Nutraceutical Role of Polyphenols and Triterpenes Present in the Extracts of Fruits and Leaves of Olea europaea as Antioxidants, Anti-Infectives and Anticancer Agents on Healthy Growth
Abstract
1. Introduction
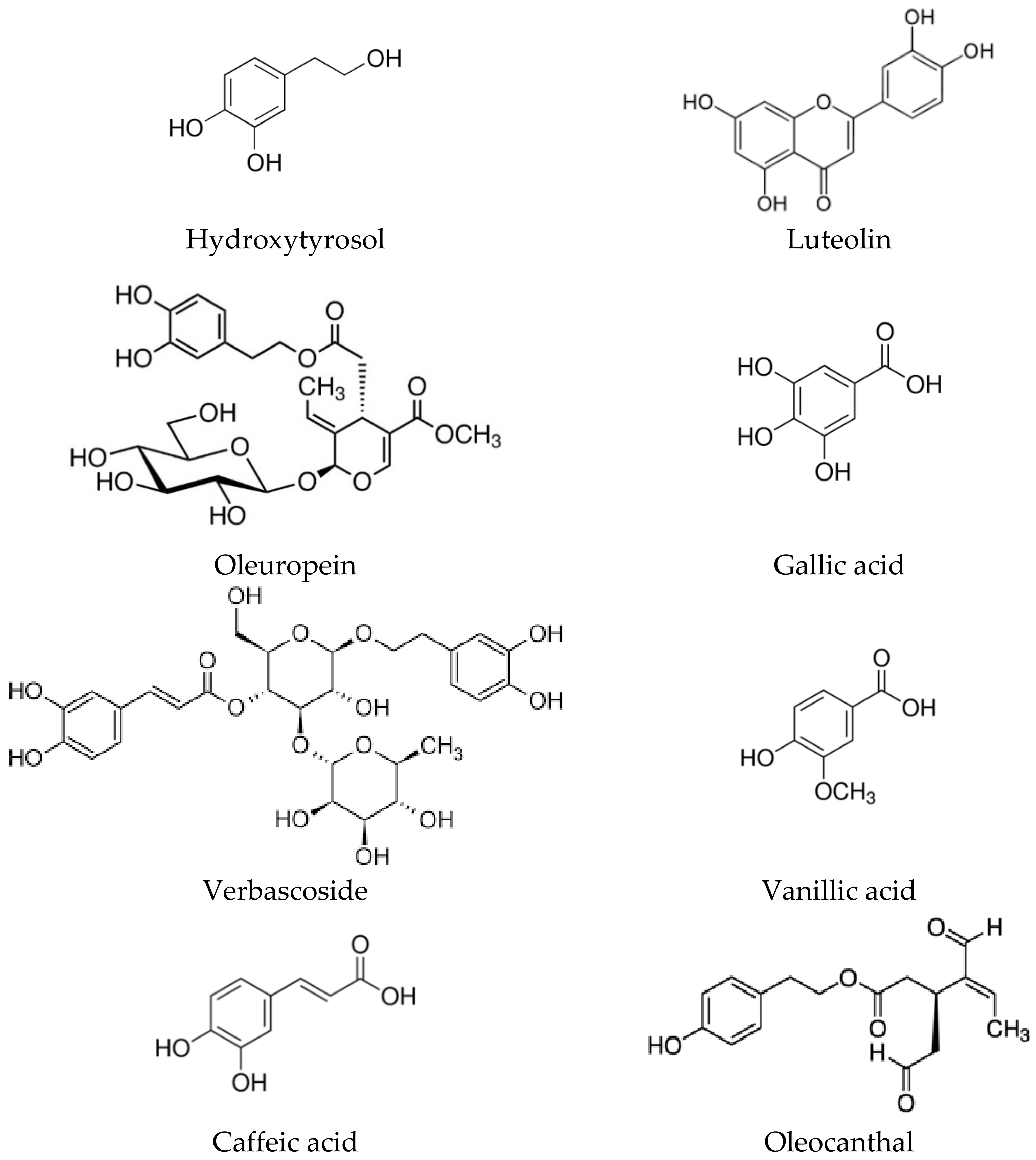
2. Biological Activities of the Polyphenolic Components of the Olive Tree
2.1. Pharmacokinetics and Toxicity
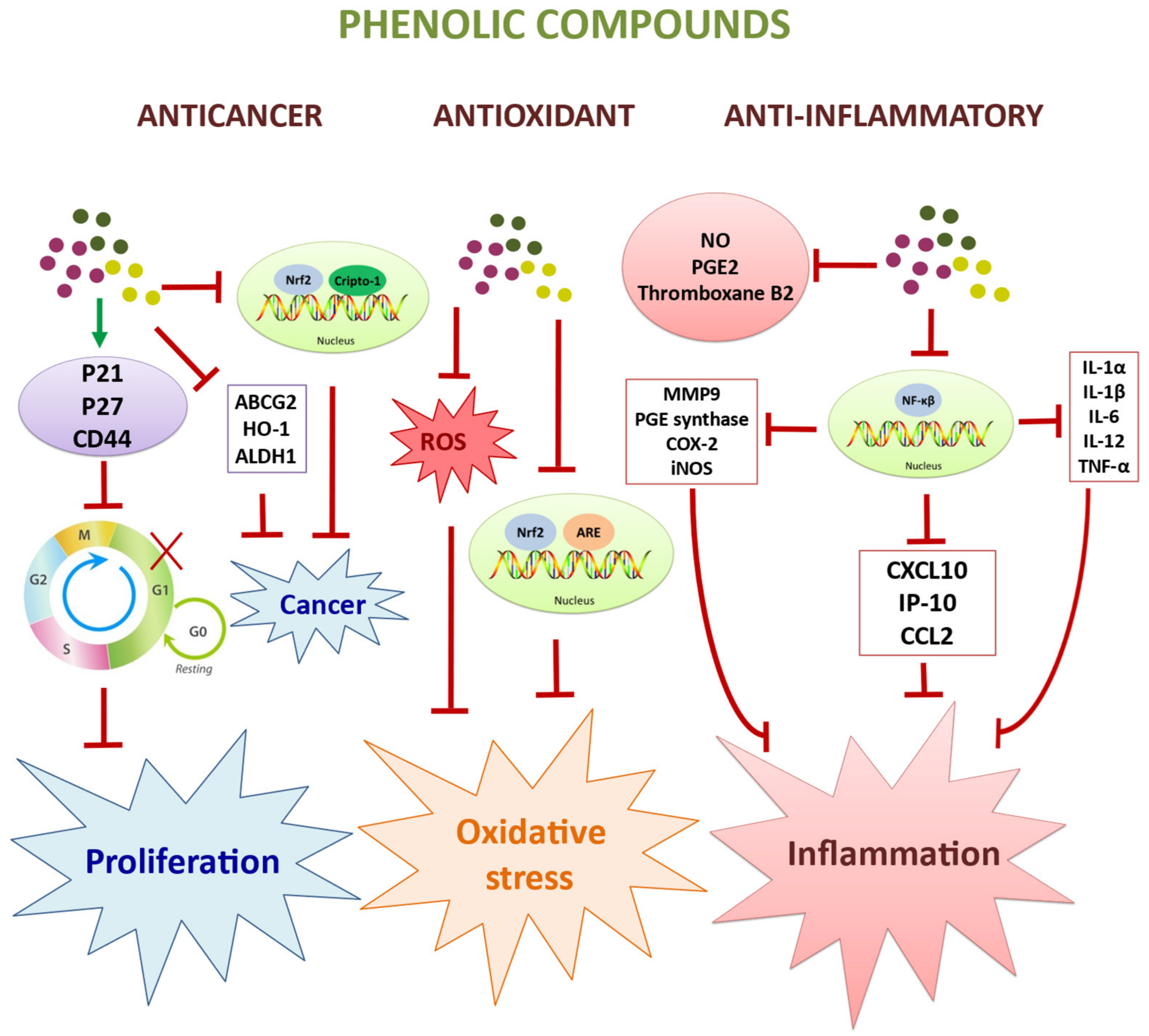
2.2. Antioxidant and Anti-Ageing Effects
2.3. Anticancer and Antiproliferaty Effects
2.4. Immunomodulatory and Anti-Inflammatory Effects
2.5. Antiviral and Antimicrobial Effects
3. Biological Activities of the Triterpenic Components of the Olive Tree
3.1. Stimulating Effects of Normal Growth
3.2. Immunomodulatory and Anti-Inflammatory Effects
3.3. Antioxidant and Anti-Ageing Effects
3.4. Anti-Proliferative and Anticancer Effects
3.5. Antiviral and Antimicrobial Effects
4. Discussion
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| 3CL-Pro | 3-Chymotripsin-like protease |
| 6PGDH | 6-Phosphogluconate dehydrogenase |
| 253J | Human bladder cancer cell line |
| ABCG2 | ATP-binding cassette transporter G2 |
| ACC-2 | Salivary adenoid cystic carcinoma cell line |
| ACC-M | Salivary adenoid cystic carcinoma cell line |
| ACE2 | Angiotensin-converting enzyme 2 |
| AIDS | Acquired immunodeficiency syndrome |
| AKT | Protein kinase B |
| ALDH1 | Aldehyde dehydrogenase 1 |
| AMP | Adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| AOM | Azoxymethane |
| AP-1 | Activator protein 1 |
| ARE | Antioxidant response elements |
| βAS | β-Amyrin synthase |
| B16F10 | B16 melanoma cell line |
| BA | Betulinic acid |
| Bad | Pro-apoptotic BH3-only protein Bad |
| Bax | Pro-apoptotic Bcl-2-associated X protein |
| Bcl-2 | Anti-apoptotic B-cell lymphoma 2 |
| BGC-803 | Gastric cancer cell line |
| Bid | Pro-apoptotic BH3 Interacting domain death agonist |
| Bim | Pro-apoptotic Bcl-2 homology 3-only protein Bim |
| BT474 | Human breast carcinoma cell line |
| CA | Caffeic acid |
| Caco-2 | Caucasian colon adenocarcinoma cell line |
| Caspase | Cysteine-dependent aspartate-directed protease |
| CAT | Catalase |
| CCL2/MPC-1 | Chemokine (C-C motif) ligand2/monocyte chemoattractant protein 1 |
| CCl4 | Carbon tetrachloride |
| CD44 | Cell-surface glycoprotein involved in cell–cell interactions, adhesion and migration |
| CNS | Catecholaminergic cell line |
| COMT | Catechol-O-methyltransferase |
| COVID-19 | Coronavirus disease 2019 |
| COX2 | Cyclooxygenase 2 |
| Cripto-1 | Epidermal growth factor CFC-1 |
| CRC | Colorectal cancer |
| CRP | C-reactive protein |
| CS | Protein-synthesis capacity |
| CSC | Cancer stem cell |
| CT | Clinical trial |
| CXCL10/IP-10 | C-X-C motif chemokine ligand 10/interferon gamma-induced protein 10 |
| DHPA | Dihydroxyphenylacetic acid |
| DMT2 | Diabetes mellitus type 2 |
| DNA | Deoxyribonucleic acid |
| DSS | Dextran sulphate sodium |
| eNOS | Endothelial nitric oxide synthase |
| EO | Erythrodiol |
| EMT | Epithelial–mesenchymal transition |
| EVOO | Extra virgin olive oil |
| ErbB2 | Receptor tyrosine-protein kinase erbB-2 |
| G6PDH | Glucose 6 phosphate dehydrogenase |
| GPX | Glutathione peroxidase |
| GR | Glutathione reductase |
| GSH | Glutathione reduced |
| GSSG | Glutathione oxidized |
| GST | Glutathione S-transferase |
| H22 | Hepatocellular cancer cell line |
| H2O2 | Hydrogen peroxide |
| HCT116 | Human colon cancer cell line |
| HepG2 | Human liver cancer cell line |
| HIV-1 | Human immunodeficiency virus-1 |
| HL60 | Human promyelocytic leukemia cell line |
| HO-1 | Heme oxygenase 1 |
| HPLC | High performance liquid chromatography |
| HSP60 | Heat shock proteins (chaperon) |
| HSP70 | Heat shock proteins (chaperon) |
| HSPA | Low molecular weight heat shock protein (chaperon) |
| HT | Hydroxytyrosol |
| HT29 | Human colorectal adenocarcinoma cell line |
| HVA | Homovanillic acid |
| HVOL | Homovanillic alcohol |
| IC50 | Concentration of a drug that gives half-maximal response |
| IL | Interleukins |
| iNOS | Inducible nitric oxide synthase |
| J774 | Macrophage cell line |
| JNK | Jun N-terminal kinase |
| KD | Protein degradation rate |
| KDNA | Protein synthesis rate/DNA unit |
| KG | Protein accumulation rate |
| KRNA | Protein synthesis efficiency |
| KS | Protein synthesis rate |
| LD50 | Median lethal dose |
| LDL | Low-density lipoprotein |
| LP | Lipoperoxide |
| LPO | Lipid peroxidation |
| LPS | Lipopolysaccharide |
| MA | Maslinic acid |
| MAPK | Mitogen-activated protein kinase |
| MCF-7 | Michigan cancer foundation-7/breast cancer cell line |
| MDA-MB-231 | Triple-negative breast cancer cell line |
| MDCK | Madin-Darby Canine Kidney cells |
| MIP1β | Macrophage inflammatory protein 1 βeta |
| miRNA, miR | Micro RNA |
| MMP | Mitochondrial membrane potential |
| MMP9 | Matrix metallopeptidase 9 |
| mRNA | Messenger ribonucleic acid |
| MS | Mass spectrometry |
| MT2 | Human T cell leukemia |
| mTOR | Mechanistic target of rapamycin kinase |
| MUFA | Monounsaturated fatty acids |
| NADP | Nicotinamide adenine-dinucleotide phosphate oxidized |
| NADPH | Nicotinamide adenine-dinucleotide phosphate reduced |
| NADP-ICDH | Isocitrate dehydrogenase NADP dependent |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated beta cells |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NTPs | Nucleoside triphosphates |
| O2•− | Superoxide anion radical |
| OA | Oleanolic acid |
| ODC | Ornithine decarboxylase |
| OE | Oleuropein |
| OS | (3S)-2,3-Epoxi-2,3-dihidroescualeno |
| OSC | (3S)-2,3-Epoxi-2,3-dihidroescualeno-lanosterol cyclase, also named lanosterol synthase |
| p21WAF/CIP1 | Cyclin-dependent kinase inhibitor A1 |
| p24 | HIV p24 protein (CA, core antigen) |
| p27Kip1 | Cyclin-dependent kinase inhibitor |
| p53 | Tumor suppressor protein 53 |
| p90Rsk | Ribosomal S MAPK-activated protein kinase-1 |
| PDCD4 | Programmed cell death protein 4 |
| PGES | Prostaglandin E synthase |
| PI3K | Phosphoinositide 3-kinase |
| PL-Pro | Papain-like protease |
| PMA | Phorbol 12-myristate 13-acetate |
| PPP | Pentose phosphate pathway |
| PRE | Protein retention efficiency |
| PUFA | Polyunsaturated fatty acids |
| RdRp | RNA-dependent RNA polymerase |
| ROS | Reactive oxygen species |
| RPS6KA2 | Ribosomal Protein S6 Kinase A2 gene |
| RT4 | Urinary bladder transitional cell carcinoma |
| SARS-CoV-2 | Severe acute respiratory syndrome Coronavirus 2 |
| SGp-RBD | Spike glycoprotein receptor binding domain |
| SH-SY5Y | Human neuroblastoma cell line |
| SK-UT-1 | Human leiomyosarcoma cell line |
| SOD | Superoxide dismutase |
| Sp | Specific protein transcription factors |
| SSDE | Steady state of dynamic equilibrium |
| STAT3 | Signal transducer and activator of transcription 3 |
| SW480 | Human colon cancer cell line |
| SW982 | Human synovial sarcoma cell line |
| T24 | Human urothelial bladder cancer cell line |
| TGF-β1 | Transforming growth factor β1 |
| Th9 | T helper type 9 cell line |
| THP-1 | Human leukemia monocytic cell line |
| TNFα | Tumor necrosis factor α |
| TXA2 | Thromboxane A2 |
| TXB2 | Thromboxane B2 |
| U-251 MG | Malignant glioblastoma cell line |
| UA | Ursolic acid |
| UO | Uvaol |
| VEGFR | Vascular endothelial growth factor receptor |
| WRL68 | Normal human liver cell line |
| YY1 | Yin Yang1 transcriptional repressor |
| ZBRB10 | Zinc finger and BTB domain-containing protein 10 |
References
- Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; García-Salguero, L.; Mokhtari, K.; Herrera-Merchán, A.; Medina, P.P.; Peragón, J.; Lupiáñez, J.A. Anti-cancer and Anti-angiogenic Properties of Various Natural Pentacyclic Tri-terpenoids and Some of their Chemical Derivatives. Curr. Org. Chem. 2015, 19, 919–947. [Google Scholar] [CrossRef]
- Vilaplana-Pérez, C.; Aunon, D.; García-Flores, L.A.; Gil-Izquierdo, A. Hydroxytyrosol and potential uses in cardiovascular diseases, cancer, and AIDS. Front. Nutr. 2014, 1, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; Quiles, J.L.; Ramírez-Tortosa, C.L.; Sánchez-Rovira, P.; Ramírez-Tortosa, M.C. Hydroxytyrosol: From laboratory investigations to future clinical trials. Nut. Rev. 2000, 68, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Merendino, N.; Romani, A.; Velotti, F. Naturally occurring hydroxytyrosol: Synthesis and anticancer potential. Curr. Med. Chem. 2013, 20, 655–670. [Google Scholar] [CrossRef]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219–224. [Google Scholar] [CrossRef]
- Hu, T.; He, X.W.; Jiang, J.G.; Xu, X.L. Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef]
- Weinbrenner, T.; Fitó, M.; Farré Albaladejo, M.; Sáez, G.T.; Rijken, P.; Tormos, C.; Coolen, S.; de La Torre, R.; Covas, M.I. Bioavailability of phenolic compounds from olive oil and oxidative/antioxidant status at postprandial state in healthy humans. Drugs Exp Clin Res. 2004, 30, 207–212. [Google Scholar] [PubMed]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.; Leenen, R.; Katan, M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef]
- Manna, C.; Galletti, P.; Maisto, G.; Cucciolla, V.; D’Angelo, S.; Zappia, V. Transport mechanism and metabolism of olive oil hydroxytyrosol in Caco-2 cells. FEBS Lett. 2000, 470, 341–344. [Google Scholar] [CrossRef]
- Bai, C.; Yan, X.J.; Takenaka, M.; Sekiya, K.; Nagata, T. Determination of synthetic hydroxytyrosol in rat plasma by GC-MS. J. Agric. Food Chem. 1998, 46, 3998–4001. [Google Scholar] [CrossRef]
- Tuck, K.L.; Freeman, M.P.; Hayball, P.J.; Stretch, G.L.; Stupans, I. The in vivo fate of hydroxytyrosol and tyrosol, antioxidant phenolic constituents of olive oil, after intravenous and oral dosing of labeled compounds to rats. J. Nut. 2001, 131, 1993–1996. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar] [PubMed]
- Edwards, D.J.; Rizk, M. Conversion of 3, 4-dihydroxyphenylalanine and deuterated 3, 4-dihydroxyphenylalanine to alcoholic metabolites of catecholamines in rat brain. J. Neurochem. 1981, 36, 1641–1647. [Google Scholar] [CrossRef]
- Xu, C.L.; Sim, M.K. Reduction of dihydroxyphenylacetic acid by a novel enzyme in the rat brain. Biochem. Pharmacol. 1995, 50, 1333–1337. [Google Scholar] [CrossRef]
- Vissers, M.N.; Zock, P.L. Katan, M.B. Bioavailability and antioxidant effects of olive oil phenols in humans: A review. Eur. J. Clin. Nutr. 2004, 58, 955–965. [Google Scholar] [CrossRef]
- Serra, A.; Rubió, L.; Borrás, X.; Maciá, A.; Romero, M.P.; Motilva, M.J. Distribution of olive oil phenolic compounds in rat tissues after administration of a phenolic extract from olive cake. Mol. Nutr. Food Res. 2012, 56, 486–496. [Google Scholar] [CrossRef]
- Christian, M.S.; Sharper, V.A.; Hoberman, A.M.; Seng, J.E.; Fu, L.; Covell, D.; Diener, R.M.; Bitler, C.M.; Crea, R. The toxicity profile of hydrolyzed aqueous olive pulp extract. Drug. Chem. Toxicol. 2004, 27, 309–330. [Google Scholar] [CrossRef]
- Soni, M.G.; Burdock, G.A.; Christian, M.S.; Bitler, C.M.; Crea, R. Safety assessment of aqueous olive pulp extract as an antioxidant or antimicrobial agent in foods. Food Chem. Toxicol. 2006, 44, 903–915. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Galli, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef]
- Visioli, F.; Bellomo, G.; Galli, C. Free radical-scavenging properties of olive oil polyphenols. Biochem. Biophys. Res. Commun. 1998, 247, 60–64. [Google Scholar] [CrossRef] [PubMed]
- De la Puerta, R.; Ruiz-Gutiérrez, V.; Hoult, J.R.S. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 1999, 57, 445–449. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.; McCord, J.M. Phytochemical Combination PB125 Activates the Nrf2 Pathway and Induces Cellular Protection against Oxidative Injury. Antioxidants 2019, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Filik, L.; Ozyilkan, O. Olive-oil consumption and cancer risk. Eur. J. Clin. Nut. 2003, 57, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. Nutritional and Biological Properties of Extra Virgin Olive Oil. J. Agric. Food Chem. 2011, 59, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F. Olive oil phenolics: Where do we stand? Where should we go? J. Sci. Food Agric. 2012, 92, 2017–2019. [Google Scholar] [CrossRef]
- Sirianni, R.; Chimento, A.; De Luca, A.; Casaburi, I.; Rizza, P.; Onofrio, A.; Iacopetta, D.; Puoci, F.; Ando, S.; Maggiolini, M.; et al. Oleuropein and hydroxytyrosol inhibit MCF-7 breast cancer cell proliferation interfering with ERK1/2 activation. Mol. Nutr. Food Res. 2010, 54, 833–840. [Google Scholar] [CrossRef]
- Bouallagui, Z.; Han, J.; Isoda, H.; Sayadi, S. Hydroxytyrosol rich extract from olive leaves modulates cell cycle progression in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2011, 49, 179–184. [Google Scholar] [CrossRef]
- Casaburi, I.; Puoci, F.; Chimento, A.; Sirianni, R.; Ruggiero, C.; Avena, P.; Pezzi, V. Potential of olive oil phenols as chemopreventive and therapeutic agents against cancer: A review of in vitro studies. Mol. Nutr. Food Res. 2013, 57, 71–83. [Google Scholar] [CrossRef]
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen peroxide sensing and signaling. Mol. Cell 2007, 26, 1–14. [Google Scholar] [CrossRef]
- Fabiani, R.; Sepporta, M.V.; Rosignoli, P.; De Bartolomeo, A.; Crescimanno, M.; Morozzi, G. Anti-proliferative and pro-apoptotic activities of hydroxytyrosol on different tumour cells: The role of extracellular production of hydrogen peroxide. Eur. J. Nutr. 2012, 51, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Della Ragione, F.; Cucciolla, V.; Borriello, A.; Della Pietra, V.; Pontoni, G.; Racioppi, L.; Manna, C.; Galletti, P.; Zappia, V. Hydroxytyrosol, a natural molecule occurring in olive oil, induces cytochrome c-dependent apoptosis. Biochem. Biophys. Res. Commun. 2000, 278, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; De Bartolomeo, A.; Rosignoli, P.; Servili, M.; Montedoro, G.F.; Morozzi, G. Cancer chemoprevention by hydroxytyrosol isolated from virgin olive oil through G1 cell cycle arrest and apoptosis. Eur. J. Cancer Prev. 2002, 11, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; Rosignoli, P.; De Bartolomeo, A.; Fuccelli, R.; Morozzi, G. Inhibition of cell cycle progression by hydroxytyrosol is associated with upregulation of cyclin-dependent protein kinase inhibitors p21(WAF1/Cip1) and p27(Kip1) and with induction of differentiation in HL60 cells. J. Nutr. 2008, 138, 42–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, J.; Talorete, T.P.; Yamada, P.; Isoda, H. Anti-proliferative and apoptotic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells. Cytotechnology 2009, 59, 45–53. [Google Scholar] [CrossRef]
- Tsai, K.J.; Tsai, H.Y.; Tsai, C.C.; Chen, T.Y.; Hsieh, T.H.; Chen, C.L.; Mbuyisa, L.; Huang, Y.B.; Lin, M.W. Luteolin Inhibits Breast Cancer Stemness and Enhances Chemosensitivity through the Nrf2-Mediated Pathway. Molecules 2021, 26, 6452. [Google Scholar] [CrossRef]
- Pei, T.; Meng, Q.; Han, J.; Sun, H.; Li, L.; Song, R.; Sun, B.; Pan, S.; Liang, D.; Liu, L. (−)-Oleocanthal inhibits growth and metastasis by blocking activation of STAT3 in human hepatocellular carcinoma. Oncotarget 2016, 7, 43475–43491. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, J.; Zhong, L. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 581–586. [Google Scholar] [CrossRef]
- Richard, N.; Arnold, S.; Hoeller, U.; Kilpert, C.; Wertz, K.; Schwager, J. Hydroxytyrosol is the major anti-inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages. Planta Med. 2011, 77, 1890–1897. [Google Scholar] [CrossRef]
- Toteda, G.; Lupinacci, S.; Vizza, D.; Bonofiglio, R.; Perri, E.; Bonofiglio, M.; Lofaro, D.; La Russa, A.; Leone, F.; Gigliotti, P.; et al. High doses of hydroxytyrosol induce apoptosis in papillary and follicular thyroid cancer cells. J. Endocrinol. Invest. 2017, 40, 153–162. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Redhu, D.; Sánchez-Hidalgo, M.; Fernández-Bolaños, J.G.; Alarcón de la Lastra, C.; Worm, M.; Babina, M. Olive-oil-derived polyphenols effectively attenuate inflammatory responses of human keratinocytes by interfering with the NF-kB pathway. Mol. Nutr. Food Res. 2019, 63, 1900019. [Google Scholar] [CrossRef] [PubMed]
- González-Correa, J.A.; López-Villodres, J.A.; Asensi, R.; Espartero, J.L.; Rodríguez-Gutiérrez, G.; De La Cruz, J.P. Virgin olive oil polyphenol hydroxytyrosol acetate inhibits in vitro platelet aggregation in human whole blood: Comparison with hydroxytyrosol and acetylsalicylic acid. Br. J. Nutr. 2009, 101, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- González-Correa, J.A.; Navas, M.D.; Muñoz-Marín, J.; Trujillo, M.; Fernández-Bolaños, J.; de la Cruz, J.P. Effects of hydroxytyrosol and hydroxytyrosol acetate administration to rats on platelet function compared to acetylsalicylic acid. J. Agric. Food Chem. 2008, 56, 7872–7876. [Google Scholar] [CrossRef] [PubMed]
- González-Correa, J.A.; Navas, M.D.; López-Villodres, J.A.; Trujillo, M.; Espartero, J.L.; de La Cruz, J.P. Neuroprotective effect of hydroxytyrosol and hydroxytyrosol acetate in rat brain slices subjected to hypoxia-reoxygenation. Neurosci. Lett. 2008, 446, 143–146. [Google Scholar] [CrossRef]
- Goya, L.; Mateos, R.; Bravo, L. Effect of the olive oil phenol hydroxytyrosol on human hepatoma HepG2 cells. Protection against oxidative stress induced by tert-butylhydroperoxide. Eur. J. Nutr. 2007, 46, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, D.G.; Ma, J. Nutritional benefit of olive oil: The biological effects of hydroxytyrosol and its arylating quinone adducts. J. Agric. Food Chem. 2008, 56, 8774–8786. [Google Scholar] [CrossRef]
- Gong, D.; Geng, C.; Jiang, L.; Cao, J.; Yoshimura, H.; Zhong, L. Effects of hydroxytyrosol-20 on carrageenan-induced acute inflammation and hyperalgesia in rats. Phytother. Res. 2009, 23, 646–650. [Google Scholar] [CrossRef]
- Fitó, M.; Cladellas, M.; de la Torre, R.; Marti, J.; Muñoz, D.; Schroder, H.; Alcántara, M.; Pujadas-Bastardes, M.; Marrugat, J.; López-Sabater, M.C.; et al. Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: A randomized, crossover, controlled trial. Eur. J. Clin. Nut. 2008, 62, 570–574. [Google Scholar] [CrossRef]
- Tzoulaki, I.; Murray, G.D.; Lee, A.J.; Rumley, A.; Lowe, G.D.; Fowkes, F.G. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation 2005, 112, 976–983. [Google Scholar] [CrossRef]
- Medina, E.; de Castro, A.; Romero, C.; Brenes, M. Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: Correlation with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 4954–4961. [Google Scholar] [CrossRef]
- Medina, E.; Brenes, M.; Romero, C.; García, A.; de Castro, A. Main antimicrobial compounds in table olives. J. Agric. Food Chem. 2007, 55, 9817–9823. [Google Scholar] [CrossRef] [PubMed]
- Karaosmanoglu, H.; Soyer, F.; Ozen, B.; Tokatli, F. Antimicrobial and Antioxidant Activities of Turkish Extra Virgin Olive Oils. J. Agr. Food Chem. 2010, 58, 8238–8245. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, G.; Tomaino, A.; Lo Cascio, R.; Crisafi, G.; Uccella, N.; Saija, A. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 1999, 51, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Furneri, P.M.; Piperno, A.; Sajia, A.; Bisignano, G. Antimycoplasmal activity of hydroxytyrosol. Antimicrob. Agents Chemother. 2004, 48, 4892–4894. [Google Scholar] [CrossRef]
- Yamada, K.; Ogawa, H.; Hara, A.; Yoshida, Y.; Yonezawa, Y.; Karibe, K.; Nghia, V.B.; Yoshimura, H.; Yamamoto, Y.; Yamada, M.; et al. Mechanism of the antiviral effect of hydroxytyrosol on influenza virus appears to involve morphological change of the virus. Antivir. Res. 2009, 83, 35–44. [Google Scholar] [CrossRef]
- Lee-Huang, S.; Zhang, L.; Huang, P.L.; Chang, Y.T.; Huang, P.L. Anti-HIV activity of olive leaf extract (OLE) and modulation of host cell gene expression by HIV-1 infection and OLE treatment. Biochem. Biophys. Res. Commun. 2003, 307, 1029–1037. [Google Scholar] [CrossRef]
- Lee-Huang, S.; Huang, P.L.; Zhang, D.; Lee, J.W.; Bao, J.; Sun, Y.T.; Chang, Y.T.; Zhang, J.; Huang, P.L. Discovery of small-molecule HIV-1 fusion and integrase inhibitors oleuropein and hydroxytyrosol: Part I. Fusion inhibition. Biochem. Biophys. Res. Commun. 2007, 354, 872–878. [Google Scholar] [CrossRef]
- Lee-Huang, S.; Huang, P.L.; Zhang, D.; Lee, J.W.; Bao, J.; Sun, Y.T.; Chang, Y.T.; Zhang, J.; Huang, P.L. Discovery of small-molecule HIV-1 fusion and integrase inhibitors oleuropein and hydroxytyrosol: Part II. Integrase inhibition. Biochem. Biophys. Res. Commun. 2007, 354, 879–884. [Google Scholar] [CrossRef]
- Khazeei Tabari, M.A.; Iranpanah, A.; Bahramsoltani, R.; Rahimi, R. Flavonoids as Promising Antiviral Agents against SARS-CoV-2 Infection: A Mechanistic Review. Molecules 2021, 26, 3900. [Google Scholar] [CrossRef]
- Wu, Y.; Hsieh, T.C.; Wu, J.M.; Wang, X.; Christopher, J.S.; Pham, A.H.; Swaby, J.D.L.; Lou, L.; Xie, Z.R. Elucidating the Inhibitory Effect of Resveratrol and Its Structural Analogs on Selected Nucleotide-Related Enzymes. Biomolecules 2020, 10, 1223. [Google Scholar] [CrossRef]
- Park, J.Y.; Yuk, H.J.; Ryu, H.W.; Lim, S.H.; Kim, K.S.; Park, K.H.; Ryu, Y.B.; Lee, W.S. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.N.; Lin, C.P.C.; Huang, K.K.; Chen, W.C.; Hsieh, H.P.; Liang, P.H.; Hsu, J.T.-A. Inhibition of SARS-CoV 3C-like protease activity by theaflavin-3,30-digallate (TF3). Evid.-Based Complement Altern. Med. 2005, 2, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.B.; Jeong, H.J.; Kim, J.H.; Kim, Y.M.; Park, J.Y.; Kim, D.; Naguyen, T.T.H.; Park, S.J.; Chang, J.S.; Park, K.H.; et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorg. Med. Chem. 2010, 18, 7940–7947. [Google Scholar] [CrossRef]
- Wu, Y.; Crich, D.; Pegan, S.D.; Lou, L.; Hansen, M.C.; Booth, C.; Desrochers, E.; Mullininx, L.N.; Starling, E.B.; Chang, K.Y.; et al. Polyphenols as Potential Inhibitors of SARS-CoV-2 RNA Dependent RNA Polymerase (RdRp). Molecules 2021, 26, 7438. [Google Scholar] [CrossRef] [PubMed]
- Stiti, N.; Triki, S.; Hartmann, M.A. Formation of triterpenoids throughout Olea europaea fruit ontogeny. Lipids 2007, 42, 55–67. [Google Scholar] [CrossRef]
- Peragón, J.; Rufino-Palomares, E.E.; Muñoz-Espada, I.; Reyes-Zurita, F.J.; Lupiáñez, J.A. A New HPLC-MS Method for Measuring Maslinic Acid and Oleanolic Acid in HT29 and HepG2 Human Cancer Cells. Int. J. Mol. Sci. 2015, 16, 21681–21694. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Palomares, E.E.; Reyes-Zurita, F.J.; Fuentes-Almagro, C.A.; de la Higuera, M.; Lupiáñez, J.A.; Peragón, J. Proteomics in liver of gilthead sea bream (Sparus aurata) to elucidate the cellular response induced by the intake of maslinic acid. Proteomics 2011, 11, 3312–3325. [Google Scholar] [CrossRef]
- Fernández-Navarro, M.; Peragón, J.; Esteban, F.J.; Amores, V.; de la Higuera, M.; Lupiáñez, J.A. Maslinic acid: A component of olive oil on growth and protein-turnover rates. In Olives and Olive Oil in Health and Disease Prevention, 1st ed.; Preedy, V.R., Watson, R.R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Chapter 157; pp. 1415–1421. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Reyes-Zurita, F.J.; García-Salguero, L.; Peragón, J.; de la Higuera, M.; Lupiáñez, J.A. The role of maslinic acid in the pentose phosphate pathway during growth of Gilthead Sea bream (Sparus aurata). Aquac. Nutr. 2013, 19, 709–720. [Google Scholar] [CrossRef]
- Fernández-Navarro, M.; Peragón, J.; Esteban, F.J.; de la Higuera, M.; Lupiáñez, J.A. Maslinic acid as a feed additive to stimulate growth and hepatic protein-turnover rates in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 144, 130–140. [Google Scholar] [CrossRef]
- Fernández-Navarro, M.; Peragón, J.; Esteban, F.J.; de la Higuera, M.; Lupiáñez, J.A. Maslinic acid added to the diet increases growth and protein-turnover rates in the white-muscle of rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 147, 158–167. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Reyes-Zurita, F.J.; García-Salguero, L.; Peragón, J.; de la Higuera, M.; Lupiáñez, J.A. Maslinic acid and ration restriction enhanced hepatic protein-turnover rates of gilthead sea bream (Sparus aurata). Aquac. Nut. 2012, 18, 138–151. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Reyes-Zurita, F.J.; García-Salguero, L.; Peragón, J.; de la Higuera, M.; Lupiáñez, J.A. Maslinic acid, a natural triterpene, and ration size increased growth and protein turnover of white muscle in gilthead sea bream (Sparus aurata). Aquac. Nut. 2012, 18, 568–580. [Google Scholar] [CrossRef]
- Peragón, J.; Barroso, J.B.; García-Salguero, L.; Aranda, F.; de la Higuera, M.; Lupiáñez, J.A. Growth, protein-turnover and nucleic acid concentrations in the white muscle of rainbow trout (Oncorhynchus mykiss) during development. Int. J. Biochem. Cell Biol. 2001, 33, 1227–1238. [Google Scholar] [CrossRef]
- Peragón, J.; Barroso, J.B.; de la Higuera, M.; Lupiáñez, J.A. Relationship between growth and protein turnover and nucleic acids in the liver of rainbow trout (Oncorhynchus mykiss) during development. Can. J. Fish. Aquat. Sci. 1998, 55, 649–657. [Google Scholar] [CrossRef]
- Huang, L.; Guan, T.; Qian, Y.; Huang, M.; Tang, X.; Li, Y.; Sun, H. Anti-inflammatory effects of maslinic acid, a natural triterpene, in cultured cortical astrocytes via suppression of nuclear factor-kappa B. Eur. J. Pharm. 2011, 672, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Martín, A.; de la Puerta, R.; Fernández-Arche, A.; Ruiz-Gutiérrez, V.; Yaqoob, P. Modulation of cytokine secretion by pentacyclic triterpenes from olive pomace oil in human mononuclear cells. Cytokine 2006, 36, 211–217. [Google Scholar] [CrossRef]
- Reyes-Zurita, F.J.; Pachón-Peña, G.; Lizárraga, D.; Rufino-Palomares, E.E.; Cascante, M.; Lupiáñez, J.A. The natural triterpene maslinic acid induces apoptosis in HT29 colon cancer cells by a JNK-p53-dependent mechanism. BMC Cancer 2011, 11, 154. [Google Scholar] [CrossRef]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; Medina, P.P.; García-Salguero, L.; Peragón, J.; Cascante, M.; Lupiáñez, J.A. Antitumor activity on extrinsic apoptotic targets of the triterpenoid maslinic acid in p53-deficient Caco-2 adenocarcinoma cells. Biochimie 2013, 95, 2198–2208. [Google Scholar] [CrossRef]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; García-Salguero, L.; Peragón, J.; Medina, P.P.; Parra, A.; Cascante, M.; Lupiáñez, J.A. Maslinic acid, a natural triterpene, induces a death receptor-mediated apoptotic mechanism in Caco-2 p53-deficient colon adenocarcinoma cells. PLoS ONE 2016, 11, e0146178. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Perona, J.S.; Herrera, M.D.; Ruiz-Gutiérrez, V. Triterpenic compounds from “orujo” olive oil elicit vasorelaxation in aorta from spontaneously hypertensive rats. J. Agric. Food Chem. 2006, 54, 2096–2102. [Google Scholar] [CrossRef]
- Nagai, N.; Yagyu, S.; Hata, A.; Nirengi, S.; Kotani, K.; Moritani, T.; Sakane, N. Maslinic acid derived from olive fruit in combination with resistance training improves muscle mass and mobility functions in the elderly. J. Clin. Biochem. Nutr. 2019, 64, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Pilz, R.B.; Willis, R.C.; Boss, G.R. The influence of ribose 5-phosphate availability on purine synthesis of cultured human lymphoblasts and mitogen-stimulated lymphocytes. J. Biol. Chem. 1984, 259, 2927–2935. [Google Scholar] [CrossRef]
- Peragón, J.; Aranda, F.; García-Salguero, L.; Vargas, A.M.; Lupiáñez, J.A. Long-term adaptive response to dietary protein of hexose monophosphate shunt dehydrogenases in rat kidney tubules. Cell Biochem. Funct. 1990, 8, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, K.; Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; Figuera, C.; García-Salguero, L.; Medina, P.P.; Peragón, J.; Lupiáñez, J.A. Maslinic Acid, a Triterpene from Olive, Affects the Antioxidant and Mitochondrial Status of B16F10 Melanoma Cells Grown under Stressful Conditions. Evid.-Based Complement. Altern. Med. 2015, 2015, 272457. [Google Scholar] [CrossRef]
- Bonel-Pérez, G.C.; Pérez-Jiménez, A.; Gris-Cárdenas, I.; Parra-Pérez, A.M.; Lupiáñez, J.A.; Reyes-Zurita, F.J.; Siles, E.; Csuk, R.; Peragón, J.; Rufino-Palomares, E.E. Antiproliferative and Pro-Apoptotic Effect of Uvaol in Human Hepatocarcinoma HepG2 Cells by Affecting G0/G1 Cell Cycle Arrest, ROS Production and AKT/PI3K Signaling Pathway. Molecules 2020, 25, 4254. [Google Scholar] [CrossRef]
- Mokhtari, K.; Pérez-Jiménez, A.; García-Salguero, L.; Lupiáñez, J.A.; Rufino-Palomares, E.E. Unveiling the Differential Antioxidant Activity of Maslinic Acid in Murine Melanoma Cells and in Rat Embryonic Healthy Cells Following Treatment with Hydrogen Peroxide. Molecules 2020, 25, 4020. [Google Scholar] [CrossRef]
- Šrejber, M.; Navrátilová, V.; Paloncýová, M.; Bazgier, V.; Berka, K.; Anzenbacher, P.; Otyepka, M. Membrane-attached mammalian cytochromes P450: An overview of the membrane’s effects on structure, drug binding, and interactions with redox partners. J. Inorg. Biochem. 2018, 183, 117–136. [Google Scholar] [CrossRef]
- Nóbrega-Pereira, S.; Fernández-Marcos, P.J.; Brioche, T.; Gómez-Cabrera, M.C.; Salvador-Pascual, A.; Flores, J.M.; Viña, J.; Serrano, M. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2006, 7, 10894. [Google Scholar] [CrossRef]
- Barroso, J.B.; García-Salguero, L.; Peragón, J.; de la Higuera, M.; Lupiáñez, J.A. The influence of dietary protein on the kinetics of NADPH production systems in various tissues of rainbow trout (Oncorhynchus mykiss). Aquaculture 1994, 124, 47–59. [Google Scholar] [CrossRef]
- Peragón, J.; Barroso, J.B.; García-Salguero, L.; Aranda, F.; de la Higuera, M.; Lupiáñez, J.A. Selective changes in the protein-turnover rates and nature of growth induced in trout liver by long-term starvation followed by re-feeding. Mol. Cell. Biochem. 1999, 201, 1–10. [Google Scholar] [CrossRef]
- Peragón, J.; Barroso, J.B.; García-Salguero, L.; de la Higuera, M.; Lupiáñez, J.A. Dietary alterations in protein, carbohydrates and fat increases liver-protein turnover rate and decrease overall growth rate in the rainbow trout (Oncorhynchus mykiss). Mol. Cell. Biochem. 2000, 20, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Lupiáñez, J.A.; Adroher, F.J.; Vargas, A.M.; Osuna, A. Differential behaviour of glucose 6-phosphate dehydrogenase in two morphological forms of Trypanosoma cruzi. Int. J. Biochem. 1987, 19, 1085–1089. [Google Scholar] [CrossRef]
- Adroher, F.J.; Osuna, A.; Lupiáñez, J.A. Differential energetic metabolism during Trypanosoma cruzi differentiation. I. Citrate synthase, NADP-isocitrate and succinate dehydrogenases. Arch. Biochem. Biophys. 1988, 267, 252–261. [Google Scholar] [CrossRef]
- Adroher, F.J.; Osuna, A.; Lupiáñez, J.A. Differential energetic metabolism during Trypanosoma cruzi differentiation. II. Hexokinase, phosphofructokinase and pyruvate kinase. Mol. Cell. Biochem. 1990, 94, 71–82. [Google Scholar] [CrossRef]
- Peragón, J.; Barroso, J.B.; García-Salguero, L.; de la Higuera, M.; Lupiáñez, J.A. Dietary protein effects on growth and fractional protein synthesis and degradation rates in rainbow trout (Oncorhynchus mykiss) liver and white muscle. Aquaculture 1994, 124, 35–46. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.J.; García-Rejón, L.; Lupiáñez, J.A.; de la Higuera, M. Long-term nutritional effects on the primary liver and kidney metabolism in rainbow trout (Oncorhynchus mykiss). II. Adaptive response of glucose 6-phosphate dehydrogenase activity to high-carbohydrate/low-protein and high-fat/non-carbohydrate diets. Aquac. Nutr. 1996, 2, 193–200. [Google Scholar] [CrossRef]
- Barroso, J.B.; Peragón, J.; García-Salguero, L.; de la Higuera, M.; Lupiáñez, J.A. Carbohydrate deprivation reduces NADPH-production in fish liver but not in adipose tissue. Int. J. Biochem. Cell Biol. 2001, 33, 785–796. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Palma, J.M.; Corpas, F.J. NADPH as a quality footprinting in horticultural crops marketability. Trends. Food Sci. Technol. 2020, 103, 152–161. [Google Scholar] [CrossRef]
- Montilla, M.; Agil, P.A.; Navarro, M.C.; Jiménez, M.I.; García-Granados, A.; Parra, A.; Cabo, M.M. Antioxidant activity of maslinic acid, a triterpene derivative obtained from Olea europaea. Planta Med. 2003, 69, 72–74. [Google Scholar] [CrossRef]
- Wang, D.; Xia, M.; Cui, Z. New Triterpenoids Isolated from the Root Bark of Ulmus pumila L. Chem. Pharm. Bull. (Tokyo) 2006, 54, 775–778. [Google Scholar] [CrossRef]
- Yang, Z.G.; Li, H.R.; Wang, L.Y.; Li, Y.H.; Lu, S.G.; Wen, X.F.; Wang, J.; Daikonya, A.; Kitanaka, S. Triterpenoids from Hippophae rhamnoides L. and their nitric oxide production-inhibitory and DPPH radical-scavenging activities. Chem. Pharm. Bull. (Tokyo) 2007, 55, 15–18. [Google Scholar] [CrossRef]
- Blanco-Cabra, N.; Vega-Granados, K.; Moya-Andérico, L.; Vukomanovic, M.; Parra, A.; Álvarez de Cienfuegos, L.; Torrents, E. Novel Oleanolic and Maslinic Acid Derivatives as a Promising Treatment against Bacterial Biofilm in Nosocomial Infections: An In Vitro and In Vivo Study. ACS Infect. Dis. 2019, 5, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Zeng, F.Q.; Wan, M.; Sim, K.Y. Anti-HIV triterpene acids from Geum japonicum. J. Nat. Prod. 1996, 59, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Kashiwada, Y.; Wang, H.K.; Nagao, T.; Kitanaka, S.; Yasuda, I.; Fujioka, T.; Yamagishi, T.; Cosentino, L.M.; Kozuka, M.; Okabe, H.; et al. Anti-AIDS Agents. 30. Anti-HIV Activity of Oleanolic Acid, Pomolic Acid, and Structurally Related Triterpenoids. J. Nat. Prod. 1998, 61, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Rivas, F.; López, P.E.; García-Granados, A.; Martínez, A.; Albericio, F.; Márquez, N.; Muñoz, E. Solution-and solid-phase synthesis and anti-HIV activity of maslinic acid derivatives containing amino acids and peptides. Bioorg. Med. Chem. 2009, 17, 1139–1145. [Google Scholar] [CrossRef]
- Reyes, F.J.; Centelles, J.J.; Lupiáñez, J.A.; Cascante, M. (2α, 3β)-2,3-dihydroxyolean-12-en-28-oic acid, anew natural triterpene from Olea europea, induces caspase dependent apoptosis selectively in colon adenocarcinoma cells. FEBS Lett. 2006, 580, 6302–6310. [Google Scholar] [CrossRef]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; Lupiáñez, J.A.; Cascante, M. Maslinic acid, a natural triterpene from Olea europaea L., induces apoptosis in HT29 human colon-cancer cells via the mitochondrial apoptotic pathway. Cancer Lett. 2009, 273, 44–54. [Google Scholar] [CrossRef]
- Sánchez-Tena, S.; Reyes-Zurita, F.J.; Díaz-Moralli, S.; Vinardell, M.P.; Reed, M.; García-García, F.; Dopazo, J.; Lupiáñez, J.A.; Günther, U.; Cascante, M. Maslinic acid-enriched diet decreases intestinal tumorigenesis in ApcMin/+ mice through transcriptomic and metabolomic reprogramming. PLoS ONE 2013, 8, e59392. [Google Scholar] [CrossRef]
- Reyes-Zurita, F.J.; Medina-O’Donnell, M.; Ferrer-Martin, R.M.; Rufino-Palomares, E.E.; Martin-Fonseca, S.; Rivas, F.; Martínez, A.; García-Granados, A.; Pérez-Jiménez, A.; García-Salguero, L.; et al. The oleanolic acid derivative, 3-O-succinyl-28-O-benzyl oleanolate, induces apoptosis in B16–F10 melanoma cells via the mitochondrial apoptotic pathway. RSC Adv. 2016, 6, 93590. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Reyes-Zurita, F.J.; García-Salguero, L.; Mokhtari, K.; Medina, P.P.; Lupiáñez, J.A.; Peragón, J. Maslinic acid, a triterpenic anti-tumoural agent, interferes with cytoskeleton protein expression in HT29 human colon-cancer cells. J. Proteomics 2013, 83, 15–25. [Google Scholar] [CrossRef]
- Martín, R.; Carvalho, J.; Ilbeas, E.; Hernández, M.; Ruiz-Gutiérrez, V.; Nieto, M.L. Acidic triterpenes compromise growth and survival of astrocytoma cell lines by regulating reactive oxygen species accumulation. Cancer Res. 2007, 67, 3741–3751. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peñas-Fuentes, J.L.; Siles, E.; Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; Lupiáñez, J.A.; Fuentes-Almagro, C.; Peragón-Sánchez, J. Effects of Erythrodiol on the Antioxidant Response and Proteome of HepG2 Cells. Antioxidants 2022, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Mena, G.; Sánchez-González, M.; Juan, M.E.; Planas, J.M. Maslinic acid, a natural phytoalexin-type triterpene from olives—A promising nutraceutical? Molecules 2014, 19, 11538–11559. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, H.; Farooq, A.A.; Nie, B.; Chen, X.; Su, S.; Yuan, R.; Qiao, G.; Li, C.; Li, X.; et al. Maslinic acid induces autophagy by down-regulating HSPA8 in pancreatic cancer cells. Phytother. Res. 2018, 32, 1320–1331. [Google Scholar] [CrossRef]
- Wu, T.W.; Chen, S.; Brinton, R.D. Membrane estrogen receptors mediate calcium signaling and MAP kinase activation in individual hippocampal neurons. Brain Res. 2011, 1379, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, X.; He, D.; Jin, X.; Guo, P. Metformin sensitizes human bladder cancer cells to TRAIL-induced apoptosis through mTOR/S6K1-mediated downregulation of c-FLIP. Anticancer Drugs 2014, 25, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, Y.Z.; Liu, B.; Wu, R.; Yang, Y.Y.; Xiao, X.Q.; Zhang, X. Pioglitazone upregulates angiotensin converting enzyme 2 expression in insulin-sensitive tissues in rats with high-fat diet-induced nonalcoholic steatohepatitis. Sci. World J. 2014, 2014, 603409. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Xing, D.; Chen, W.R. Inhibition of the JNK/Bim pathway by Hsp70 prevents Bax activation in UV-induced apoptosis. FEBS Lett. 2010, 584, 4672–4678. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, B.; Li, P.; Wen, X.; Yang, J. Maslinic Acid Inhibits Colon Tumorigenesis by the AMPK-mTOR Signaling Pathway. J. Agr. Food Chem. 2019, 67, 4259–4272. [Google Scholar] [CrossRef]
- Yurasakpong, L.; Nantasenamat, C.; Nobsathian, S.; Chaithirayanon, K.; Apisawetakan, S. Betulinic Acid Modulates the Expression of HSPA and Activates Apoptosis in Two Cell Lines of Human Colorectal Cancer. Molecules 2021, 26, 6377. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Lendeckel, W.; Tuschl, T. Identification of novel genes coding for small expressed RNAs. Science 2001, 294, 853–8588. [Google Scholar] [CrossRef] [PubMed]
- Selbach, M.; Schwanhäusser, B.; Thierfelder, N.; Fang, Z.; Khanin, R.; Rajewsky, N. Widespread changes in protein synthesis induced by microRNAs. Nature 2008, 455, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Palomares, E.E.; Reyes-Zurita, F.J.; Lupiáñez, J.A.; Medina, P.P. miRNAs as oncogenes and tumor suppressors. In MicroRNAs in Medicine, 1st ed.; Lawrie, C.H., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; Chapter 14; pp. 223–243. [Google Scholar] [CrossRef]
- Sayeed, M.A.; Bracci, M.; Lucarini, G.; Lazzarini, R.; Di Primio, R.; Santarelli, L. Regulation of microRNA using promising dietary phytochemicals: Possible preventive and treatment option of malignant mesothelioma. Biomed. Pharmacother. 2017, 94, 1197–1224. [Google Scholar] [CrossRef]
- Liu, X.; Jutooru, I.; Lei, P.; Kim, K.; Lee, S.; Brents, L.K.; Prather, P.L.; Safe, S. Betulinic Acid Targets YY1 and ErbB2 through Cannabinoid Receptor-Dependent Disruption of MicroRNA-27a:ZBTB10 in Breast Cancer. Mol. Cancer Ther. 2012, 11, 1421–1431. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Noratto, G.D.; Li, X.; Angel-Morales, G.; Bertoldi, M.C.; Safe, S. Betulinic Acid Decreases ER-Negative Breast Cancer Cell Growth In Vitro and In Vivo: Role of Sp Transcription Factors and MicroRNA-27a:ZBTB10. Mol. Carcinog. 2013, 52, 591–602. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Wang, X.; Jiang, C. Ursolic Acid Inhibits Proliferation and Induces Apoptosis in Human Glioblastoma Cell Lines U251 by Suppressing TGF-b1/miR-21/PDCD4 Pathway. Basic Clin. Pharmacol. Toxicol. 2012, 111, 106–112. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, F.; Yang, L.; Mei, Y.; Long, H.; Zhang, X.; Zhang, J.; Qimuge-Suyila; Su, X. Ursolic acid inhibits proliferation and induces apoptosis of cancer cells in vitro and in vivo. J. Biomed. Biotechnol. 2011, 2011, 419343. [Google Scholar] [CrossRef]
- Huang, H.C.; Huang, C.Y.; Lin-Shiau, S.Y.; Lin, J.K. Ursolic acid inhibits IL-1beta or TNF-alpha-induced C6 glioma invasion through suppressing the association ZIP/p62 with PKC-zeta and downregulating the MMP-9 expression. Mol. Carcinog. 2009, 48, 517–531. [Google Scholar] [CrossRef]
- Serra, C.; Lampis, G.; Pompei, R.; Pinza, M. Antiviral activity of new triterpenic derivatives. Pharmacol. Res. 1994, 29, 359–366. [Google Scholar] [CrossRef]
- Parra, A.; Martín-Fonseca, S.; Rivas, F.; Reyes-Zurita, F.J.; Medina-O’Donnell, M.; Rufino-Palomares, E.E.; Martínez, A.; García-Granados, A.; Lupiáñez, J.A.; Albericio, F. Solid-phase library synthesis of bi-functional derivatives of oleanolic and maslinic acids and their cytotoxicity on three cancer cell lines. ACS Comb. Sci. 2014, 16, 428–447. [Google Scholar] [CrossRef] [PubMed]
- Medina-O’Donnell, M.; Rivas, F.; Reyes-Zurita, F.J.; Martínez, A.; Martin-Fonseca, S.; García-Granados, A.; Ferrer-Martin, R.; Lupiáñez, J.A.; Parra, A. Semi-synthesis and antiproliferative evaluation of PEGylated pentacyclic triterpenes. Eur. J. Med. Chem. 2016, 118, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Medina-O’Donnell, M.; Rivas, F.; Reyes-Zurita, F.J.; Martínez, A.; Galisteo-González, F.; Lupiáñez, J.A.; Parra, A. Synthesis and in vitro antiproliferative evaluation of PEGylated triterpene acids. Fitoterapia 2017, 120, 25–40. [Google Scholar] [CrossRef]
- Medina-O’Donnell, M.; Rivas, F.; Reyes-Zurita, F.J.; Martínez, A.; Lupiáñez, J.A.; Parra, A. Diamine and PEGylated-diamine conjugates of triterpenic acids as potential anticancer agents. Eur. J. Med. Chem. 2018, 148, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Martín-Fonseca, S.; Rivas, F.; Lupiáñez, J.A.; Reyes-Zurita, F.J.; Albericio, F. Semi-synthesis of acylated triterpenes from olive-oil industry wastes for the development of anticancer and anti-HIV agents. Eur. J. Med. Chem. 2014, 74, 278–301. [Google Scholar] [CrossRef]
- Medina-O’Donnell, M.; Rivas, F.; Reyes-Zurita, F.J.; Cano-Muñoz, M.; Martínez, A.; Lupiáñez, J.A.; Parra, A. Oleanolic Acid Derivatives as Potential Inhibitors of HIV-1 Protease. J. Nat. Prod. 2019, 82, 2886–2896. [Google Scholar] [CrossRef]
- Vega-Granados, K.; Medina-O’Donnell, M.; Rivas, F.; Reyes-Zurita, F.J.; Martínez, A.; Álvarez de Cienfuegos, L.; Lupiáñez, J.A.; Parra, A. Synthesis and Biological Activity of Triterpene-Coumarin Conjugates. J. Nat. Prod. 2021, 84, 1587–1597. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, X.; Ma, Q.; Zheng, Y.; Xu, F.; Peng, H.; Dai, H.; Zhou, J.; Zhao, Y. Terpenoids and their anti-HIV-1 activities from Excoecaria acerifolia. Fitoterapia 2013, 91, 224–230. [Google Scholar] [CrossRef]
- Vardhan, S.; Sahoo, S.K. In silico ADMET and molecular docking study on searching potential inhibitors from limonoids and triterpenoids for COVID-19. Comput. Biol. Med. 2020, 124, 103936. [Google Scholar] [CrossRef]
- Soltane, R.; Chrouda, A.; Mostafa, A.; Al-Karmalawy, A.A.; Chouaïb, K.; Dhahri, A.; Pashameah, R.A.; Alasiri, A.; Kutkat, O.; Shehata, M.; et al. Strong Inhibitory Activity and Action Modes of Synthetic Maslinic Acid Derivative on Highly Pathogenic Coronaviruses: COVID-19 Drug Candidate. Pathogens 2021, 10, 623. [Google Scholar] [CrossRef]
- Hisham Shady, N.; Youssif, K.A.; Sayed, A.M.; Belbahri, L.; Oszako, T.; Hassan, H.M.; Abdelmohsen, U.R. Sterols and Triterpenes: Antiviral Potential Supported by In-Silico Analysis. Plants 2021, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Alhadrami, H.A.; Sayed, A.M.; Sharif, A.M.; Azhar, E.I.; Rateb, M.E. Olive-Derived Triterpenes Suppress SARS-CoV-2 Main Protease: A Promising Scaffold for Future Therapeutics. Molecules 2021, 26, 2654. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Liu, H.M.; Liu, X.M.; Yuan, X.Y.; Xu, C.; Wang, F.; Lin, J.Z.; Xu, R.C.; Zhang, D.K. Screening S protein-ACE2 blockers from natural products: Strategies and advances in the discovery of potential inhibitors of COVID-19. Eur. J. Med. Chem. 2021, 226, 113857. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Rather, M.A.; Kumar Jha, A.; Shashank, A.; Singhal, S.; Sharma, M.; Pathak, U.; Sharma, D.; Mastinu, A. Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam. Flowers: New Sources of Bioactive Compounds. Plants 2020, 9, 1329. [Google Scholar] [CrossRef]
- Abate, G.; Zhang, L.; Pucci, M.; Morbini, G.; Mac Sweeney, E.; Maccarinelli, G.; Ribaudo, G.; Gianoncelli, A.; Uberti, D.; Memo, M.; et al. Phytochemical Analysis and Anti-Inflammatory Activity of Different Ethanolic Phyto-Extracts of Artemisia annua L. Biomolecules 2021, 11, 975. [Google Scholar] [CrossRef]
- Escobar, E.; Fernández, M.; Betancur, L.I.; Muñoz, D.L.; Acín, S.; Orozco, J.; Balcázar, N. Development of a phytotherapeutic prototype based on triterpenes encapsulated in nanocarriers for the treatment of obesity and T2DM. Planta Med. 2021, 87, 1252–1253. [Google Scholar] [CrossRef]


| Effects Parameters | Pharmaco-Kinetics and Toxicity [7,8,9,10,11,12,13,14,15,16,17,18,19] | Antioxidant and Anti-Aging Activity [20,21,22,23] | Anticancer and Antiproliferative Activity [24,25,26,27,28,29,30,31,32,33,34,35,36,37] | Immunomodulatory and Anti-Inflammatory [38,39,40,41,42,43,44,45,46,47,48,49] | Antiviral and Antimicrobial Activity [50,51,52,53,54,55,56,57,58,59,60,61,62,63,64] |
|---|---|---|---|---|---|
| Organisms, tissues, and cell lines studied in this review | Wistar rats Sprague-Dawley rats Caco-2 cells Human tissues Rat tissues | In vitro models Leucocyte cells HepG2 cells | HL60 cells HT29 cells MCF-7 cells MDA-MB-231 cells CSC cells HepG2 cells | RatsTHP-1 cells J774 cells Mouse macrophages | MT2 cells MDCK cells Th9 cells HIV virus SARS-CoV-2 Different types of bacteria |
| Molecular markers and target processes involved in the activity of olive’s fruit and leaf polyphenols | Bioavailability: Dose-dependent Rat tissues: Liver, Heart Spleen, Thymus Testicle, Brain Time absorption: 5–10 min after ingestion Recovery: 75% by aqueous solution 90% by oil solution Excretion: Glucuronide conjugates Toxicity: Nontoxic LD50 >>5 g/kg | Transcription factor: Nrf2 Redox homeostasis: ROS H2O2 O2− LDL Glutathione NADPH Platelet Aggregation Chelating: Iron and copper metals Diseases: Cardiovascular Diabetes mellitus Metabolic syndrome Cancer Enzymes: GPX GR G6PDH 6PGDH | Cell cycle phases: G0/G1 G2/M S Cyclins: p21WAF/Cip1 p27Kip1 CD44 Pro-apoptotic Anti-metastatic Anti-angiogenic Enzymes and factors: Nrf2 ABCG2 HO-1 Cripto-1 ALDH1 Nuclear parameters: AMP biosynthesis Transport of mature mRNA | Parameters: NO PGE2 LPS Thromboxane B2 NF-κB Cytokines: IL-1α IL-1β IL-6 IL-12 TNF-α Chemokines: CXCL10/IP-10 CCL2/MPC-1 Enzymes: MMP9 PGE Synthase COX-2 iNOS | Viruses, bacteria: Escherichia coli Candida albicans Kluyveromyces marxianus, Clostridium perfringens Streptococcus mutans, Shigella sonnei Salmonella enterica Vibrio parahaemolyticus, Vibrio cholerae, Salmonella typhi, Haemophilus influenzae, Staphylococcus aureus, Moraxella catarrhalis, Mycoplasma pneumoniae Influenza A viruses: H1N1, H3N2, H5N1, H9N2, Newcastle virus SARS-CoV-2 DNA polymerase α, Reverse transcriptase, RdRp 3CL-Pro HIV virus p24 expression Viral proteases |
| Effects Parameters | Stimulating Effects of Normal Growth [67,68,69,70,71,72,73,74,75] | Immunomodulatory and Anti-Inflammatory [76,77,78,79,80,81,82] | Antioxidant and Anti-Aging Activity [83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113] | Anticancer and Antiproliferative Activity [114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130] | Antiviral and Antimicrobial Activity [131,132,133,134,135,136,137,138,139,140,141,142,143,144] |
|---|---|---|---|---|---|
| Organisms, tissues, and cell lines studied in this review | Sparus aurata Oncorhynchus mykiss Liver White muscle | Catecholaminergic cells Macrophages Aortic tissue | Hepatocyte Macrophage B16F10 cells Rats Plasma | HT29, Caco-2 cells HepG2 cells B16-F10 cells MCF-7, ACC cells T24, RT4 cells HCT116 cells SW982, 253J cells SK-UT-1 cells ACC2, ACCM cells WRL68 cells BT474, H22 cells BGC-803 cells | Excoecaria acerifolia sp. AIDS virus HIV-1 SARS-CoV-2 |
| Molecular markers and target processes involved in the activity of olive’s fruit and leaf triterpenes | Hyperplasia Hypertrophy Liver White muscle NADPH Growth parameters: CS KD KS KG KDNA KRNA PRE Protein turnover: KS/KD | Molecular parameters: NF-κB AP-1 Bcl-2 family Bax, Bid Bcl2 Cytokines: IL-6 TNFα Enzymes: COX-2 iNOS eNOS JNK MMP9 or Collagenase type IV ODC Resistance training | Oxidative stress Parameters: ROS H2O2 NADP NADPH MMP CCl4 PMA GSH GSSG Lipid peroxidation (LPO) Enzymes as: GST CAT SOD G6PDH 6PGDH NADP-ICDH | Parameters: MAPKs, JNK activating p53 AMPK/mTOR Rps6ka2 gene p90Rsk p38 MAPK AKT/PI3K ZBTB10, VEGFR ErbB2, Sp YY1, PDCD4 TGF-β1 Apoptotic proteins: Bcl2, Bax Bid, Bim Caspases: Caspase-3, -9 Caspase-8, -7 Chaperones: HSPA HSP70 HSP60 MicroRNAs miRNA-27a miRNA-21 | Protease inhibitory activity: 3CL-Pro PL-Pro Antiviral capacity: SGp-RBD Virus replication: RdRp Viral repression: ACE2 Other triterpenic markers: Epipomolic acid Euscamphenic acid Tormentic acid Chemical derivatives: Maslinic isoxazole chlorinated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rufino-Palomares, E.E.; Pérez-Jiménez, A.; García-Salguero, L.; Mokhtari, K.; Reyes-Zurita, F.J.; Peragón-Sánchez, J.; Lupiáñez, J.A. Nutraceutical Role of Polyphenols and Triterpenes Present in the Extracts of Fruits and Leaves of Olea europaea as Antioxidants, Anti-Infectives and Anticancer Agents on Healthy Growth. Molecules 2022, 27, 2341. https://doi.org/10.3390/molecules27072341
Rufino-Palomares EE, Pérez-Jiménez A, García-Salguero L, Mokhtari K, Reyes-Zurita FJ, Peragón-Sánchez J, Lupiáñez JA. Nutraceutical Role of Polyphenols and Triterpenes Present in the Extracts of Fruits and Leaves of Olea europaea as Antioxidants, Anti-Infectives and Anticancer Agents on Healthy Growth. Molecules. 2022; 27(7):2341. https://doi.org/10.3390/molecules27072341
Chicago/Turabian StyleRufino-Palomares, Eva E., Amalia Pérez-Jiménez, Leticia García-Salguero, Khalida Mokhtari, Fernando J. Reyes-Zurita, Juan Peragón-Sánchez, and José A. Lupiáñez. 2022. "Nutraceutical Role of Polyphenols and Triterpenes Present in the Extracts of Fruits and Leaves of Olea europaea as Antioxidants, Anti-Infectives and Anticancer Agents on Healthy Growth" Molecules 27, no. 7: 2341. https://doi.org/10.3390/molecules27072341
APA StyleRufino-Palomares, E. E., Pérez-Jiménez, A., García-Salguero, L., Mokhtari, K., Reyes-Zurita, F. J., Peragón-Sánchez, J., & Lupiáñez, J. A. (2022). Nutraceutical Role of Polyphenols and Triterpenes Present in the Extracts of Fruits and Leaves of Olea europaea as Antioxidants, Anti-Infectives and Anticancer Agents on Healthy Growth. Molecules, 27(7), 2341. https://doi.org/10.3390/molecules27072341










