Comparative Analysis of VOCs from Winter Melon Pomace Fibers before and after Bleaching Treatment with H2O2
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Aldehydes
3.2. Alcohols
3.3. Ketones
3.4. Esters
3.5. Acids
3.6. Sulfur-Derived Compounds
3.7. Other Compounds
- (i)
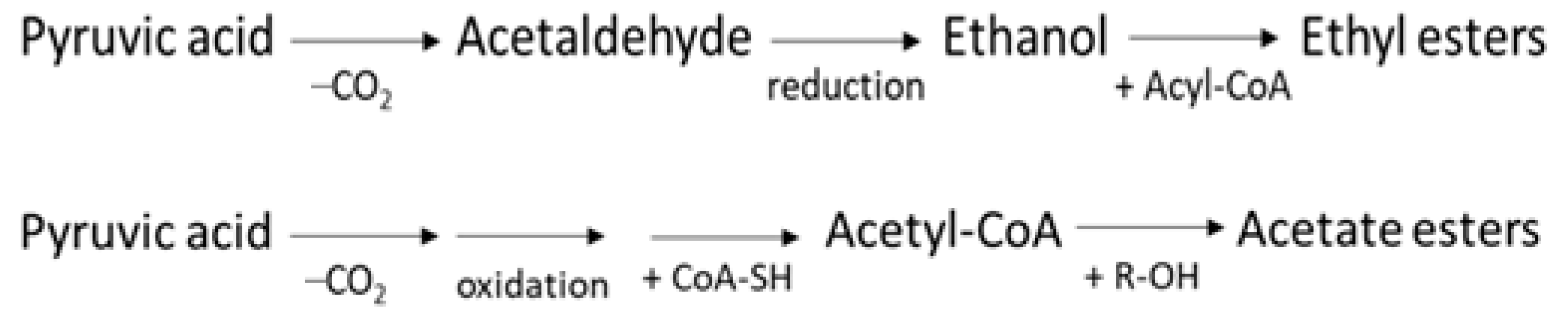
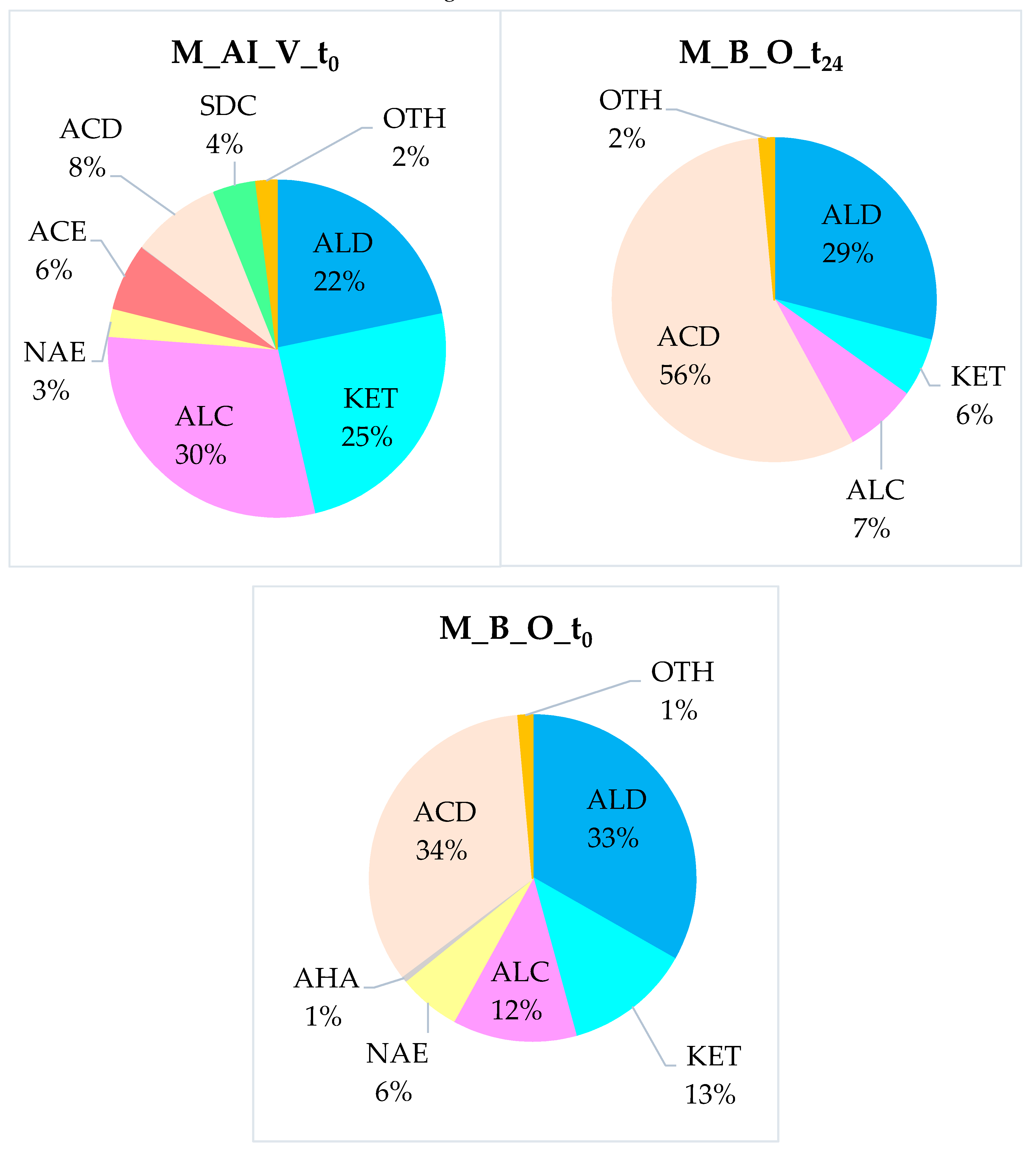
- Volatile compounds typical of the ripe fruit (alcohols, esters): Alcohols covered about one third of the VOCs of the M_AI_V_t0 fiber, while immediately after the bleaching treatment, they represented ≈12 % of the volatiles (M_B_O_t0) and only ≈7 % in the aged sample (M_B_O_t24). Acetate esters were present only in the M_AI_V_t0 sample at a relative percentage of about 7 %, and completely disappeared in the treated ones. On the other hand, non-acetate esters persisted even after the chemical whitening process of the melon pomace fiber, reaching ≈6 % in the M_B_O_t0 sample, but not after the long storage period.
- (ii)
- Volatiles completely lost during the bleaching process (Sulfur-Derived Compounds): We report that six SDCs were detected only in the crude fiber (M_AI_V_t0), at around 4.5%, while none of them were identified in the bleached ones. The total loss of this class of analytes may have been caused by their intrinsic volatility in combination with the hydrolysis processes in which they can be involved, due to being in the presence of organic acids and residual moisture. Finally, even the repeated washing of the fibers may have contributed to the removal of some analytes present in the “As-it-Is” pomace.
- (iii)
- Molecules developed from the DF treatment with H2O2 (organic acids): The fraction of acids was the largest in the M_B_O_t24 sample (≈57%), while it covered only about 10% of the total volatile organic fraction of the M_AI_V_t0 and reached ≈34% in the M_B_O_t0 ones. Certainly, the action of H2O2 strongly contributed to the development of these analytes, oxidizing some of the molecules originally present in the starting matrix, to give products with acid functional groups. In addition, the oxygen present in the residual atmosphere of the preservation vials also led to an increasing quantity of organic acids over time, with O2 acting as a starter for radical oxidation processes.
4. Materials and Methods
4.1. Sample Preparation
4.2. Volatile Organic Compounds Sampling—HS SPME
4.3. GC-MS Analysis
4.4. Chemicals and Reagents
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- FAO. Fruit and Vegetables—Your Dietary Essentials: The International Year of Fruits and Vegetables 2021; Background Paper; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Cui, S.W.; Roberts, K.T. Dietary Fiber: Fulfilling the Promise of Added-Value Formulations. In Modern Biopolymer Science, 1st ed.; Kasapis, S., Norton, I.T., Ubbink, J.B., Eds.; Chapter 13; Academic Press: San Diego, CA, USA, 2009; pp. 399–448. [Google Scholar] [CrossRef]
- O’Shea, N.; Arendt, E.K.; Gallagher, E. Dietary fibre and phytochemical characteristics of fruit and vegetable by-products and their recent applications as novel ingredients in food products. Innov. Food Sci. Emerg. Technol. 2012, 16, 1–10. [Google Scholar] [CrossRef]
- Singh, A.; Kaur, V.; Kaler, R.S.S. A review on dietary fiber in cereals and its characterization. J. Appl. Nat. Sci. 2018, 10, 1216–1225. [Google Scholar] [CrossRef]
- Lipinski, B.; Hanson, C.; Lomax, J.; Kitinoja, L.; Waite, R.; Searchinger, T. Reducing Food Loss and Waste, Installment 2 of Creating a Sustainable Food Future; Working Paper 1; World Resources Institute: Washington, DC, USA, 2013; pp. 1–40. [Google Scholar]
- Kassim, F.O.; Thomas, C.L.P.; Afolabi, O.O.D. Integrated conversion technologies for sustainable agri-food waste valorization: A critical review. Biomass Bioenergy 2022, 156, 106314. [Google Scholar] [CrossRef]
- FAO. Get Involved : Stop Food Loss and Waste. For the People. For the Planet. International Day of Awareness of Food Loss and Waste; FAO: Rome, Italy, 2020; Available online: https://www.fao.org/documents/card/en/c/cb0641en/ (accessed on 28 September 2021).
- Messner, R.; Johnson, H.; Richards, C. From surplus-to-waste: A study of systemic overproduction, surplus and food waste in horticultural supply chains. J. Clean. Prod. 2021, 278, 123952. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. Vegetables from the Cucurbitaceae family and their products: Positive effects on human health. Nutrition 2020, 78, 110788. [Google Scholar] [CrossRef]
- Shi, J.; Wu, H.; Xiong, M.; Chen, Y.; Chen, J.; Zhou, B.; Wang, H.; Li, L.; Fu, X.; Bie, Z.; et al. Comparative analysis of volatile compounds in thirty nine melon cultivars by headspace solid-phase microextraction and gas chromatography-mass spectrometry. Food Chem. 2020, 316, 26342. [Google Scholar] [CrossRef] [PubMed]
- Esteras, C.; Rambla, J.L.; Sánchez, G.; López-Gresa, M.P.; González-Mas, M.C.; Fernández-Trujillo, J.P.; Bellés, J.M.; Granell, A.; Picó, M.B. Fruit flesh volatile and carotenoid profile analysis within the Cucumis melo L. species reveals unexploited variability for future genetic breeding. J. Sci. Food Agric. 2018, 98, 3915–3925. [Google Scholar] [CrossRef] [PubMed]
- Kourkoutas, D.; Elmore, J.S.; Mottram, D.S. Comparison of the volatile compositions and flavour properties of cantaloupe, Galia and honeydew muskmelons. Food Chem. 2006, 97, 95–102. [Google Scholar] [CrossRef]
- Perry, P.L.; Wang, Y.; Lin, J. Analysis of honeydew melon (Cucumis melo var. inodorus) flavour and GC-MS/MS identification of (E,Z)-2,6-nonadienyl acetate. Flavour Fragr. J. 2009, 24, 341–347. [Google Scholar] [CrossRef]
- Plutowska, B.; Wardencki, W. Aromagrams–Aromatic profiles in the appreciation of food quality. Food Chem. 2007, 101, 845–872. [Google Scholar] [CrossRef]
- Chambers, E.; Koppel, K. Associations of Volatile Compounds with Sensory Aroma and Flavor: The Complex Nature of Flavor. Molecules 2013, 18, 4887–4905. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.D. Removing Green Color from and Reducing Flavor Levels of Fibrous and Other Granular Material. U.S. Patent No. 825,072, 4 January 1994. [Google Scholar]
- Monsalve-Gonzales, A.; Metzger, L.E.; Prakash, A.; Valanju, M.; Roufs, J.G. Bleached Bran and Bran Products and Methods of Preparation. Patent Cooperation Treaty (PCT). U.S. Patent No. WO2002021936A2, 12 December 2002. [Google Scholar]
- Renard, C.M.G.C.; Rohou, Y.; Hubert, C.; Della Valle, G.; Thibault, J.F.; Savina, J.P. Bleaching of Apple Pomace by Hydrogen Peroxide in Alkaline Conditions: Optimisation and Characterisation of the Products. LWT Food Sci. Technol. 1997, 30, 398–405. [Google Scholar] [CrossRef]
- Dos-Santos, N.; Bueso, M.C.; Fernández-Trujillo, J.P. Aroma volatiles as biomarkers of textural differences at harvest in non-climacteric near-isogenic lines of melon. Food Res. Int. 2013, 54, 1801–1812. [Google Scholar] [CrossRef]
- Dima, G.; Tripodi, G.; Condurso, C.; Verzera, A. Volatile constituents of mini-watermelon fruits. J. Essent. Oil Res. 2014, 26, 323–327. [Google Scholar] [CrossRef]
- Saftner, R.; Luo, Y.; McEvoy, J.; Abbott, J.A.; Vinyard, B. Quality characteristics of fresh-cut watermelon slices from non-treated and 1-methylcyclopropene- and/or ethylene-treated whole fruit. Postharvest Biol. Technol. 2007, 44, 71–79. [Google Scholar] [CrossRef]
- Hasbullah, U.H.A.; Supriyadi; Daryono, B.S. Aroma Volatile Compounds Profile of Melon (Cucumis melo L.) cv. Gama Melon Parfum. IOP Conf. Ser. Earth Environ. Sci. 2019, 292, 012027. [Google Scholar] [CrossRef]
- Ntsoane, M.L.; Luca, A.; Zude-Sasse, M.; Sivakumar, D.; Mahajan, P.V. Impact of low oxygen storage on quality attributes including pigments and volatile compounds in ‘Shelly’ mango. Sci. Hortic. 2019, 250, 174–183. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Grimm, C.C. Identification of Volatile Compounds in Cantaloupe at Various Developmental Stages Using Solid Phase Microextraction. J. Agric. Food Chem. 2001, 49, 1345–1352. [Google Scholar] [CrossRef]
- Yabumoto, K.; Yamaguchi, M.; Jennings, W.G. Production of Volatile Compounds by Muskmelon, Cucumis melo. Food Chem. 1978, 3, 7–16. [Google Scholar] [CrossRef]
- Sun, X.; Baldwin, E.; Plotto, A.; Cameron, R.; Manthey, J.; Dorado, C.; Bai, J. The effect of cultivar and processing method on the stability, flavor, and nutritional properties of winter melon juice. LWT 2018, 97, 223–230. [Google Scholar] [CrossRef]
- Maletti, L. Stability and aging of dietary fiber from waste watermelon. Acad. Lett. 2021, 2386. [Google Scholar] [CrossRef]
- Allwood, J.W.; Cheung, W.; Xu, Y.; Mumm, R.; De Vos, R.C.H.; Deborde, C.; Biais, B.; Maucourt, M.; Berger, Y.; Schaffer, A.A.; et al. Metabolomics in melon: A new opportunity for aroma analysis. Phytochemistry 2014, 99, 61–72. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y.; Wang, B.; Song, H.; Zou, T. Screening of the volatile compounds in fresh and thermally treated watermelon juice via headspace-gas chromatography-ion mobility spectrometry and comprehensive two-dimensional gas chromatography-olfactory-mass spectrometry analysis. LWT 2021, 137, 110478. [Google Scholar] [CrossRef]
- Sun, X.; Baldwin, E.A.; Plotto, A.; Manthey, J.A.; Duan, Y.; Bai, J. Effects of thermal processing and pulp filtration on physical, chemical and sensory properties of winter melon juice: Processing and sensory properties of winter melon juice. J. Sci. Food Agric. 2017, 97, 543–550. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, Y.; Qui, J.; Cao, J.; Sun, Y.; Li, H.; Kong, F. Coupled multidimensional GC and odor activity value calculation to identify off-odors in thermally processed muskmelon juice. Food Chem. 2019, 301, 125307. [Google Scholar] [CrossRef]
- Güler, Z.; Karaca, F.; Yetisir, H. Volatile Compounds and Sensory Properties in Various Melons, which were Chosen from Different Species and Different Locations, Grown in Turkey. Int. J. Food Prop. 2013, 16, 168–179. [Google Scholar] [CrossRef]
- Frankvoort, W. The reaction between diacetyl and hydrogen peroxide: Its mechanism and kinetic constants. Thermochim. Acta 1978, 25, 35–49. [Google Scholar] [CrossRef]
- Pearson, T.W.; Dawson, H.J.; Lackey, H.B. Naturally occurring levels of dimethyl sulfoxide in selected fruits, vegetables, grains, and beverages. J. Agric. Food Chem. 1981, 29, 1089–1091. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhang, W.; Lao, F.; Mi, R.; Liao, X.; Luo, D.; Wu, J. Isolation and identification of putative precursors of the volatile sulfur compounds and their inhibition methods in heat-sterilized melon juices. Food Chem. 2021, 343, 128459. [Google Scholar] [CrossRef] [PubMed]
- Nishibori, S.; Osawa, T.; Kawakishi, S. Volatile Components Formed from Various Sugars with p-alanine in Actual Cookies. Flavor Chem. Ethn. Foods 1999, 23, 239–249. [Google Scholar]
- Čechovská, L.; Cejpek, K.; Konečný, M.; Velíšek., J. On the role of 2,3-dihydro-3,5-dihydroxy-6-methyl-(4H)-pyran-4-one in antioxidant capacity of prunes. Eur. Food Res. Technol. 2011, 233, 367–376. [Google Scholar] [CrossRef]
- Hu, Z.; Ge, S.; Yang, J.; Li, Y.; Bi, H.; Zheng, D.; Zhao, Y.; Peng, W.; Zhang, Z. Molecular characteristics and function of elliptical Kiwifruit. J. King Saud Univ. Sci. 2020, 32, 1884–1888. [Google Scholar] [CrossRef]
- Genthner, E.R. Identification of Key Odorants in Fresh-Cut Watermelon Aroma and Structure-Odor Relationships of cis,cis-3,6-Nonadienal and Ester Analogs with cis,cis-3,6-nonadiene, cis-3-nonene and cis-6-nonene Backbone Structures. Master’s Thesis, Graduate College of the University of Illinois, Urbana, IL, USA, 2010. [Google Scholar]
- Mathew, A.K.; Abraham, A.; Mallapureddy, K.K.; Sukumaran, R.K. Lignocellulosic Biorefinery Wastes, or Resources? In Waste Biorefinery; Bhaskar, T., Pandey, A., Mohan, S.V., Lee, D.J., Khanal, S.K., Eds.; Chapter 9; Elsevier: Amsterdam, The Netherlands, 2018; pp. 267–297. [Google Scholar] [CrossRef]
- El Hadi, M.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in Fruit Aroma Volatile Research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed]
- Xisto, A.L.R.P.; Boas, E.V.d.B.V.; Nunes, E.E.; Federal, B.M.V.B.; Guerreiro, M.C. Volatile profile and physical, chemical, and biochemical changes in fresh cut watermelon during storage. Food Sci. Techno 2012, 32, 173–178. [Google Scholar] [CrossRef] [Green Version]
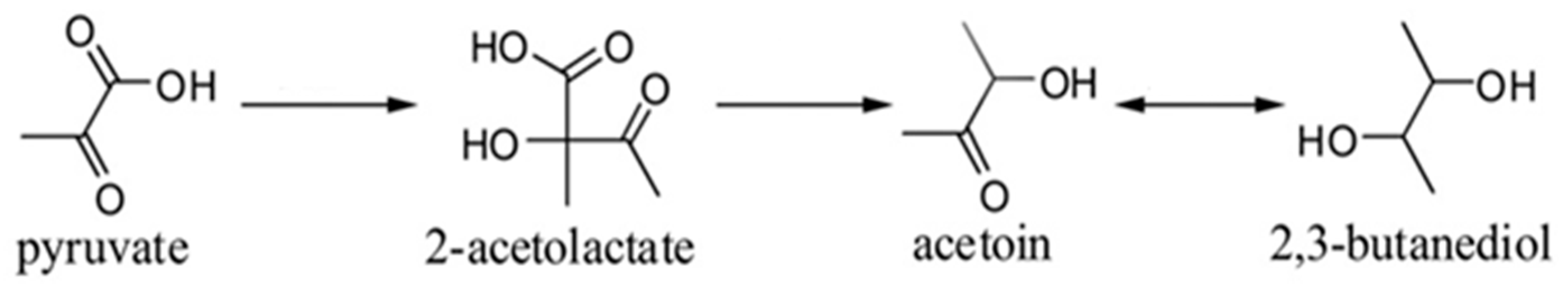
- Xiao, Z.; Lu, J.R. Generation of Acetoin and Its Derivatives in Foods. J. Agric. Food Chem. 2014, 62, 6487–6497. [Google Scholar] [CrossRef] [PubMed]
- Senesi, E.; Scalzo, R.L.; Prinzivalli, C.; Testoni, A. Relationships between volatile composition and sensory evaluation in eight varieties of netted muskmelon (Cucumis melo L. var. reticulatus Naud). J. Sci. Food Agric. 2002, 82, 655–662. [Google Scholar] [CrossRef]
- Xiao, Z.; Lu, J.R. Strategies for enhancing fermentative production of acetoin: A review. Biotechnol. Adv. 2014, 32, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Celińska, E.; Grajek, W. Biotechnological production of 2,3-butanediol—Current state and prospect. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Peinado, R. Formation of Butanedione, Acetoin, and 2,3-butanediol. In Enological Chemistry, 1st ed.; Chapter 11; Academic Press, Elsevier: London, UK, 2012; pp. 168–169. [Google Scholar]
- Lewinsohn, E.; Yaron, S.; Einat, B.; Yaniv, A.; Mwafaq, I.; Ayala, M.; Emanuel, Y.; Dani, Z.; Yaakov, T. Not just colors—carotenoid degradation as a link between pigmentation and aroma in tomato and watermelon fruit. Trends Food Sci. Technol. 2005, 16, 407–415. [Google Scholar] [CrossRef]
- Song, J.; Fan, L.; Beaudry, R.M. Application of Solid Phase Microextraction and Gas Chromatography/Time-of-Flight Mass Spectrometry for Rapid Analysis of Flavor Volatiles in Tomato and Strawberry Fruits. J. Agric. Food Chem. 1998, 46, 3721–3726. [Google Scholar] [CrossRef]
- Studart-Guimarães, C.; Fait, A.; Nunes-Nesi, A.; Carrari, F.; Usadel, B.; Fernie, A.R. Reduced Expression of Succinyl-Coenzyme A Ligase Can Be Compensated for by Up-Regulation of the γ-Aminobutyrate Shunt in Illuminated Tomato Leaves. Plant Physiol. 2007, 145, 626–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toro, G.; Pinto, M. Plant respiration under low oxygen. Chil. J. Agric. Res. 2015, 75, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Verzera, A.; Dima, G.; Tripodi, G.; Ziino, M.; Lanza, C.M.; Mazzaglia, A. Fast Quantitative Determination of Aroma Volatile Constituents in Melon Fruits by Headspace-Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry. Food Anal. Methods 2011, 4, 141–149. [Google Scholar] [CrossRef]

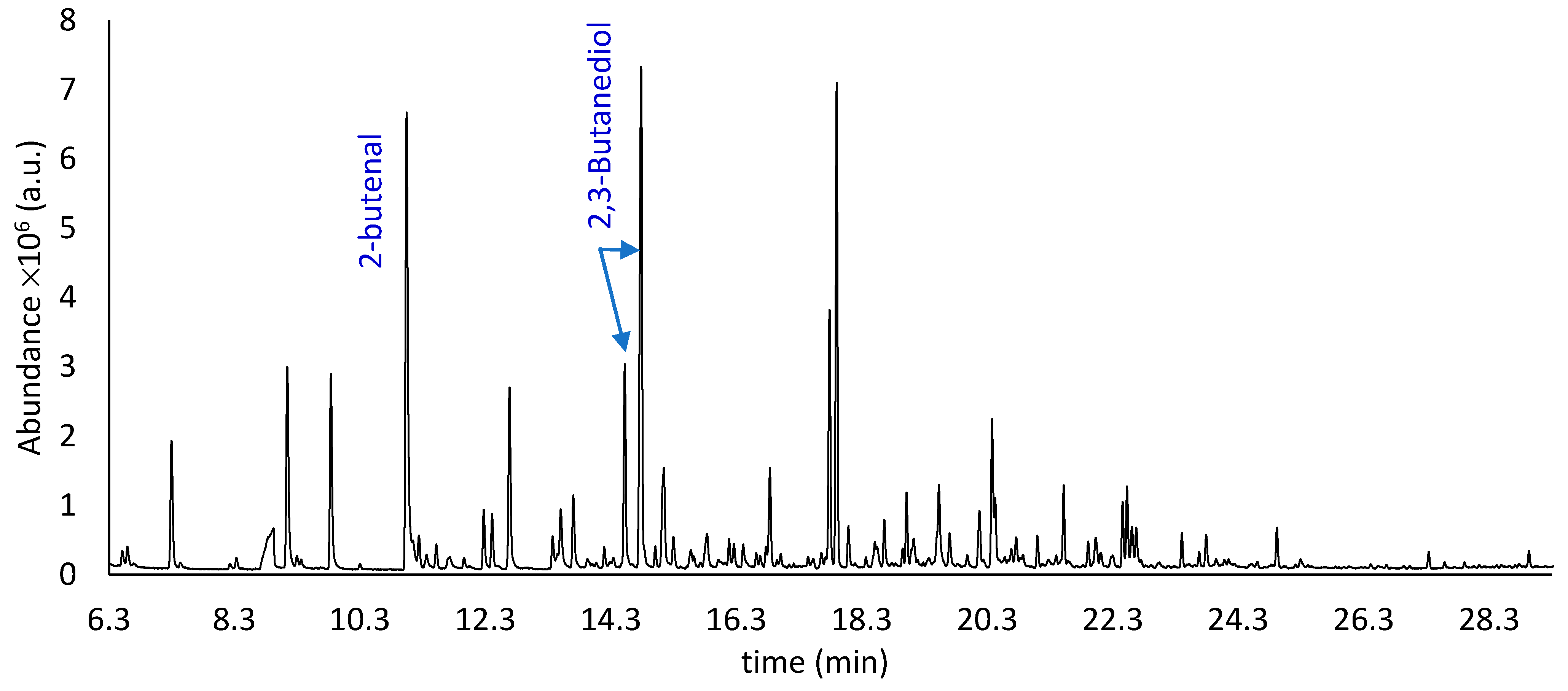
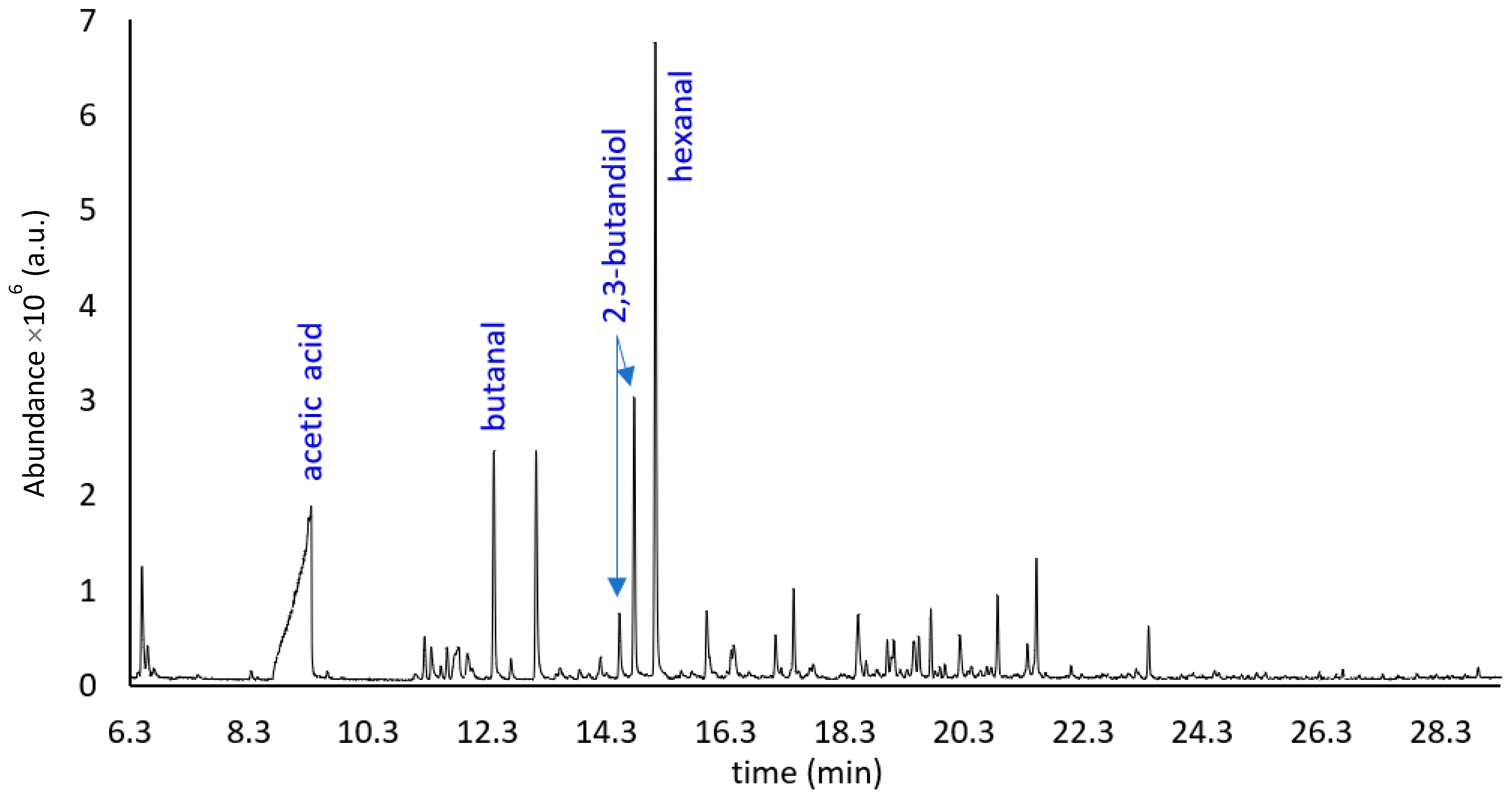



| Compound | LRI | ID # | Aroma | Area | Ref |
|---|---|---|---|---|---|
| Alcohols | |||||
| 1-Propanol | 538 | A, B | Alcoholic, fermented | 0.22 a | - |
| 2-methyl-1-Propanol | 639 | A, B, C | Ethereal | 0.24 a | [19] |
| 1-Penten-3-ol | 720 | A, B | Ethereal, green, vegetable | 0.74 a | [20,21] |
| Propylene glycol | 789 | A, B | - | 1.2 ± 0.1 | [22,23] |
| 1-Pentanol | 829 | A, B, C | Pungent | 0.78 a | [20,21,24,25,26] |
| (R,R)-2,3-Butanediol | 845 | A, B, D | Creamy, fruity, buttery | 7.4 ± 0.2 | [26,27] |
| (R,S)-2,3-Butanediol | 858 | A, B, D | Creamy, fruity, buttery | 20.0 ± 0.3 | [26,27] |
| 3-Pentanol | 875 | A, B | Sweet, herbal, oily, nutty | 5.19 ± 0.1 | [20,21,24,25,26] |
| 3-Hexen-1-ol | 937 | A, B | Green, leafy | 1.2 ± 0.1 | [10,28,29] |
| 1-Hexanol | 948 | A, B | Green, flowery | 0.53 a | [10,20,24,26,28,30] |
| 1-acetoxy-2-Propanol | 955 | A, B | - | 0.67 a | - |
| 2-acetoxy-1-Propanol | 967 | A, B | - | 0.49 a | - |
| 1-Octen-3-ol | 1065 | A, B | Mushroom, earthy, green | 2.4 ± 0.1 | [10,24,29] |
| 2-ethyl-1-Hexanol | 1112 | A, B, C | Citrus, fresh, floral, sweet | 0.63 a | [10,20] |
| Benzyl alcohol | 1131 | A, B | Sweet, floral, fruity | 4.9 ± 0.2 | [10,22,24,29,31] |
| Phenylethyl alcohol | 1212 | A, B, C | Floral, sweet | 1.4 ± 0.1 | [10,31] |
| 2,6-dimethyl-Cyclohexanol | 1216 | A, B | - | 0.72 a | [19] |
| 3-Nonen-1-ol | 1233 | A, B | Green, melon | 1.9 ± 0.1 | [10,20,21,29,31] |
| 3,6-Nonadienol | 1236 | A, B | Fresh, green, melon, cucumber | 2.7 ± 0.1 | [10,24,28] |
| 1-Nonanol | 1243 | A, B | Fresh, fatty, floral | 1.8 ± 0.1 | [10,21,24,29,31] |
| 2-Phenoxyethanol | 1306 | A, B, C | Floral, balsamic | 0.46 a | [31] |
| Aldehydes | |||||
| Propanal | 458 | A, B | Ethereal, pungent, earthy, alcoholic | 0.64 a | [24] |
| 2-methyl-Propanal | 543 | A, B | Malty | 0.49 a | [19,31] |
| 2-Butenal | 675 | A, B | Flower | 19.8 ± 0.3 | [20] |
| 3-methyl-Butanal | 685 | A, B, C | Fruity, green, cocoa | 1.3 ± 0.1 | [20,29,30,31] |
| 2-methyl-Butanal | 698 | A, B | Malty, cocoa, almond | 0.90 a | [19,20,30,31] |
| Pentanal (valeraldehyde) | 742 | A, B | Fermented, bready, almond, malt | 2.1 ± 0.1 | [24,26,29,30,32] |
| 2-methyl-2-Butenal | 805 | A, B | Pungent, green, ethereal | 2.9 ± 0.1 | - |
| 2-ethyl-2-Butenal | 899 | A, B, C | - | 0.35 a | - |
| Furfural | 918 | A, B | Woody, almond, sweet, fruity, floral | 0.63 a | [19] |
| 2-ethenyl-2-Butenal | 930 | A, B | - | 1.01 a | - |
| Heptanal | 988 | A, B | Fresh, fatty, green, herbal | 0.55 a | [10,20,21,26,29,30] |
| 2-ethyl-3-methyl-Butanal | 992 | A, B | - | 0.45 a | |
| 2,4-Hexadienal | 1002 | A, B | Fatty, sweet, green | 0.39 a | [29] |
| 2-Heptenal | 1047 | A, B | Green, fatty | 1.6 ± 0.1 | [10,20,21,24,29] |
| Benzaldehyde | 1069 | A, B | Bitter, almond-like, fruity | 0.7 ± 0.1 | [10,20,22,24,28,30] |
| Benzeneacetaldehyde | 1146 | A, B | Green, floral, honey | 1.0 ± 0.1 | [10,24,31] |
| Nonanal | 1187 | A, B | Waxy, fresh, green, citrus | 3.2 ± 0.1 | [10,20,24,29,30] |
| Decanal | 1279 | A, B, C | Sweet, waxy, citrus, green melon | 1.0 ± 0.1 | [10,20,24,29] |
| Benzeneacetaldehyde, α-ethylidene | 1353 | A, B | - | 1.6 ± 0.1 | - |
| Esters | |||||
| Methyl acetate | 499 | A, B, C | Ethereal, fruity | 0.32 a | [10,24,25,32] |
| Ethyl acetate | 616 | A, B | Ethereal, fruity, sweet | 7.2 ± 0.2 | [10,24,25,29,32] |
| Ethyl butanoate | 869 | A, B, C | Fruity | 0.8 ± 0.1 | [10,22,24,31] |
| Ethyl 2-methyl-Butanoate | 926 | A, B | Fruity | 1.0 ± 0.1 | [31] |
| Ethyl 3-hydroxy-Butanoate | 1019 | A, B, C | - | 1.2 ± 0.1 | - |
| 1-Butanol,3-methyl-, propanoate | 1061 | A, B | - | 0.62 a | - |
| Ethyl 2,3-epoxybutyrate | 1098 | A, B | - | 1.4 ± 0.1 | [23] |
| 2,3-Butanediol diacetate | 1121 | A, B | Earthy, soil-like odor | 2.2 ± 0.1 | [24,28] |
| 2,3-Butanediol diacetate | 1134 | A, B | Earthy, soil-like odor | 2.2 ± 0.1 | [24,28] |
| Ketones | |||||
| Acetone | 454 | A, B | Solvent, ethereal | 0.46 a | [30] |
| 2,3-Butanedione | 582 | A, B | Buttery, sweet, creamy | 7.9 ± 0.3 | [31,33] |
| 2-Pentanone | 590 | A, B | Sweet, fruity, ethereal | 0.58 a | - |
| 1-hydroxy-2-Propanone | 691 | A, B | Sweet, caramel-like | 0.8 ± 0.1 | - |
| 2,3-Pentanedione | 735 | A, B | Buttery, sweet, caramel-like | 2.1 ± 0.1 | [19,31,33] |
| 3-hydroxy-2-Butanone (Acetoin) | 755 | A, B, C | Acid, yogurt, creamy, fruity | 6.4 ± 0.2 | [22,29] |
| 3-Penten-2-one | 795 | A, B | Fruity | 3.2 ± 0.2 | - |
| 4-hydroxy-5-methyl-2-Hexanone | 964 | A, B | - | 0.30 a | - |
| Ketones (Continued) | |||||
| 6-methyl-5-Hepten-2-one | 1070 | A, B | Citrus, green, fruity | 1.0 ± 0.1 | [10,20,29] |
| 3,5-Octadien-2-one | 1155 | A, B | Fruity, fatty, mushroom | 0.62 a | [24,29,31] |
| Geranylacetone | 1484 | A, B | Floral, fresh, green | 0.27 a | [10,31] |
| Acids | |||||
| Acetic acid | 571 | A, B | Sour, pungent | 5.3 ± 0.2 | [22,29,31] |
| Propanoic acid | 709 | A, B | Pungent, acidic | 1.1 a | [33] |
| 2-methyl-Propanoic acid | 793 | A, B | Acidic, sour | 0.50 a | [19] |
| 3-methyl-Butanoic acid | 897 | A, B | Sour, cheesy | 1.3 ± 0.1 | - |
| 2-methyl-Butanoic acid | 909 | A, B, C | Cheesy | 2.1 ± 0.1 | [22,31] |
| 2-Hydroxy-2-methylbutyric acid | 958 | A, B | - | 3.5 ± 0.2 | - |
| Hexanoic acid | 1040 | A, B | Sour, fatty, sweet, cheesy | 1.8 ± 0.1 | [33] |
| Nonanoic acid | 1312 | A, B | Waxy, cheesy, dairy | 0.34 a | [10] |
| Sulfur-derived compounds | |||||
| Dimethyl sulfide | 492 | A, B | Sulfurous, onion, corn, vegetable | 4.7 ± 0.2 | [30,31,34,35] |
| Dimethyl disulfide | 816 | A, B | Sulfurous, onion, cabbage | 0.73 a | [25,26,30,31,35] |
| Dimethyl Sulfoxide | 923 | A, B | Fatty, oily, cheesy | 0.26 a | [34] |
| Methional | 998 | A, B | Cooked potato, earthy, vegetable | 0.58 a | [10,22,29,31] |
| Ethyl (methylthio)acetate | 1073 | A, B | Fruity, sulfurous, green | 0.24 a | [10,24] |
| 2,3-dihydro-Thiophene | 1298 | A, B | - | 1.2 ± 0.1 | - |
| Alkanes | |||||
| Tridecane | 1269 | A, B | - | 0.16 a | [31] |
| Tetradecane | 1438 | A, B, C | Waxy | 0.11 a | [24,28] |
| Others | |||||
| Methoxy-phenyl-oxime | 950 | A, B | - | 0.6 ± 0.1 | - |
| 3-ethyl-2,5-dimethyl-Pyrazine | 1167 | A, B | Nutty, potato, cocoa, rosted | 0.98 a | - |
| 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-Pyran-4-one | 1240 | A, B | - | 1.6 ± 0.1 | [26,36,37,38] |
| Benzothiazole | 1338 | A, B, C | vegetable, cooked, coffe-like | 0.24 a | - |
| 1-Tridecene | 1499 | A, B | 0.20 a | [38] | |
| Compound | LRI | ID # | Aroma | Area | REF |
|---|---|---|---|---|---|
| Alcohols | |||||
| 1-Penten-3-ol | 720 | A, B | Ethereal, green, vegetable | 14.9 ± 0.2 | [20,21,22] |
| 1-Pentanol | 829 | A, B, C | Pungent, | 7.6 ± 0.1 | [10,20,24,25,26] |
| (R,R)-2,3-Butanediol | 845 | A, B, D | Creamy, fruity, buttery | 19.4 ± 0.3 | [26,27] |
| (R,S)-2,3-Butanediol | 857 | A, B, D | Creamy, fruity, buttery | 71.4 ± 0.4 | [26,27] |
| 2-Furanmethanol | 933 | A, B | Alcoholic, bready, caramel-like | 1.7 a | - |
| 1-Octen-3-ol | 1065 | A, B | Mushroom, earthy, green | 10.4 ± 0.2 | [10,24,29] |
| 2-ethyl-Hexanol | 1112 | A, B, C | Citrus, fresh, floral, sweet | 4.2 a | [10,20] |
| 2,6-dimethyl-Cyclohexanol | 1215 | A, B | - | 3.69 a | [19] |
| Aldehydes | |||||
| Propanal | 458 | A, B | Ethereal, pungent, alcoholic | 9.6 ± 0.2 | [24] |
| 2-methyl-Propanal | 543 | A, B | Malty | 2.11 a | [19,20] |
| 2-Butenal | 677 | A, B | Flower | 2.03 a | [20] |
| 3-methyl-Butanal | 685 | A, B, C | Fruity, green, cocoa | 10.2 ± 0.3 | [20,29,30,31] |
| 2-methyl-Butanal | 698 | A, B | Malty, cocoa, almond | 3.66 a | [19,20,31] |
| Pentanal (valeraldehyde) | 742 | A, B | Fermented, bread, almond, malt | 58.6 ± 0.4 | [19,24,26,29,32] |
| Hexanal | 874 | A, B | Grass, green, fat | 155 ± 1 | [10,19,20,21,22,23,24,25,26,27,28,29,30,31,32] |
| Furfural | 916 | A, B | Wood, almond, sweet, fruit | 26.6 ± 0.4 | [19] |
| Heptanal | 988 | A, B | Fresh, fatty, green, herbal | 24.5 ± 0.5 | [10,20,21,24,26,29,30] |
| 2-Heptenl | 1047 | A, B | Green, fatty | 5.1 ± 0.2 | [10,20,21,24,29,31] |
| 5-methyl-2-Furancarboxaldehyde | 1056 | A, B | Sweet, caramel-like, spicy | 3.9 ± 0.1 | - |
| Octanal | 1091 | A, B | Waxy, fatty, citrus, green | 11.1 ± 0.3 | [10,24,26,31] |
| 2,4-Heptadienal | 1104 | A, B | Green, pungent, fruity, spicy | 1.89 a | - |
| Benzeneacetaldehyde | 1146 | A, B | Green, floral, honey | 3.4 ± 0.1 | [10,24,31] |
| Nonanal | 1187 | A, B | Waxy, fresh, green, cucumber | 30.5 ± 0.5 | [10,20,24,29,30] |
| Decanal | 1279 | A, B, C | Sweet, waxy, green, melon | 12.4 ± 0.3 | [10,20,24,29] |
| Esters | |||||
| 2-oxo-Propanoic acid, methyl ester | 776 | A, B | - | 59.9 ± 0.4 | - |
| Isopropyl 3-methylbutanoate | 1134 | A, B | - | 6.5 ± 0.2 | [24,28] |
| Ketones | |||||
| Acetone | 453 | A, B | Solvent, ethereal | 30.3 ± 0.4 | [30] |
| 1-hydroxy-2-Propanone | 690 | A, B | Sweet, caramel-like | 10.6 ± 0.2 | - |
| 3-hydroxy-2-Butanone (Acetoin) | 756 | A, B, C | Acid, yogurt, creamy, fruity | 5.7 ± 0.1 | [22,29] |
| 3-Penten-2-one | 796 | A, B | Fruity | 4.2 ± 0.1 | - |
| 1-(acetyloxy)-2-Propanone | 939 | A, B | Fruity, buttery | 8.7 ± 0.2 | [28] |
| 2-Heptanone | 973 | A, B | Fruity, spicy, sweet, herbal | 12.8 ± 0.3 | [29] |
| 6-methyl-5-Hepten-2-one | 1070 | A, B | Citrus, green, fruity | 14.8 ± 0.4 | [10,20,29] |
| 2-Octanone | 1075 | A, B | Earthy, woody, herbal | 3.20 a | - |
| 3-Octen-2-one | 1124 | A, B | Earthy, herbal, spicy | 13.6 ± 0.4 | - |
| (E,E)-3,5-Octadien-2-one | 1155 | A, B, D | Fruity, fatty, mushroom | 20.2 ± 0.5 | [24,29,31] |
| (E,Z)-3,5-Octadien-2-one | 1180 | A, B, D | Fruity, fatty, mushroom | 11.2 ± 0.2 | [24,29,31] |
| Acids | |||||
| Acetic acid | 591 | A, B | Sour, pungent | 326 ± 2.4 | [22,29,31,33] |
| Propanoic acid | 713 | A, B | Pungent, acidic | 22.3 ± 0.5 | - |
| 2-methyl-Propanoic acid | 793 | A, B | Acidic, sour | 1.69 a | - |
| 3-methyl-Butanoic acid | 896 | A, B | Sour, cheesy | 4.8 ± 0.1 | - |
| Hexanoic acid | 1041 | A, B | Sour, fatty, sweet, cheesy | 12.3 ± 0.3 | - |
| 2-methyl-Propanoic acid | 793 | A, B | Acidic, sour | 1.69 a | - |
| 3-methyl-Butanoic acid | 896 | A, B | Sour, cheesy | 4.8 ± 0.1 | - |
| Hexanoic acid | 1041 | A, B | Sour, fatty, sweet, cheesy | 12.3 ± 0.3 | - |
| Alkanes | |||||
| Tridecane | 1269 | A, B | - | 3.7 ± 0.1 | [19] |
| 3-methyl-Dodecane | 1355 | A, B | - | 0.73 a | - |
| Tetradecane | 1438 | A, B, C | Waxy | 1.96 a | [24,28] |
| Others | |||||
| Pyrrole | 812 | A, B | Sweet, nutty | 3.6 ± 0.1 | - |
| Methoxy-phenyl-oxime | 951 | A, B | - | 4.6 ± 0.2 | - |
| 2(5H)-Furanone | 1001 | A, B | Buttery | 3.0 ± 0.1 | [31] |
| 2-pentyl-Furan | 1081 | A, B | Fruity, green, earthy | 3.1 ± 0.1 | [20,24,26,29,30] |
| 1-Tridecene | 1499 | A, B | - | 1.12 a | [28] |
| Compound | LRI | Identification # | Aroma | Area | REF |
|---|---|---|---|---|---|
| Alcohols | |||||
| 1-Pentanol | 831 | A, B, C | Pungent | 34.1 ± 0.4 | [20,21,24,25,26] |
| (R,R)-2,3-Butanediol | 848 | A, B, D | Creamy, fruity, buttery, green, leafy | 16.6 ± 0.3 | [26,27] |
| (R,S)-2,3-Butanediol | 859 | A, B, D | Creamy, fruity, buttery, green, leafy | 45.8 ± 0.5 | [26,27] |
| 1-Octen-3-ol | 1065 | A, B | Mushroom, earthy, green | 49.5 ± 0.6 | [10,24,29] |
| 2-ethyl-1-Hexanol | 1112 | A, B, C | Citrus, fresh, floral, sweet | 5.3 ± 0.1 | [10,20] |
| 2,6-dimethyl-Cyclohexanol | 1215 | A, B | 1.62 a | [19] | |
| Aldehydes | |||||
| 3-methyl-Butanal | 688 | A, B, C | Fruity, cocoa, green | 28.5 ± 0.4 | [20,29,30,31] |
| 2-methyl-Butanal | 701 | A, B | Malty, cocoa, almond | 9.4 ± 0.2 | [19,20,30,31] |
| Pentanal | 744 | A, B | Fermented, bready, almond, malt | 64.0 ± 0.5 | [24,26,29,30,32] |
| Hexanal | 876 | A, B | Grass, green, fat | 448 ± 4.8 | [10,24,26,30,31,32] |
| Furfural | 918 | A, B | Woody, almond, sweet, fruity, floral | 9.2 ± 0.3 | [19] |
| 2-Hexenal | 938 | A, B | Green, fruity, pungent, vegetable-like | 13.1 ± 0.3 | - |
| Heptanal | 988 | A, B | Fresh, fatty, green, herbal | 35.4 ± 0.4 | [10,20,21,24,29,30] |
| Octanal | 1091 | A, B | Waxy, fatty, citrus, green | 13.1 ± 0.3 | [10,24,26,31] |
| Ketones | |||||
| Acetone | 464 | A, B | Solvent, ethereal | 32.9 ± 0.4 | [30] |
| 2-Heptanone | 973 | A, B | Fruity, spicy, sweet, herbal | 30.2 ± 0.5 | [29] |
| 2-Octanone | 1075 | A, B | Earthy, woody, herbal | 6.8 ± 0.1 | - |
| 3-Octen-2-one | 1124 | A, B | Earthy, herbal, spicy | 21.2 ± 0.4 | - |
| (E,E)-3,5-Octadien-2-one | 1155 | A, B, D | Fruity, fatty, mushroom | 13.3 ± 0.3 | [24,29,31] |
| Acetophenone | 1170 | A, B | Sweet, pungent, almond | 8.4 ± 0.2 | |
| (E,Z)-3,5-Octadien-2-one | 1179 | A, B, D | Fruity, fatty, mushroom | 12.2 ± 0.28 | [24,29,31] |
| Acids | |||||
| Formic acid | 477 | A, B | Pungent, vinegar | 21.3 ± 0.4 | |
| Acetic acid | 630 | A, B | Sour, pungent | 823 ± 6.5 | [22,29,31] |
| Propanoic acid | 736 | A, B | Pungent, acidic | 143 ± 2.5 | [33] |
| 2-methyl-Propanoic acid | 802 | A, B | Acidic, sour | 12.2 ± 0.3 | - |
| 3-methyl-Butanoic acid | 900 | A, B | Sour, cheesy | 13.9 ± 0.3 | - |
| 2-methyl-Butanoic acid | 911 | A, B, C | Cheesy | 10.4 ± 0.2 | [22,31] |
| Pentanoic acid | 945 | A, B | Acidic, cheesy | 29.5 ± 0.5 | - |
| Hexanoic acid | 1048 | A, B | Sour, fatty, sweet, cheesy | 137 ± 1.8 | [22] |
| Heptanoic acid | 1134 | A, B | Sour, cheesy | 16.2 ± 0.4 | - |
| Compound Class | M_AI_V_t0 | M_B_O_t0 | M_B_O_t24 | p-Value |
|---|---|---|---|---|
| ALC (alcohols) | 33.8 ± 3.2 | 12.3 ± 2.3 | 7.3 ± 0.9 | p < 0.05 |
| ALD (aldehydes) | 24.7 ± 2.4 | 33.2 ± 3.9 | 29.5 ± 2.7 | p < 0.05 |
| KET (ketones) | 14.5 ± 2.6 | 12.5 ± 2.2 | 5.9 ± 0.8 | p < 0.05 |
| ACD (acids) | 9.7 ± 1.7 | 33.8 ± 3.7 | 57.3 ± 4.4 | p < 0.05 |
| ACE (acetate esters) | 7.2 ± 1.0 | --- | --- | |
| SDC (sulfur compounds) | 4.7 ± 0.8 | --- | --- | |
| NAE (non-acetate esters) | 3.0 ± 0.6 | 6.1 ± 0.7 | --- | p < 0.05 |
| AHA (alkanes) | 0.2 ± 0.2 | 0.6 ± 0.4 | --- | p > 0.05 |
| OTH (other compounds) | 2.2 ± 0.5 | 1.4 ± 0.9 | --- | p > 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maletti, L.; D’Eusanio, V.; Durante, C.; Marchetti, A.; Pincelli, L.; Tassi, L. Comparative Analysis of VOCs from Winter Melon Pomace Fibers before and after Bleaching Treatment with H2O2. Molecules 2022, 27, 2336. https://doi.org/10.3390/molecules27072336
Maletti L, D’Eusanio V, Durante C, Marchetti A, Pincelli L, Tassi L. Comparative Analysis of VOCs from Winter Melon Pomace Fibers before and after Bleaching Treatment with H2O2. Molecules. 2022; 27(7):2336. https://doi.org/10.3390/molecules27072336
Chicago/Turabian StyleMaletti, Laura, Veronica D’Eusanio, Caterina Durante, Andrea Marchetti, Luca Pincelli, and Lorenzo Tassi. 2022. "Comparative Analysis of VOCs from Winter Melon Pomace Fibers before and after Bleaching Treatment with H2O2" Molecules 27, no. 7: 2336. https://doi.org/10.3390/molecules27072336
APA StyleMaletti, L., D’Eusanio, V., Durante, C., Marchetti, A., Pincelli, L., & Tassi, L. (2022). Comparative Analysis of VOCs from Winter Melon Pomace Fibers before and after Bleaching Treatment with H2O2. Molecules, 27(7), 2336. https://doi.org/10.3390/molecules27072336






