar] [CrossRef]
- Sati, S.; Chanotiya, C.S.; Mathela, C.S. Comparative investigations on the leaf and root oils of Valeriana wallichii DC from Northwestern Himalaya. J. Essent. Oil Res. 2005, 17, 408–409. [Google Scholar] [CrossRef]
- Singh, S.K.; Katoch, R.; Kapila, R.K. Genetic and biochemical diversity among Valeriana jatamansi populations from Himachal Pradesh. Sci. World J. 2015, 863913. [Google Scholar] [CrossRef] [Green Version]
- Rajkumar, S.; Singh, S.K.; Nag, A.; Ahuja, P.S. Genetic structure of Indian valerian (Valeriana jatamansi) populations in Western Himalaya revealed by AFLP. Biochem. Genet. 2011, 49, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Jugran, A.K.; Bahukhandi, A.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Nandi, S.K. Impact of altitudes and habitats on valerenic acid, total phenolics, flavonoids, tannins, and antioxidant activity of Valeriana jatamansi. Appl. Biochem. Biotechnol. 2016, 179, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, I.D.; Dauthal, P.; Rawat, S.; Gaira, K.S.; Jugran, A.K.; Rawal, R.S.; Dhar, U. Characterization of essential oil composition, phenolic content, and antioxidant properties in wild and planted individuals of Valeriana jatamansi Jones. Sci. Hortic. 2012, 136, 61–68. [Google Scholar] [CrossRef]
- Mahajan, M.; Pal, P.K. Flower yield and chemical composition of essential oil from Rosa damascena under foliar application of Ca(NO3)2 and seasonal variation. Acta Physiol. Plant. 2020, 42, 23. [Google Scholar] [CrossRef]
- Thusoo, S.; Gupta, S.; Sudan, R.; Kaur, J.; Bhagat, S.; Hussain, R.; Bhagat, M. Antioxidant activity of essential oil and extracts of Valeriana jatamansi roots. BioMed. Res. Int. 2014, 614187. [Google Scholar]
- Zheljazkov, V.D.; Astatkie, T.; Schlegel, V. Effects of distillation time on the Pinus ponderosa essential oil yield, composition, and antioxidant activity. HortScience 2012, 47, 785–789. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Horgan, T.; Schlegel, V.; Schlegel, X. Distillation time effect on essential oil yield, composition, and antioxidant capacity of Sweet Sagewort (Artemisia annua L.) Oil. HortScience 2013, 48, 1288–1292. [Google Scholar] [CrossRef] [Green Version]
- Valtcho, D.; Zheljazkov, V.D.; Astatkie, T. Effect of distillation time on Mentha canadensis essential oil yield and composition. HortScience 2012, 47, 643–647. [Google Scholar] [CrossRef]
- Cannon, J.B.; Cantrell, C.L.; Astatkie, T.; Zheljazkov, V.D. Modification of yields and composition of essential oils by distillation time. Ind. Crops Prod. 2013, 41, 214–220. [Google Scholar] [CrossRef]
- Verma, R.S.; Verma, R.K.; Padalia, R.C.; Chauhan, A.; Singh, A.; Singh, H.P. Chemical diversity in the essential oil of Indian Valerian (Valeriana jatamansi Jones). Chem. Biodivers. 2011, 8, 1921–1929. [Google Scholar] [CrossRef]
- Rawat, S.; Jugran, A.K.; Bhatt, I.D.; Rawal, R.S.; Andola, H.C.; Dhar, U. Essential oil composition and antioxidant activity in Valeriana jatamansi Jones: Influence of seasons and growing sources. J. Essent. Oil Res. 2017, 29, 101–107. [Google Scholar] [CrossRef]
- Harsal, A.E.; Mansour, A.I.; Senhaji, N.S.; Khay, E.O.; Bouhdid, S.; Amajoud, N.; Farah, A.; Belmehdi, O.; Abrini, J. Influence of extraction time on the yield, chemical composition, and antibacterial activity of the essential oil from Origanum elongatum (E. & M.) harvested at Northern Morocco. J. Essent. Oil-Bear. Plants 2018, 21, 1460–1474. [Google Scholar] [CrossRef]
- Baydar, H.; Schulz, H.; Krüger, H.; Erbas, S.; Kineci, S. Influences of fermentation time, hydro-distillation time and fractions on essential oil composition of Damask Rose (Rosa damascena Mill.). J. Essent. Oil-Bear. Plants 2008, 11, 224–232. [Google Scholar] [CrossRef]
- Lawrence, B.M. Essential oil production. A discussion of influencing factors. In Biogeneration of Aromas; Parliament, T.H., Croteau, R., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1986; pp. 363–369. [Google Scholar] [CrossRef]
- Telci, I.; Bayram, E.; Yılmaz, G.; Avcı, B. Variability in essential oil composition of Turkish basils (Ocimum basilicum L.). Biochem. Syst. Ecol. 2006, 34, 489–497. [Google Scholar] [CrossRef]
- Telci, I.; Sahbaz, N.; Yilmaz, G.; Tugay, M.E. Agronomical and chemical characterization of Spearmint (Mentha spicata L.) originating in Turkey. Econ. Bot. 2004, 58, 721–728. [Google Scholar] [CrossRef]
- Rapposelli, E.; Melito, S.; Barmina, G.G.; Foddai, M.; Azara, E.; Scarpa, G.M. Relationship between soil and essential oil profiles in Salvia desoleana populations: Preliminary results. Nat. Prod. Commun. 2015, 10, 1615–1618. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.; Kyotani, Y.; Manabe, S.; Suzuki, M. Total synthesis of (±)-patchouli alcohol and (±)-seychellene via a common homoisotwistane intermediate. Tetrahedron 1979, 35, 293–298. [Google Scholar] [CrossRef]
- Das, J.; Mao, A.A.; Handique, P.J. Terpenoid compositions and antioxidant activities of two Indian valerian oils from the Khasi hills of north-east India. Nat. Prod. Commun. 2011, 6, 129–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguel, G.; Cruz, C.; Faleiro, M.L.; Simões, M.T.F.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Salvia officinalis L. essential oils: Effect of hydrodistillation time on the chemical composition, antioxidant and antimicrobial activities. Nat. Prod. Res. 2011, 25, 526–541. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Cantrell, C.L.; Astatkie, T.; Jeliazkova, E. Distillation time effect on lavender essential oil yield and composition. J. Oleo Sci. 2013, 62, 195–199. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Shiwakoti, S.; Poudyal, S.; Horgan, T.; Kovatcheva, N.; Dobreva, A. Essential oil yield and composition of garden sage as a function of different steam distillation times. Hort. Sci. 2014, 49, 785–790. [Google Scholar] [CrossRef]
- Liu, X.; Yang, D.; Liu, J.; Ren, N. Analysis of essential oils from Voacanga africana seeds at different hydro distillation extraction stages: Chemical composition, antioxidant activity and antimicrobial activity. Nat. Prod. Res. 2015, 29, 1950–1953. [Google Scholar] [CrossRef] [PubMed]
- Ratri, P.J.; Ayurini, M.; Khumaini, K.; Rohbiya, A. Clove oil extraction by steam distillation and utilization of clove buds waste as potential candidate for eco-friendly packaging. J. Bahan Alam Terbarukan 2020, 9, 47–54. [Google Scholar] [CrossRef]
- Jugran, A.K.; Rawat, S.; Bhatt, I.D.; Rawal, R.S. Essential oil composition, phenolics and antioxidant activities of Valeriana jatamansi at different phenological stages. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2020, 155, 1–14. [Google Scholar] [CrossRef]
- Moghrani, H.; Maachi, R. Valorization of Myrtus communis essential oil obtained by steam driving distillation. Asian J. Sci. Res. 2008, 1, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Dieckmann, R.H.; Palamand, S.R. Autoxidation of some constituents of hops. I. The monoterpene hydrocarbon, myrcene. J. Agric. Food Chem. 1974, 22, 498–503. [Google Scholar] [CrossRef]
- Velasco, J.; Dobarganes, C. Oxidative stability of virgin olive oil. Eur. J. Lipid Sci. Technol. 2002, 104, 661–676. [Google Scholar] [CrossRef]
- Raharjo, J.S.; Chanif, M.; Nurdiana, N.; Takheshi, K.; Fatchiyah, F. In vitro and in silico: Selectivities of seychellene compound as candidate cyclooxygenase isoenzyme inhibitor on pre-osteoblast cells. Curr. Enzym. Inhib. 2017, 13, 2–10. [Google Scholar] [CrossRef]
- Dwivedi, C.; Guan, X.; Harmsen, W.L.; Voss, A.L.; Goetz-Parten, D.E.; Koopman, E.M.; Johnson, K.M.; Valluri, H.B.; Matthees, D.P. Chemopreventive effects of alpha-santalol on skin tumor development in CD-1 and SENCAR mice. Cancer Epidemiol. Biomark. Prev. 2003, 12, 151–156. [Google Scholar]
- Baldovini, N.; Delasalle, C.; Joulain, D. Phytochemistry of the heartwood from fragrant Santalum species: A review. Flavour Fragr. J. 2010, 26, 7–26. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Y.; Wu, J.; Gao, M.; Wang, A.; Xu, B. Beta-elemene inhibits melanoma growth and metastasis via suppressing vascular endothelial growth factor-mediated angiogenesis. Cancer Chemother. Pharmacol. 2011, 67, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall: Englewood Cliffs, NJ, USA, 1967. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–37. [Google Scholar] [CrossRef]
- Subbiah, B.V.; Asija, G.L. A rapid procedure for the determination of available nitrogen in soils. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Mehlich, A. Mehlich-3 soil test extractant: A modification of Mehlich-2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Stein, S.E. Mass Spectral Database Software, Version 3.02; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2005.
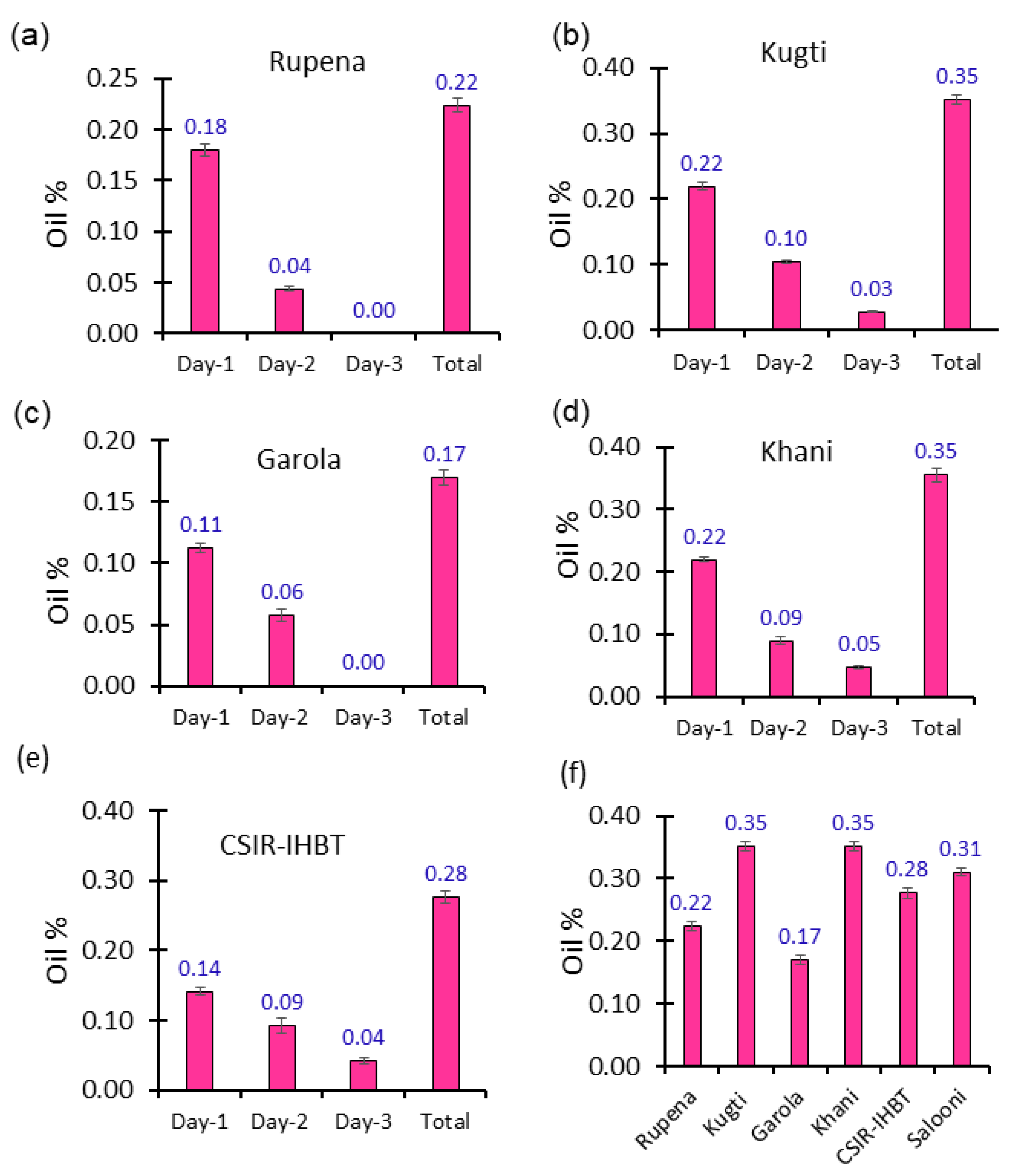
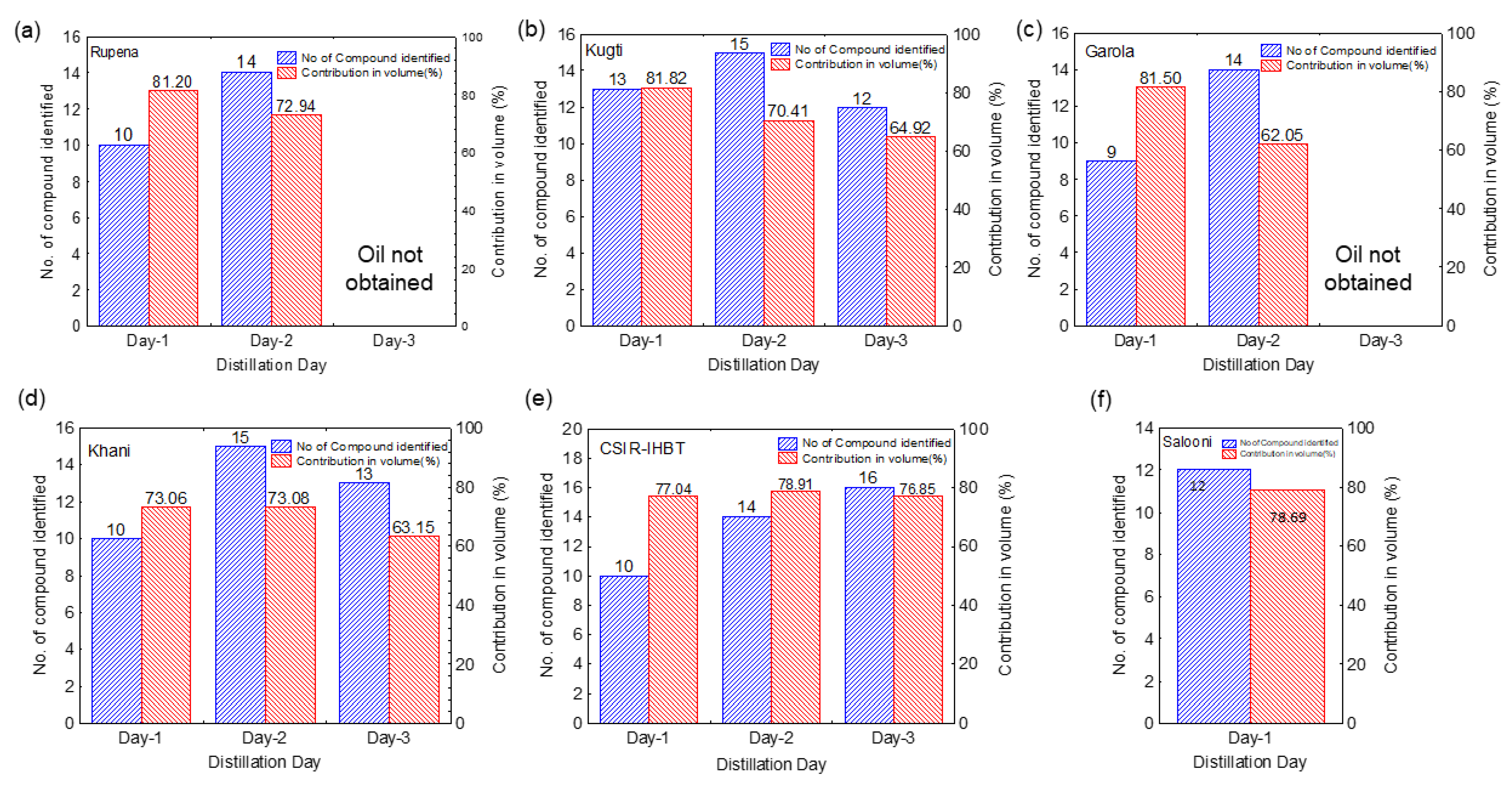
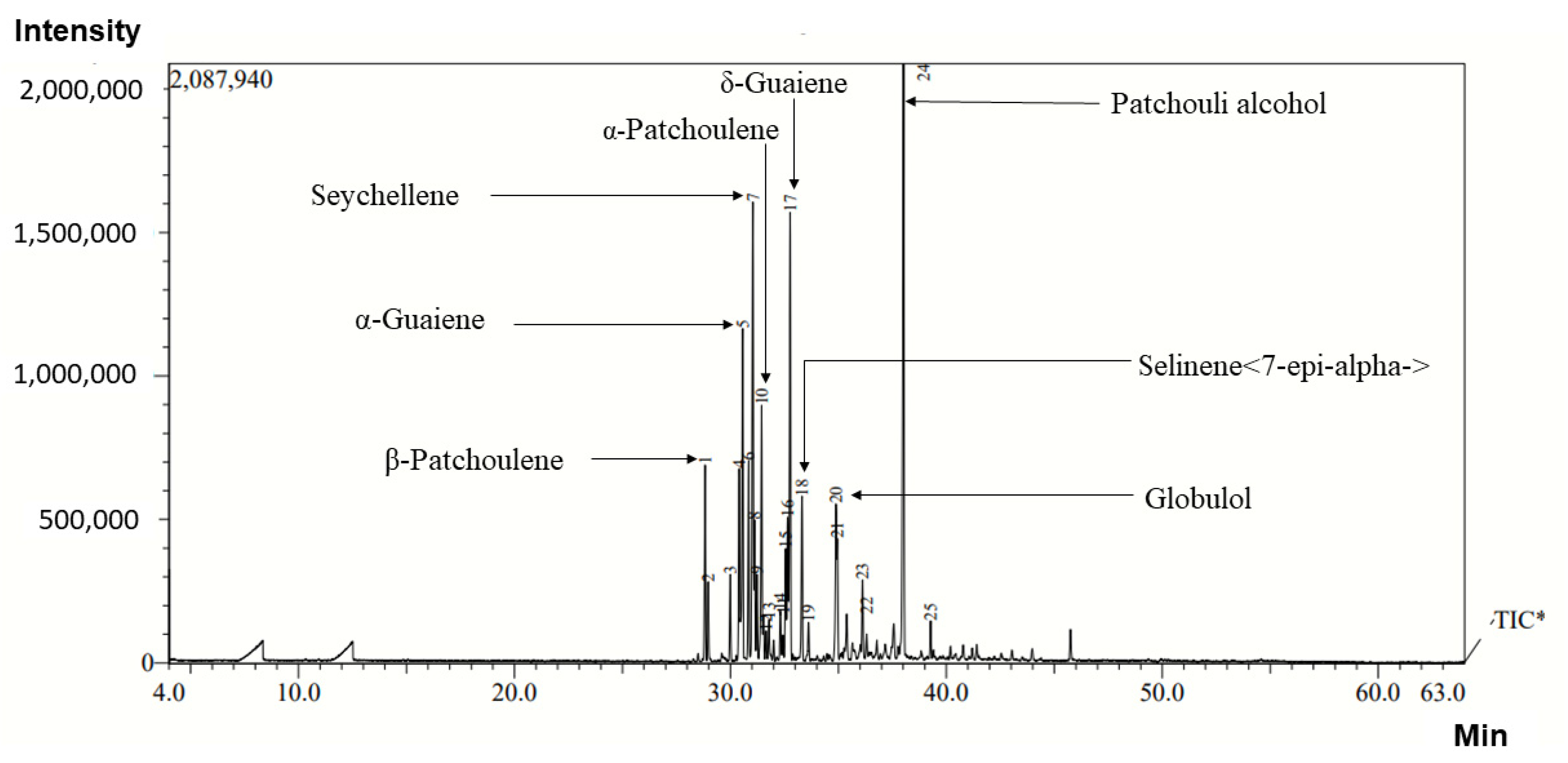
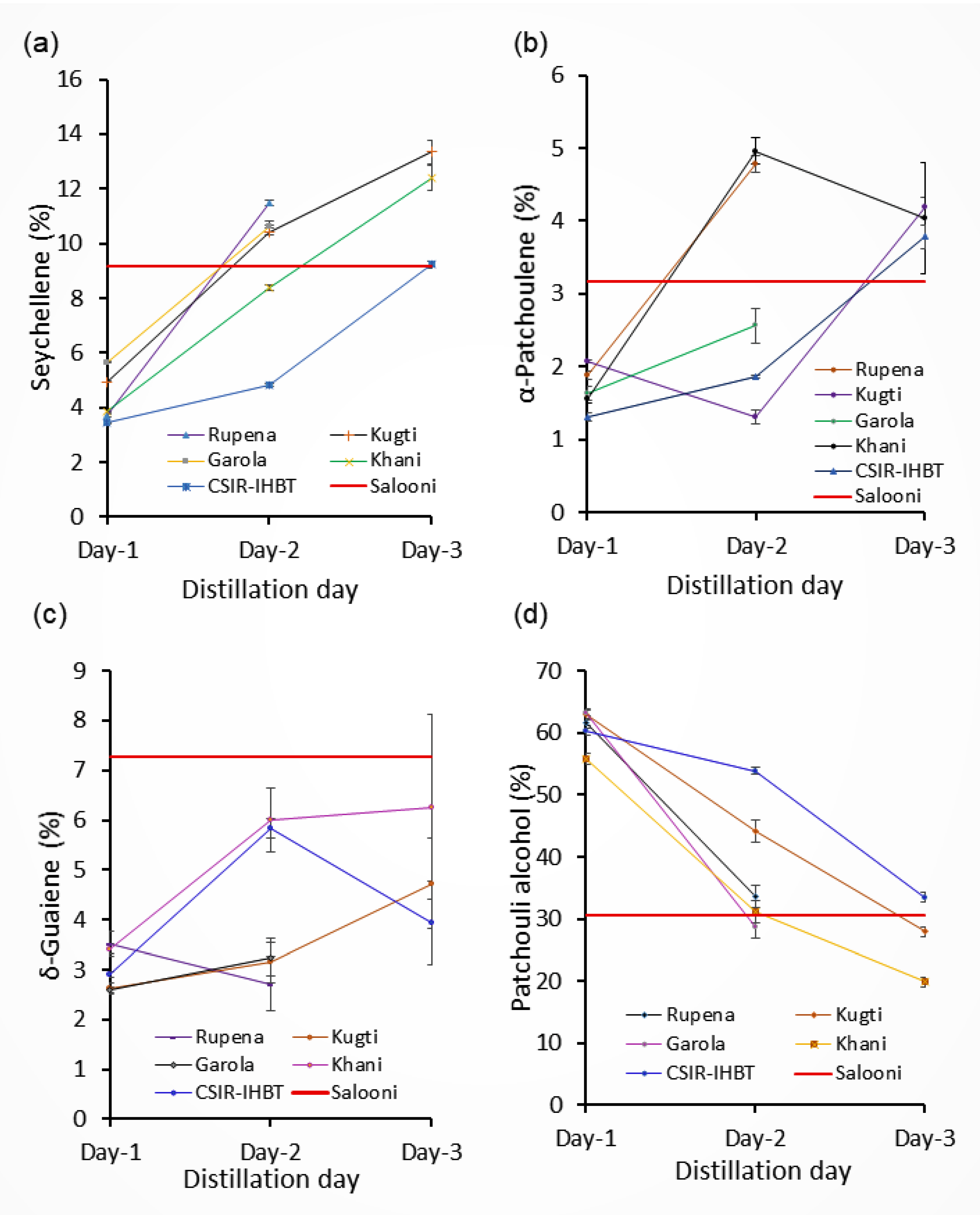

| Compound | Rupena | Kugti | Garola | Khani | CSIR-IHBT | Salooni | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day-1 | Day-2 | Day-1 | Day-2 | Day-3 | Day-1 | Day-2 | Day-1 | Day-2 | Day-3 | Day-1 | Day-2 | Day-3 | Day-1 | |
| β-Patchoulene | 4.5 ± 0.33 | 3.8 ± 0.27 | 1.9 ± 0.09 | - | - | 1.9 ± 0.11 | - | 2.0 ± 0.07 | 2.5 ± 0.12 | - | 1.7 ± 0.01 | 2.0 ± 0.08 | - | 10.2 ± 0.05 |
| β-Elemene | - | 1.2 ± 0.00 | 0.4 ± 0.01 | 0.7 ± 0.03 | 1.5 ± 0.03 | - | 1.3 ± 0.16 | - | 0.9 ± 0.02 | 1.7 ± 0.06 | - | 0.7 ± 0.02 | 2.9 ± 0.14 | 0.8 ± 0.03 |
| Santalene <alpha> | - | 1.1 ± 0.04 | - | 1.2 ± 0.01 | 1.2 ± 0.02 | - | 0.8 ± 0.20 | - | 1.1 ± 0.02 | 1.5 ± 0.05 | - | 0.7 ± 0.02 | 1.7 ± 0.04 | - |
| β-Gurjunene | - | - | - | 0.9 ± 0.03 | - | - | - | 0.9 ± 0.02 | 2.8 ± 0.05 | - | - | - | 1.5 ± 0.03 | - |
| α-Guaiene | 1.7 ± 0.02 | 2.2 ± 0.14 | 1.8 ± 0.08 | 0.7 ± 0.02 | 1.1 ± 0.03 | 1.9 ± 0.05 | 1.2 ± 0.16 | 1.7 ± 0.02 | 2.9 ± 0.15 | 4.1 ± 0.24 | 1.9 ± 0.18 | 1.1 ± 0.03 | 2.3 ± 0.13 | 5.5 ± 0.29 |
| Trans(.Beta.)-Caryophyllene | 0.9 ± 0.01 | 3.0 ± 0.01 | 1.1 ± 0.01 | 2.3 ± 0.04 | 3.1 ± 0.08 | - | 1.6 ± 0.02 | 0.9 ± 0.02 | 2.5 ± 0.03 | 3.8 ± 0.22 | 1.3 ± 0.02 | 1.8 ± 0.04 | 3.8 ± 0.06 | 1.8 ± 0.03 |
| Seychellene | 3.7 ± 0.05 | 11.5 ± 0.10 | 4.9 ± 0.04 | 10.4 ± 0.39 | 13.3 ± 0.40 | 5.7 ± 0.01 | 10.6 ± 0.04 | 3.8 ± 0.03 | 8.4 ± 0.10 | 12.4 ± 0.47 | 3.4 ± 0.04 | 4.8 ± 0.03 | 9.2 ± 0.14 | 9.2 ± 0.11 |
| α-Humulene | 1.0 ± 0.02 | - | 0.8 ± 0.01 | 1.5 ± 0.15 | - | 2.3 ± 0.03 | 0.8 ± 0.05 | 1.7 ± 0.03 | 3.4 ± 0.34 | 1.2 ± 0.04 | 1.5 ± 0.02 | 1.3 ± 0.04 | 3.3 ± 0.46 | 5.0 ± 0.06 |
| α-Patchoulene | 1.9 ± 0.06 | 4.8 ± 0.12 | 2.1 ± 0.02 | 1.3 ± 0.09 | 4.2 ± 0.14 | 1.6 ± 0.09 | 2.6 ± 0.24 | 1.6 ± 0.06 | 4.9 ± 0.18 | 4.0 ± 0.76 | 1.3 ± 0.05 | 1.8 ± 0.02 | 3.8 ± 0.16 | 3.2 ± 0.00 |
| β-Selinene | - | 0.9 ± 0.03 | - | 0.6 ± 0.13 | 0.8 ± 0.04 | - | 1.9 ± 0.01 | - | - | 1.7 ± 0.11 | - | - | 2.2 ± 0.04 | - |
| α-Selinene | - | 3.2 ± 0.33 | 0.5 ± 0.09 | 0.8 ± 0.03 | 2.3 ± 0.20 | - | 2.3 ± 0.11 | - | 2.4 ± 0.04 | 2.3 ± 0.10 | - | 1.1 ± 0.00 | 1.7 ± 0.24 | - |
| δ-Guaiene | 3.5 ± 0.26 | 2.7 ± 0.55 | 2.6 ± 0.12 | 3.14 ± 0.41 | 4.7 ± 0.90 | 2.6 ± 0.06 | 3.2 ± 0.37 | 3.4 ± 0.09 | 6.0 ± 0.64 | 6.3 ± 1.86 | 2.9 ± 0.06 | 5.8 ± 0.20 | 3.9 ± 0.84 | 7.3 ± 0.23 |
| Selinene<7-epi-alpha-> | 0.5 ± 0.10 | 3.1 ± 0.12 | 1.1 ± 0.02 | 1.0 ± 0.14 | 3.5 ± 0.06 | 1.4 ± 0.04 | 3.0 ± 0.01 | - | 1.5 ± 0.01 | 3.3 ± 0.10 | - | 1.9 ± 0.03 | 2.6 ± 0.07 | 2.2 ± 0.04 |
| Kessane | - | - | 0.4 ± 0.00 | 0.9 ± 0.06 | - | - | - | 1.1 ± 0.00 | 1.9 ± 0.07 | 1.2± 0.17 | 0.9 ± 0.02 | 0.4 ± 0.00 | 1.0 ± 0.03 | - |
| Spathulenol | - | - | - | - | - | - | 0.7 ± 0.02 | - | - | - | - | - | 2.8 ± 0.06 | 1.6 ± 0.08 |
| Epiglobulol/ Globulol | 1.8 ± 0.07 | 1.3 ± 0.03 | 1.2 ± 0.01 | 0.9 ± 0.06 | 1.1 ± 0.07 | 1.1 ± 0.09 | - | - | - | - | 1.9 ± 0.02 | 1.5 ± 0.04 | 0.7 ± 0.05 | 1.3 ± 0.01 |
| Humulene epoxide II | - | 0.7 ± 0.02 | - | - | - | - | 2.5 ± 0.04 | - | 0.6 ± 0.04 | - | - | - | - | - |
| Patchouli alcohol | 61.7 ± 0.49 | 33.6 ± 1.78 | 63.0 ± 0.91 | 44.1 ± 1.82 | 27.9 ± 0.77 | 63.1 ± 0.65 | 28.8 ± 1.85 | 55.8 ± 0.47 | 31.2 ± 1.05 | 19.83 ± 0.46 | 60.2 ± 0.63 | 53.8 ± 0.56 | 33.5 ± 0.68 | 30.7 ± 0.49 |
| Location | Soil Texture | pH | EC (25 °C) (dS m−1) | Organic Carbon (%) | Bulk Density (g cm−3) | Available N (kg ha−1) | Available P (kg ha−1) | Available K (kg ha−1) |
|---|---|---|---|---|---|---|---|---|
| Rupena (Chamba) | Sandy Loam | 7.61 | 0.847 | 3.22 | 1.25 | 284.53 | 18.54 | 1231.83 |
| Kugti (Chamba) | Loam | 8.85 | 0.484 | 1.23 | 1.48 | 80.22 | 12.45 | 642.54 |
| Garola (Chamba) | Silty Clay Loam | 7.81 | 1.074 | 0.71 | 1.47 | 61.12 | 33.75 | 1044.37 |
| Khani (Chamba) | Loam | 6.67 | 0.411 | 2.05 | 1.48 | 139.29 | 14.76 | 185.38 |
| CSIR-IHBT, Palampur | Silty Clay | 5.59 | 0.057 | 1.35 | 1.48 | 77.47 | 13.90 | 295.98 |
| Salooni (Chamba) | Sandy Loam | 6.40 | 0.210 | 3.34 | 1.34 | 190.89 | 37.62 | 218.68 |
| SEm (±) | 0.12 | 0.00 | 0.04 | 0.03 | 7.25 | 1.45 | 19.35 | |
| CD (p = 0.05) | 0.38 | 0.01 | 0.12 | 0.09 | 23.15 | 4.63 | 61.76 |
| Source of Variation | df | Parameters Tested | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Oil% | pH | EC (25 °C) (dS m−1) | Organic Carbon (%) | Bulk Density (g cm−3) | Available N (kg ha−1) | Available P (kg ha−1) | Available K (kg ha−1) | ||
| Location | 5 | 0.016 ** | 4.07 ** | 0.442 ** | 3.592 ** | 0.027 ** | 22,306.07 ** | 361.875 ** | 604,904.63 ** |
| Error | 12 | 0.00 | 0.042 | 0.00 | 0.003 | 0.002 | 157.491 | 6.529 | 960.476 |
| Location | Altitude (m) | Latitude | Longitude |
|---|---|---|---|
| Rupena (Chamba) | 1810 | 32°27′68″ N | 76°04′54″ E |
| Kugti (Chamba) | 2140 | 32°34′35″ N | 72°27′82″ E |
| Garola (Chamba) | 2003 | 32°25′11″ N | 72°28′32″ E |
| Khani (Chamba) | 2014 | 32°27′39″ N | 76°29′1″ E |
| CSIR-IHBT, Palampur | 1354 | 32°06′61″ N | 76°33′77″ E |
| Salooni (Chamba) | 2032 | 32°26′43″ N | 76°32′10″ E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, B.K.; Shivani; Mahajan, M.; Pal, P.K. Chemical Diversity of Essential Oil of Valeriana jatamansi from Different Altitudes of Himalaya and Distillation Methods. Molecules 2022, 27, 2387. https://doi.org/10.3390/molecules27082387
Thakur BK, Shivani, Mahajan M, Pal PK. Chemical Diversity of Essential Oil of Valeriana jatamansi from Different Altitudes of Himalaya and Distillation Methods. Molecules. 2022; 27(8):2387. https://doi.org/10.3390/molecules27082387
Chicago/Turabian StyleThakur, Babit Kumar, Shivani, Mitali Mahajan, and Probir Kumar Pal. 2022. "Chemical Diversity of Essential Oil of Valeriana jatamansi from Different Altitudes of Himalaya and Distillation Methods" Molecules 27, no. 8: 2387. https://doi.org/10.3390/molecules27082387
APA StyleThakur, B. K., Shivani, Mahajan, M., & Pal, P. K. (2022). Chemical Diversity of Essential Oil of Valeriana jatamansi from Different Altitudes of Himalaya and Distillation Methods. Molecules, 27(8), 2387. https://doi.org/10.3390/molecules27082387







