Antibacterial Activity of a Novel Oligosaccharide from Streptomyces californics against Erwinia carotovora subsp. Carotovora
Abstract
:1. Introduction
2. Results and Discussion
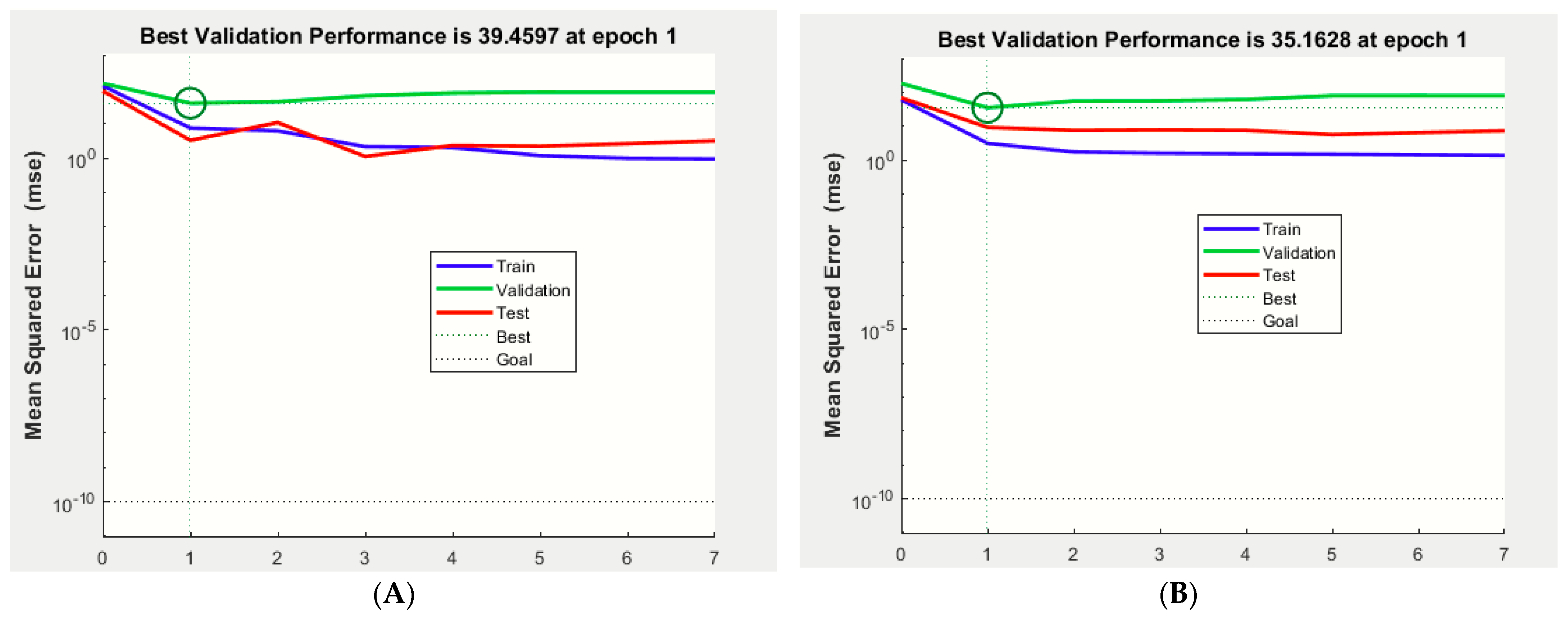
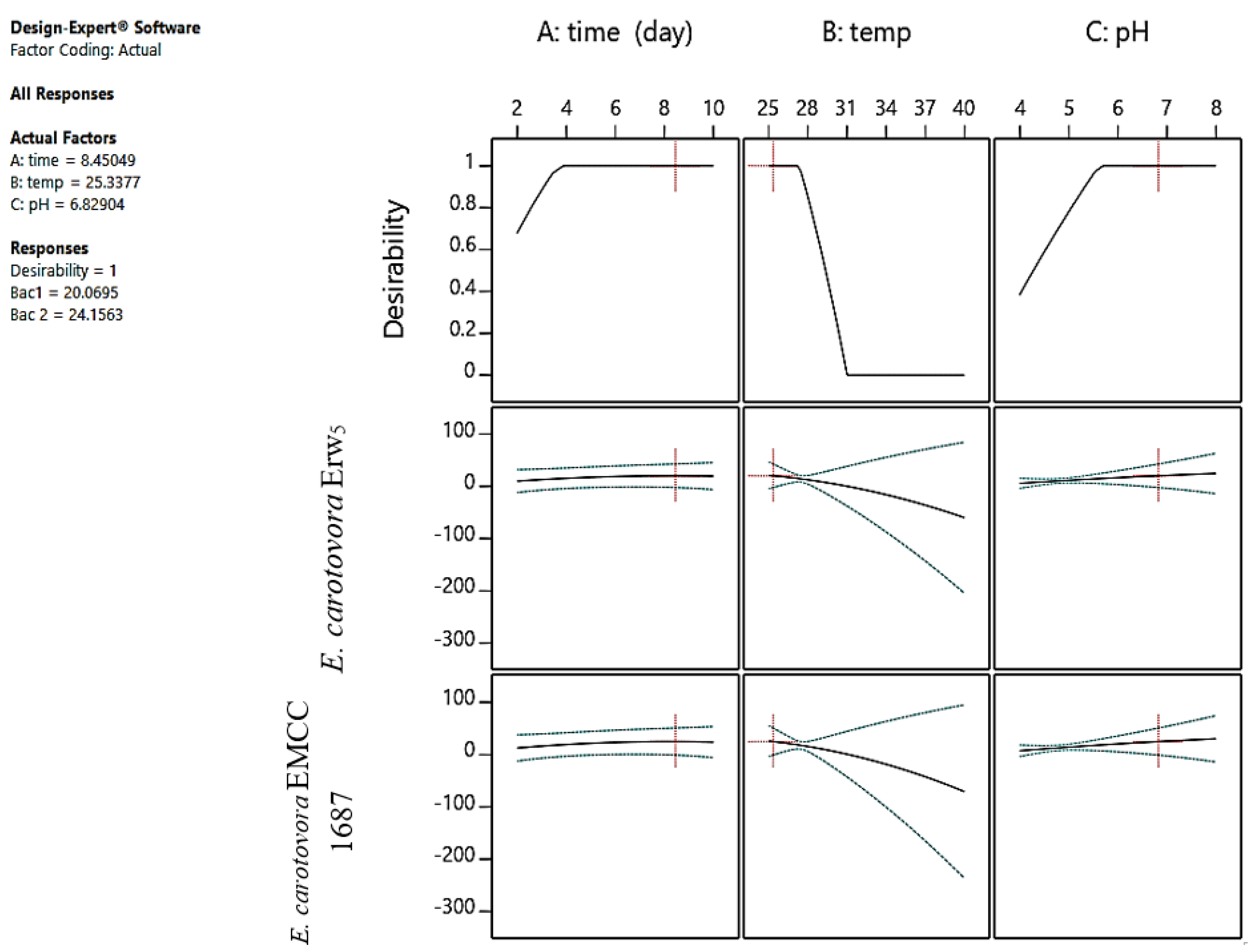
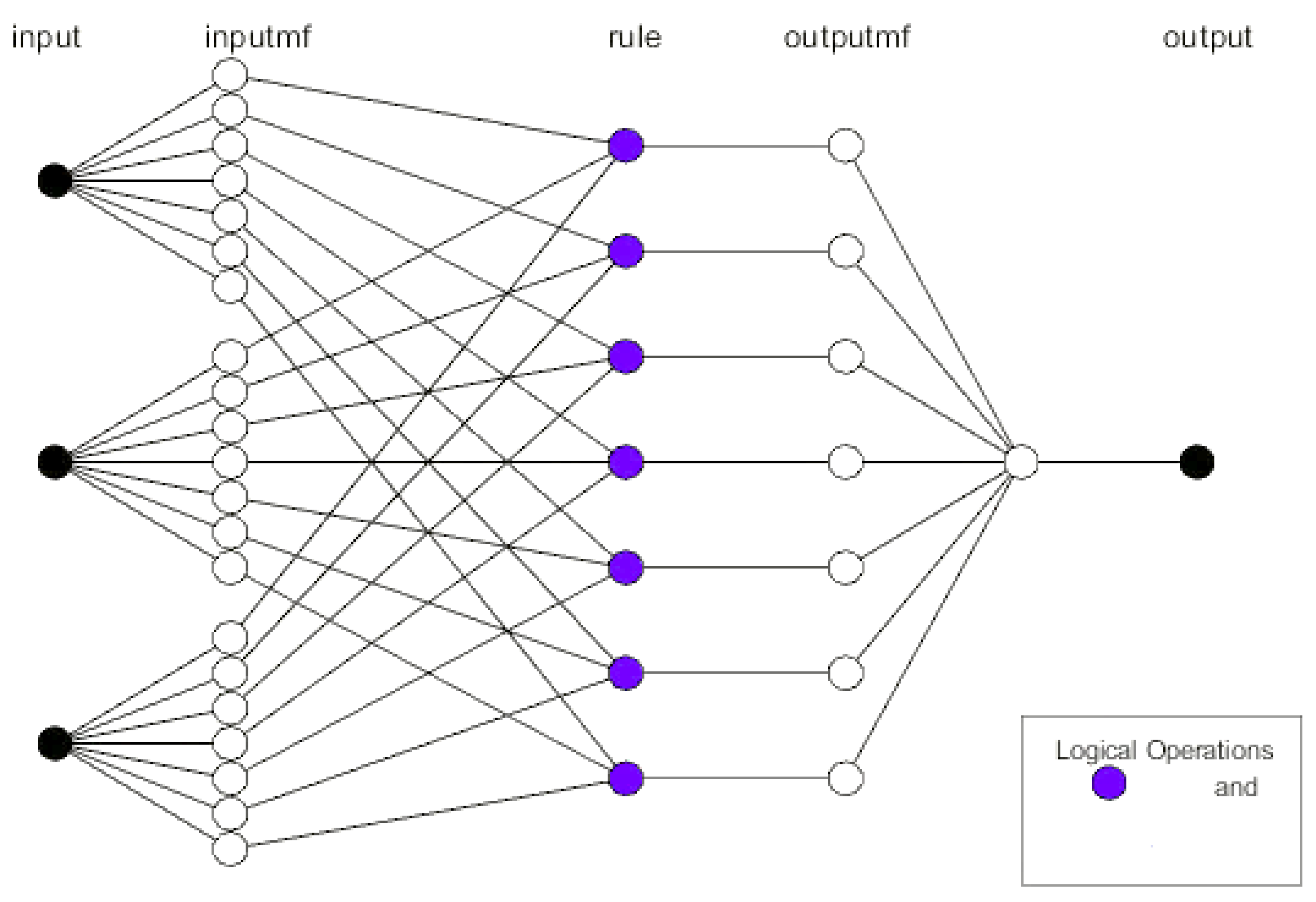
2.1. Optimization and Prediction Models Using ANFIS and ANN Simulations
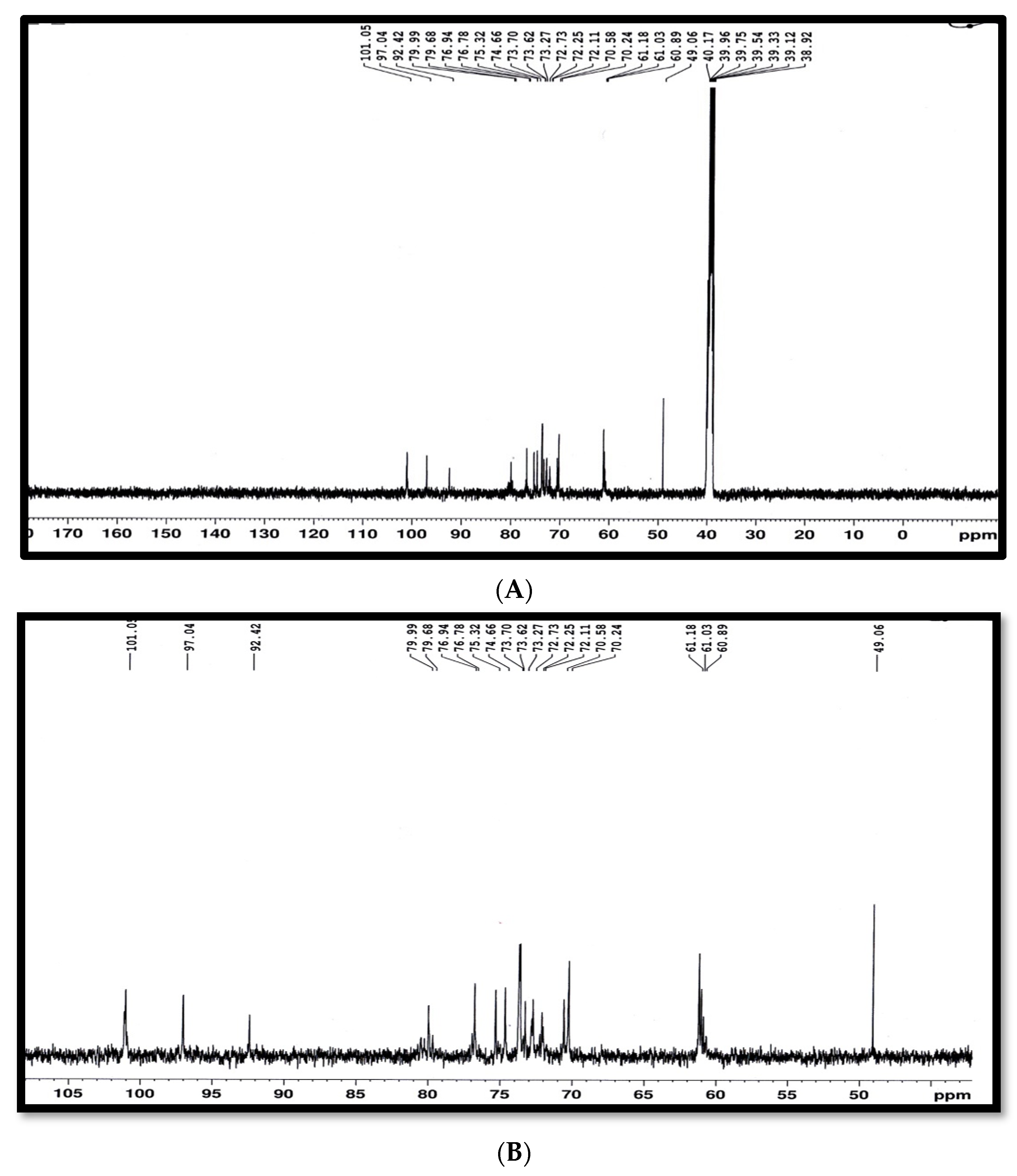
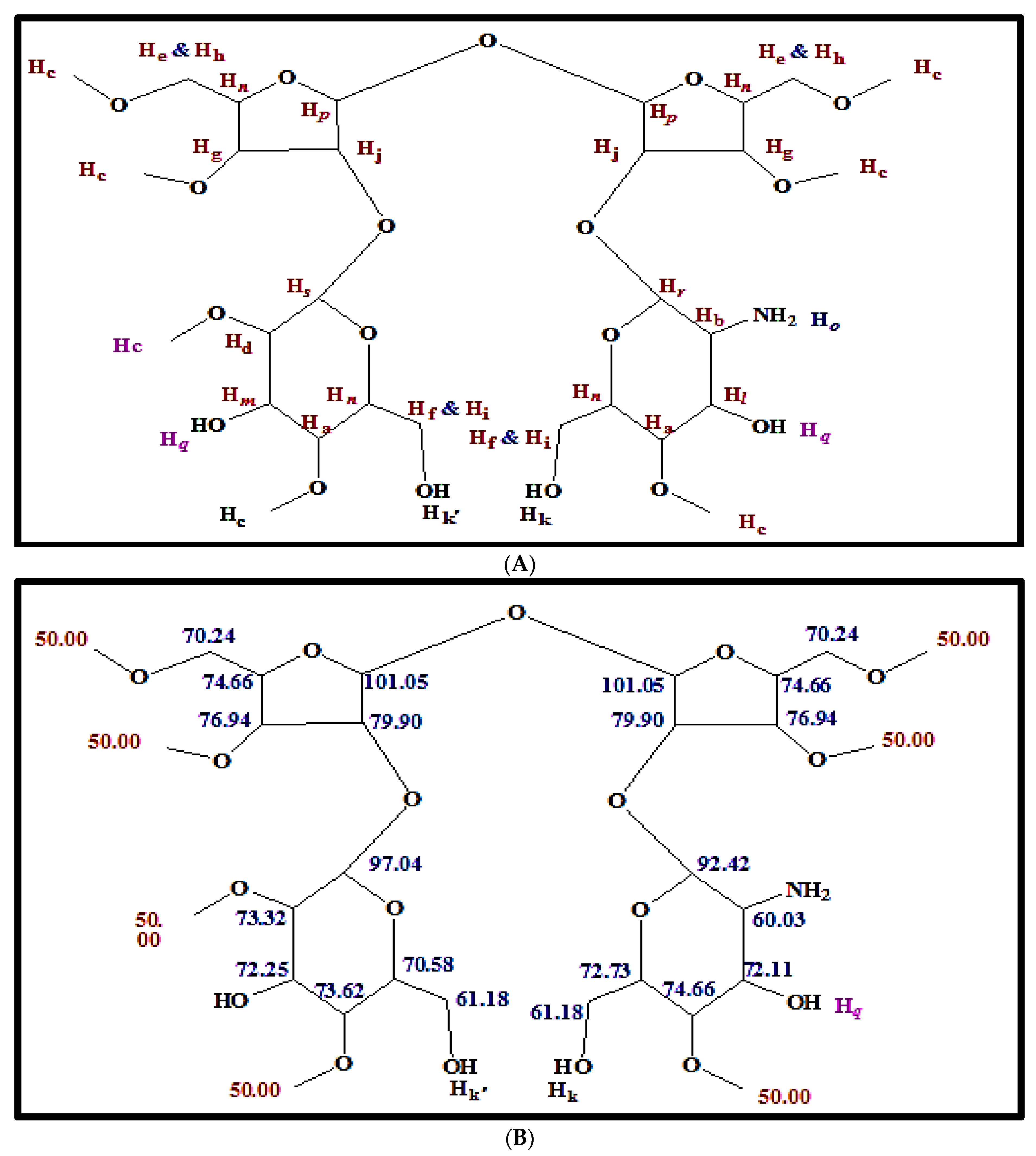
2.2. Characteristics of Antibacterial Agent from S. californics (22/30a)
3. Materials and Methods
3.1. Streptomyces sp. Strain
3.2. Test Bacterial Strain
3.3. Production of Antibacterial Agent from Streptomyces sp. Strain
3.4. Adaptive Neuro-Fuzzy Inference System (ANFIS) and Artificial Neural Network (ANN) Analysis
3.5. Extraction, Separation, and Purification
3.6. Characterization and Structural Elucidation of the Purified Antibacterial Agent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Balagurunathan, R.; Subramanian, A. Antagonistic streptomycetes from marine sediments. Adv. Biosci. 2001, 20, 71–76. [Google Scholar]
- Quinn, G.A.; Banat, A.M.; Abdelhameed, A.M.; Banat, I.M. Streptomyces from traditional medicine: Sources of new innovations in antibiotic discovery. J. Med. Microbiol. 2020, 69, 1040. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaibani, M.M.; Radin Mohamed, R.M.S.; Sidik, N.M.; Enshasy, H.A.E.; Al-Gheethi, A.; Noman, E.; Al-Mekhlafi, N.A.; Zin, N.M. Biodiversity of secondary metabolites compounds isolated from phylum actinobacteria and its therapeutic applications. Molecules 2021, 26, 4504. [Google Scholar] [CrossRef] [PubMed]
- Rather, I.A.; Koh, W.Y.; Paek, W.K.; Lim, J. The sources of chemical contaminants in food and their health implications. Front. Pharmacol. 2017, 8, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, M.; Vasebi, Y.; Safaie, N. Microbial antagonists against plant pathogens in Iran: A review. Open Agric. 2020, 5, 404–440. [Google Scholar] [CrossRef]
- Kyeremeh, A.G.; Kikumoto, T.; Chuang, D.Y.; Gunji, Y.; Takahara, Y.; Ehara, Y. Biological control of soft rot of Chinese Cabbage using single and mixed treatments of bacteriocin-producing avirulent mutants of Erwinia carotovora subsp. carotovora. J. Gen. Plant Pathol. 2000, 66, 264–268. [Google Scholar] [CrossRef]
- Salem, E.A.; Abd El-Shafea, Y.M. Biological control of potato soft rot caused by Erwinia carotovora subsp. carotovora. Egypt. J. Biol. Pest Control. 2018, 28, 1–5. [Google Scholar] [CrossRef]
- Fiers, M.; Edel-Hermann, V.; Chatot, C.; Le Hingrat, Y.; Alabouvette, C.; Steinberg, C. Potato soil-borne diseases. A review. Agron. Sustain. Dev. 2012, 32, 93–132. [Google Scholar] [CrossRef] [Green Version]
- Gnanamanickam, S.S. (Ed.) Plant-Associated Bacteria; Springer: Dordrecht, The Netherlands, 2006; Volume 1. [Google Scholar]
- Hauben, L.; Moore, E.R.; Vauterin, L.; Steenackers, M.; Mergaert, J.; Verdonck, L.; Swings, J. Phylogenetic position of phytopathogens within the Enterobacteriaceae. Syst. Appl. Microbiol. 1998, 21, 384–397. [Google Scholar] [CrossRef]
- McGregor, I.; Vreugdenhil, D. The Fresh Potato Market, Potato Biology and Biotechnology: Advances and Perspectives; Publisher-Elsevier B.V: Amsterdam, The Netherlands, 2007; p. 823. [Google Scholar]
- Darsanaki, R.K.; Rokhi, M.L.; Aliabadi, M.A.; Issazadeh, K. Antimicrobial activities of Lactobacillus strains isolated from fresh vegetables. Middle-East J. Sci. Res. 2012, 11, 1216–1219. [Google Scholar]
- Ho, M.Y.; Chung, W.C.; Huang, H.C.; Chung, W.H.; Chung, W.H. Identification of endophytic fungi of medicinal herbs of Lauraceae and Rutaceae with antimicrobial property. Taiwania 2012, 57, 229–241. [Google Scholar]
- Niu, B.; Vater, J.; Rueckert, C.; Blom, J.; Lehmann, M.; Ru, J.J.; Chen, X.H.; Wang, Q.; Borriss, R. Polymyxin P is the active principle in suppressing phytopathogenic Erwinia spp. by the biocontrol rhizobacterium Paenibacillus polymyxa M-1. BMC Microbiol. 2013, 13, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, M.; Chowdhury, M.S.M.; Sultana, N. In-vitro screening of some chemicals and biocontrol agents against Erwinia carotovora subsp. carotovora, the causal agent of soft rot of potato (Solanum tuberosum). Agriculturists 2013, 11, 1–9. [Google Scholar]
- Algeblawi, A.; Adam, F. Biological control of Erwinia carotovora subsp. carotovora by Pseudomonas fluorescens, Bacillus subtilis and Bacillus thuringiensis. Int. J. Chem. Environ. Biol. Sci. IJCEBS Vol. 2013, 1, 770–774. [Google Scholar]
- Iqbal, M.; Amin, M.; Iqbal, Z.; Bibi, H.; Iqbal, A.; Din, Z.U.; Suleman, M.; Shah, H.U. Antimicrobial, cytotoxic and phytotoxic potency of ethyl acetate extract of Rhizopus stolonifer culture. Trop. J. Pharm. Res. 2014, 13, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Baz, M.; Tran, D.; Kettani-Halabi, M.; Samri, S.E.; Jamjari, A.; Biligui, B.; Meimoun, P.; El-Maarouf-Bouteau, H.; Garmier, M.; Saindrenan, P.; et al. Calcium-and ROS-mediated defence responses in BY2 tobacco cells by nonpathogenic Streptomyces sp. J. Appl. Microbiol. 2012, 112, 782–792. [Google Scholar] [CrossRef]
- Li, J.; Hu, M.; Xue, Y.; Chen, X.; Lu, G.; Zhang, L.; Zhou, J. Screening, identification and efficacy evaluation of antagonistic bacteria for biocontrol of soft rot disease caused by Dickeya zeae. Microorganisms 2020, 8, 697. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [Green Version]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the antibacterial mechanism of plant-derived compounds against multidrug-resistant bacteria (MDR). Evid.-Based Complement. Altern. Med. 2021, 2021, 3663315. [Google Scholar] [CrossRef]
- Maget-Dana, R.; Peypoux, F. Iturins, a special class of pore-forming lipopeptides: Biological and physicochemical properties. Toxicology 1994, 87, 151–174. [Google Scholar] [CrossRef]
- Bekemakhanova, N.E.; Shemshura, O.N. Alkaloids of Microscopic Fungi for Plant Protection. Bioactive Fungal Metabolites. Impact and Exploitation; International Symposium by British Mycological Society: Manchester, UK, 2001; pp. 22–27. [Google Scholar]
- Cladera-Olivera, F.; Caron, G.R.; Motta, A.S.; Souto, A.A.; Brandelli, A. Bacteriocin-like substance inhibits potato soft rot caused by Erwinia carotovora. Can. J. Microbiol. 2006, 52, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Al-Gheethi, A.; Noman, E.; Mohamed, R.M.S.R.; Talip, B.; Vo, D.V.N.; Algaifi, H.A. Cephalexin removal by a novel Cu–Zn bionanocomposite biosynthesized in secondary metabolic products of Aspergillus arenarioides EAN603 with pumpkin peels medium: Optimization, kinetic and artificial neural network models. J. Hazard. Mater. 2021, 419, 126500. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Das, A.K. A utilization of GEP (gene expression programming) metamodel and PSO (particle swarm optimization) tool to predict and optimize the forced convection around a cylinder. Energy 2016, 95, 447–458. [Google Scholar] [CrossRef]
- Shahmansouri, A.A.; Bengar, H.A.; Jahani, E. Predicting compressive strength and electrical resistivity of eco-friendly concrete containing natural zeolite via GEP algorithm. Constr. Build. Mater. 2019, 229, 116883. [Google Scholar] [CrossRef]
- Zamanian, S.; Shahidi, G.H.; Saadoun, I. First report of antibacterial properties of a new strain of Streptomyces plicatus (strain 101) against Erwinia carotovora subsp. carotovora from Iran. Biotechnology 2005, 4, 114–120. [Google Scholar]
- Kang, Y.S.; Lee, Y.; Cho, S.K.; Lee, K.H.; Kim, B.J.; Kim, M.; Lim, Y.; Cho, M. Antibacterial activity of a disaccharide isolated from Streptomyces sp. strain JJ45 against Xanthomonas sp. FEMS Microbiol. Lett. 2009, 294, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.R.; Tzeng, D.D.; Yang, C.H. Generation of PCR-based DNA fragments for specific detection of Streptomyces saraceticus N45. Proc. Natl. Sci. Counc. Repub. China. Part B Life Sci. 2001, 25, 119–127. [Google Scholar]
- Vilella, D.; Sánchez, M.; Platas, G.; Salazar, O.; Genilloud, O.; Royo, I.; Cascales, C.; Martin, I.; Diez, T.; Silverman, K.C.; et al. Inhibitors of farnesylation of Ras from a microbial natural products screening program. J. Ind. Microbiol. Biotechnol. 2000, 25, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Arasu, M.V.; Duraipandiyan, V.; Agastian, P.; Ignacimuthu, S. In vitro antimicrobial activity of Streptomyces spp. ERI-3 isolated from Western Ghats rock soil (India). J. Mycol. Médicale 2009, 19, 22–28. [Google Scholar] [CrossRef]
- Valanarasu, M.; Kannan, P.; Ezhilvendan, S.; Ganesan, G.; Ignacimuthu, S.; Agastian, P. Antifungal and antifeedant activities of extracellular product of Streptomyces spp. ERI-04 isolated from Western Ghats of Tamil Nadu. J. De Mycol. Médicale 2010, 20, 290–297. [Google Scholar] [CrossRef]
- Saha, M.R.; Ripa, F.A.; Islam, M.Z.; Khondkar, P. Optimization of conditions and in vitro antibacterial activity of secondary metabolite isolated from Streptomyces sp. MNK7. J. Appl. Sci. Res. 2010, 6, 453–459. [Google Scholar]
- Singh, N.E.H.A.; Rai, V.I.B.H.U.T.I. Optimization of cultural parameters for antifungal and antibacterial metabolite from microbial isolate; Streptomyces rimosus MTCC 10792 from soil of Chhattisgarh. Int. J. Pharm. Pharmaceut. Sci. 2012, 4, 94–101. [Google Scholar]
- Noaman, N.H.; Fattah, A.; Khaleafa, M.; Zaky, S.H. Factors affecting antimicrobial activity of Synechococcus leopoliensis. Microbiol. Res. 2004, 159, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zeng, W.; Huang, Y.; Yang, Z.; Li, J.; Cai, H.; Su, W. Detection of antitumor and antimicrobial activities in marine organism associated actinomycetes isolated from the Taiwan Strait, China. FEMS Microbiol. Lett. 2000, 188, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Matsumoto, A.; Takahashi, Y.; Tomoda, H.; Ōmura, S. Phenatic acids A and B, new potentiators of antifungal miconazole activity produced by Streptomyces sp. K03-0132. J. Antibiot. 2005, 58, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Xie, R.; Li, M.; Ma, S.; Liu, J.; Long, M. Efficient analysis of monosaccharides and oligosaccharides from hydrolyzed hemicellulose of Spartina anglica. Bioresources 2020, 15, 7628–7639. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, Y.; Cai, M.; Guan, R.; Neng, J.; Pi, X. In vitro prebiotic activities of oligosaccharides from the by-products in Ganoderma lucidum spore polysaccharide extraction. R. Soc. Chem. 2020, 10, 14794–14802. [Google Scholar] [CrossRef] [Green Version]
- Křen, V.; Řezanka, T. Sweet antibiotics—The role of glycosidic residues in antibiotic and antitumor activity and their randomization. FEMS Microbiol. Rev. 2008, 32, 858–889. [Google Scholar] [CrossRef] [Green Version]
- Ritter, T.K.; Wong, C.H. Carbohydrate-based antibiotics: A new approach to tackling the problem of resistance. Angew. Chem. Int. Ed. 2001, 40, 3508–3533. [Google Scholar] [CrossRef]
- Agnelli, F.; Sucheck, S.J.; Marby, K.A.; Rabuka, D.; Yao, S.L.; Sears, P.S.; Liang, F.S.; Wong, C.H. Dimeric aminoglycosides as antibiotics. Angew. Chem. 2004, 116, 1588–1592. [Google Scholar] [CrossRef]
- Johnson, L.F.; Curl, E.A. Methods for Research on the Ecology of Soil-Borne Plant Pathogens; Minneapolis, Burgess Publishing Co. CABI: Wallingford, UK, 1972; p. 247. [Google Scholar]
- Johnson, S.B. Blackleg and Bacterial Soft Rot; Maine Cooperative Extension Service; University of Maine: Orono, ME, USA, 1999; Volume 2493. [Google Scholar]
- Pisano, M.A.; Sommer, M.J.; Taras, L. Bioactivity of chitinolytic actinomycetes of marine origin. Appl. Microbiol. Biotechnol. 1992, 36, 553–555. [Google Scholar] [CrossRef]
- Al-Shaibani, M.M.; Radin Mohamed, R.M.S.; Zin, N.M.; Al-Gheethi, A.; Al-Sahari, M.; El Enshasy, H.A. Enhanced Pharmaceutically Active Compounds Productivity from Streptomyces SUK 25: Optimization, Characterization, Mechanism and Techno-Economic Analysis. Molecules 2021, 26, 2510. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zubairy, M.A.; Hussein, K.; Alkhyat, S.H.; Al-Mahdi, A.Y.; Alghalibi, S.M.; Al-Gheethi, A.A.; Al-Shaibani, M.M.; El Enshasy, H.A.; Sidik, N.M. Antibacterial Activity of a Novel Oligosaccharide from Streptomyces californics against Erwinia carotovora subsp. Carotovora. Molecules 2022, 27, 2384. https://doi.org/10.3390/molecules27082384
Al-Zubairy MA, Hussein K, Alkhyat SH, Al-Mahdi AY, Alghalibi SM, Al-Gheethi AA, Al-Shaibani MM, El Enshasy HA, Sidik NM. Antibacterial Activity of a Novel Oligosaccharide from Streptomyces californics against Erwinia carotovora subsp. Carotovora. Molecules. 2022; 27(8):2384. https://doi.org/10.3390/molecules27082384
Chicago/Turabian StyleAl-Zubairy, Maysoon Abdulrahman, Khaled Hussein, Salwa H. Alkhyat, Abdullah Yahya Al-Mahdi, Saeed Munassar Alghalibi, Adel Ali Al-Gheethi, Muhanna Mohammed Al-Shaibani, Hesham Ali El Enshasy, and Nik Marzuki Sidik. 2022. "Antibacterial Activity of a Novel Oligosaccharide from Streptomyces californics against Erwinia carotovora subsp. Carotovora" Molecules 27, no. 8: 2384. https://doi.org/10.3390/molecules27082384
APA StyleAl-Zubairy, M. A., Hussein, K., Alkhyat, S. H., Al-Mahdi, A. Y., Alghalibi, S. M., Al-Gheethi, A. A., Al-Shaibani, M. M., El Enshasy, H. A., & Sidik, N. M. (2022). Antibacterial Activity of a Novel Oligosaccharide from Streptomyces californics against Erwinia carotovora subsp. Carotovora. Molecules, 27(8), 2384. https://doi.org/10.3390/molecules27082384







