Triacylglycerols and Other Lipids Profiling of Hemp By-Products
Abstract
:1. Introduction
2. Results
2.1. Total Lipid Content and Oil Extraction Yield
2.2. Separation of Lipid Classes and 1H NMR Analysis
2.3. Triacylglycerols (TAGs) Analysis of Neutral Lipid Using UPLC-HRMS
2.4. Fatty Acid Analysis
2.5. Pigment Analysis
2.6. Terpene and Cannabinoid Analysis
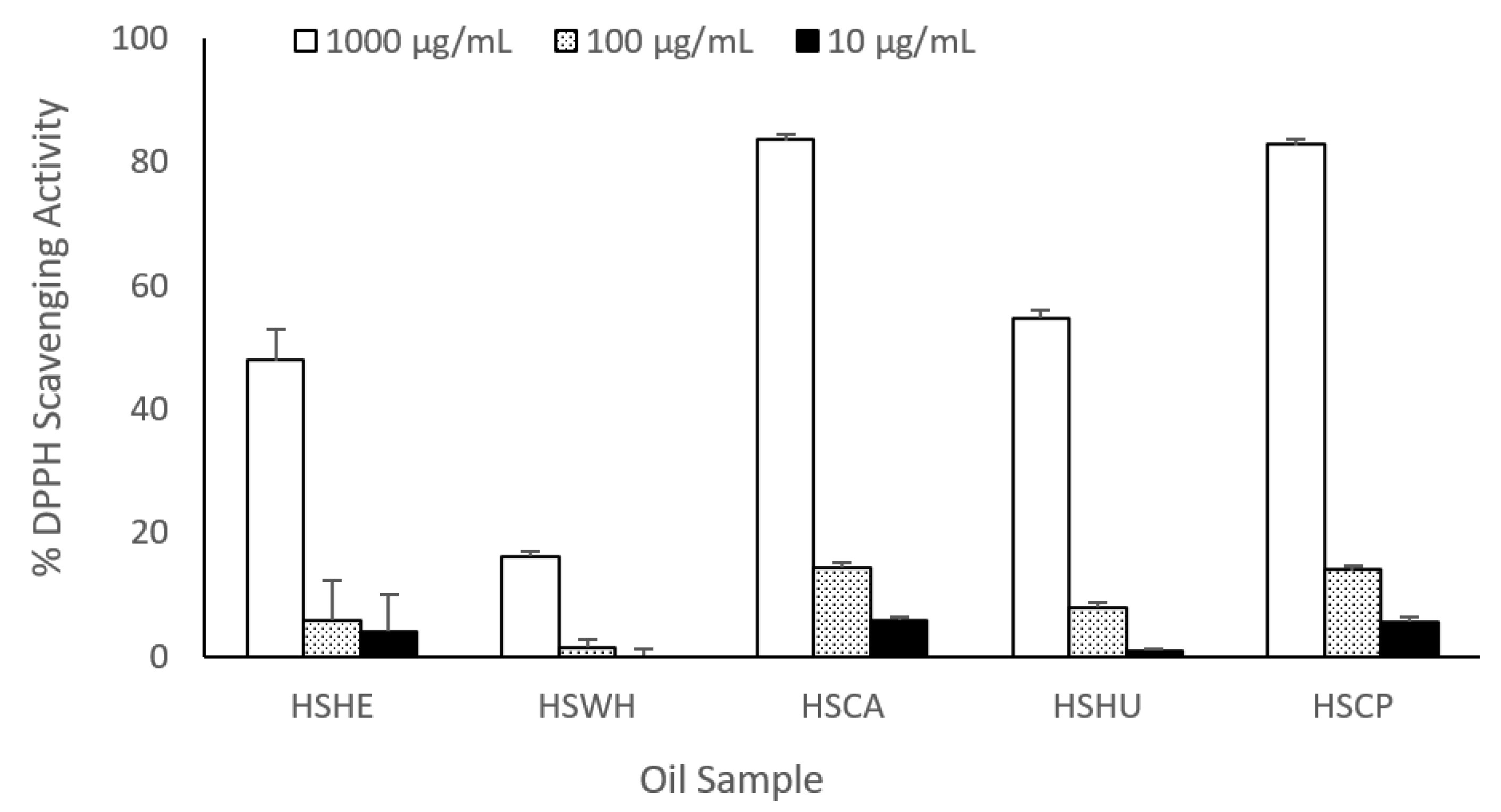
2.7. DPPH Radical Scavenging Activity
3. Discussion
4. Materials and Methods
4.1. General
4.2. Research Materials
4.3. Total Lipid Content and Oil Extraction
4.4. Lipid Class Separation by Solid Phase Extraction (SPE)
4.5. Triacylglycerols (TAGs) Analysis by UPLC-HRMS
4.6. Fatty Acid Analysis by GC
4.7. Pigment Analysis
4.8. Terpene and Cannabinoids Analysis
4.9. DPPH Radical Scavenging Activity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Lupescu, M. Industrial hemp production tread and regulation. In Global Agriculture Information Network; GAIN Report Number: CA19030; USDA Foreign Agriculture Service: Ottawa, ON, Canada, 2019. [Google Scholar]
- Pojić, M.; Mišan, A.; Sakač, M.; Hadnađev, T.D.; Šarić, B.; Milovanović, I.; Hadnađev, M. Characterization of byproducts originating from hemp oil processing. J. Agric. Food Chem. 2014, 62, 12436–12442. [Google Scholar] [CrossRef] [PubMed]
- Pojić, M.; Hadnađev, T.D.; Hadnađev, M.; Rakita, S.; Brlek, T. Bread supplementation with hemp seed cake: A by-product of hemp oil processing. J. Food Qual. 2015, 38, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Semwogerere, F.; Katiyatiya, C.L.F.; Chikwanha, O.C.; Marufu, M.C.; Mapiye, C. Bioavailability and bioefficacy of hump by-products in ruminant meat production and preservation: A review. Front. Vet. Sci. 2020, 7, 572906. [Google Scholar] [CrossRef] [PubMed]
- Parger, F.; Talukdar, S.; Pal, K.; Zanotti, C. Hemp waste valorization as biofuel and cement replacement in cement and concrete production. Waste Biomass Valori. 2021, 12, 913–923. [Google Scholar] [CrossRef]
- Porto, C.D.; Decorti, D.; Tubaro, F. Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide. Ind. Crops Prod. 2012, 36, 401–404. [Google Scholar] [CrossRef]
- Vonapartis, E.; Aubin, M.-P.; Seguin, P.; Mustafa, A.F.; Charron, J.-B. Seed composition of ten industrial hemp cultivars approved for production in Canada. J. Food Compos. Anal. 2015, 39, 8–12. [Google Scholar] [CrossRef]
- Cottrell, R.S.; Blanchard, J.L.; Halpern, B.S.; Metian, M.; Froehlich, H.E. Global adoption of novel aquaculture feeds could substantially reduce forage fish demand by 2030. Nat. Food 2020, 1, 302–308. [Google Scholar] [CrossRef]
- Hua, K.H.; Cobcroft, J.M.; Cole, A.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vocko, M.J.; Zeng, C.; Zenger, K.; Strugnell, J.M. The future of aquatic protein: Implications for protein sources in aquaculture diets. One Earth 2019, 1, 316–319. [Google Scholar] [CrossRef] [Green Version]
- Sarker, P.K.; Bureau, D.P.; Hua, K.; Drew, M.D.; Forster, I.; Were, K.; Hicks, B.; Vandenberg, G.W. Sustainability issues related to feeding salmonids: A Canadian perspective. Rev. Aquac. 2013, 5, 199–219. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Muylaert, K.; Foubert, I. Optimization of an analytical procedure for extraction of lipids from microalgae. J. Am. Oil Chem. Soc. 2012, 89, 189–198. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. A method based on 1H NMR spectral data useful to evaluate the hydrolysis level in complex lipid mixtures. Food Res. Int. 2014, 66, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Crescnte, G.; Piccolella, S.; Esposito, A.; Scognamiglio, M.; Fiorentino, A.; Pacifico, S. Chemical composition and nutraceutical properties of hempseed: An ancient food with actual functional value. Phytochem. Rev. 2018, 17, 733–749. [Google Scholar] [CrossRef]
- Oseyko, M.; Sova, N.; Lutsenko, M.; Kalyna, V. Chemical aspects of the composition of industrial hemp products. Food Technol. 2019, 8, 544–558. [Google Scholar] [CrossRef]
- Leizer, C.; Ribnicky, D.; Poulev, A.; Dushenkov, S.; Raskin, I. The composition of hemp seed oil and its potential as an important source of nutrition. J. Nutraceuticals Funct. Med. Foods 2000, 2, 35–53. [Google Scholar]
- Malomo, S.A.; Aluko, R.E. Conversion of a low protein hemp seed meal into a functional protein concentrate through enzymatic digestion of fibre coupled with membrane ultrafiltration. Innov. Food Sci. Emerg. Technol. 2015, 31, 151–159. [Google Scholar] [CrossRef]
- Hadnađev, M.; Dapčević-Hadnađev, T.; Lazaridou, A.; Moschakis, T.; Michaelidou, A.-M.; Poppvić, S.; Biliaderis, C.G. Hempseed meal protein isolates prepared by different isolation techniques. Part I. physicochemical properties. Food Hydrocoll. 2018, 79, 526–533. [Google Scholar] [CrossRef]
- Aladić, K.; Jarni, K.; Barbir, T.; Vidović, S.; Vladić, J. Supercritical CO2 extraction of hemp (Cannabis sativa L.) seed oil. Ind. Crops Prod. 2015, 76, 472–478. [Google Scholar] [CrossRef]
- Oomah, B.D.; Bussonb, M.; Godfrey, D.V.; Drovera, J.C.G. Characteristics of hemp (Cannabis sativa L.) seed oil. Food Chem. 2002, 76, 33–43. [Google Scholar] [CrossRef]
- Kim, D.-K.; Kim, K.-D.; Seo, J.-Y.; Lee, S.-M. Effects of dietary lipid source and level on growth performance, blood parameters and fresh quality of sub-adult olive flounder (Paralichthys olivaceus). Asian-Aust. J. Anim. Sci. 2012, 6, 869–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taşbozan, O.; Gökçe, M.A. Fatty Acids in Fish; Catala, A., Ed.; Intech Open Book Series; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Bell, J.G. Alternative lipid sources for fish feed and farmed fish quality. In Improving Farmed Fish and Quality and Safety; Woodhead Publishing: Sawston, UK, 2008; pp. 275–299. [Google Scholar]
- Nasopoulou, C.; Zabetakis, I. Benefits of fish oil replacement by plant originated oils in compounded fish feeds, a review. Food Sci. Technol. 2012, 47, 217–224. [Google Scholar] [CrossRef]
- Montoya, C.; Cochard, B.; Flori, A.; Cros, D.; Lopes, R.; Cuellar, T.; Espeout, S.; Syaputra, I.; Villeneuve, P.; Pina, M.; et al. Genetic architecture of palm oil fatty acid composition in cultivated oil palm (Elaeis guineensis Jacq.) compared to its wild relative E. oleifera (H.B.K.) Cortés. PLoS ONE 2014, 9, 95412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramezani, F.E.; Sallesh, K.M.; Ehteshami, F.; Shakiba, Z.S.; Roos, S.C.; Zokaeifar, H. Effect of dietary linolenic acid (18:3n-3)/linoleic acid (18:2n-6) ratio on growth performance, tissue fatty acid and histological alterations in the liver of juvenile Tor. tambroides. Iran. J. Fish. Sci. 2014, 13, 185–200. [Google Scholar]
- Rosenlund, G.; Obach, A.; Sandberg, M.G.; Standal, H.; Tveit, K. Effect of alternative lipid sources on long-term growth performance and quality of Atlantic salmon (Salmo salar L.). Aquac. Res. 2001, 32, 323–328. [Google Scholar] [CrossRef]
- Foroutani, M.B.; Parrish, C.C.; Wells, J.; Taylor, R.G.; Rise, M.L.; Shahidi, F. Minimizing marine ingredients in diets of farmed Atlantic salmon (Salmo salar): Effects on growth performance and muscle lipid and fatty acid composition. PLoS ONE 2018, 13, e0198538. [Google Scholar]
- Xue, M.; Luo, L.; Wu, X.; Ren, Z.; Gao, P.; Yu, Y.; Pearl, G. Effects of six alternative lipid on growth and tissue fatty acid composition in Japanese sea brass (Lateolabrax japonicas). Aquaculture 2006, 260, 206–214. [Google Scholar] [CrossRef]
- Sankian, Z.; Khosravi, S.; Kim, Y.-O.; Lee, S.-M. Total replacement of dietary oil with alternative lipid sources in a practical diet for mandarin fish, Siniperca scherzeri, juveniles. Fish. Aquatic Sci. 2019, 22, 8. [Google Scholar] [CrossRef]
- Clemente, T.M.; Cahoon, E.B. Soybean oil: Genetic approaches for modification of functionality and total content. Plant Physiol. 2009, 151, 1030–1040. [Google Scholar] [CrossRef] [Green Version]
- Walker, C.G.; Jebb, S.A.; Calder, P.C. Stearidonic acid as a supplemental source of ω-3 polyunsaturated fatty acids to enhance status for improved human health. Nutrition 2013, 29, 363–369. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omgga-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Phamacother. 2006, 60, 502–507. [Google Scholar] [CrossRef]
- Selvam, C.; Powell, M.D.; Liland, N.S.; Rosenlund, G.; Sissener, N.H. Impact of dietary level and ration of n-6 and n-3 fatty acids on disease progression and nRNA expression of immune and inflammatory markers in Atlantic salmon (Salmo salar) challenged with Paramoeba perurans. PeerJ 2021, 9, e12028. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.; Pacifico, S.; Piccolella, S.; Castaldo, L.; Narváez, A.; Grosso, M.; Ritieni, A. Chemical analysis of minor bioactive components and cannabidiolic acid in commercial hemp seed oil. Molecules 2020, 25, 3710. [Google Scholar] [CrossRef]
- Dyerberg, J.; Madsen, P.; Moller, J.M.; Aardesrup, I.; Schmidt, E.B. Bioavailability of marine n-3 fatty acid formulations. Prostagandins Leukot. Essent. Fatty Acids 2010, 83, 137–141. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, K.M.; McNichol, J.; McGinn, P.J.; O’Leary, S.J.B.; Melanson, J.E. Triacylglycerol profiling of microalgae strains for biofuel feedstock by liquid chromatography-high-resolution mass spectrometry. Anal. Bioannal. Chem. 2011, 401, 2609–2616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahy, E.; Sud, M.; Cotter, D.; Subramaniam, S. LIPID MAPS online tools for lipid research. Nucl. Acids Res. 2007, 35, W606–W612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattila, P.H.; Pihlava, J.-M.; Hellström, J.; Nurmi, M.; Eurola, M.; Mäkinen, S.; Jalava, T.; Pihlanto, A. Contents of phytochemicals and antinutritional factors in commercial protein-rich plant. Food Qual. Saf. 2018, 2, 213–219. [Google Scholar] [CrossRef]
- Alonso-Esteban, J.I.; Torija-Isasa, M.E.; Sánchez-Mata, M.C. Mineral elements and related antinutrients, in whole and hulled hemp (Cannabis sativa L.) seeds. J. Food Compost. Anal. 2022, 109, 104516. [Google Scholar] [CrossRef]
- Xuan, T.D.; Gangqiang, G.; Minh, T.N.; Quy, T.N.; Khanh, T.D. An overview of chemical profiles, antioxidant and antibacterial activities of commercial vegetable edible oils marketed in Japan. Foods 2018, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- AOAC Official Method 991.39. Fatty Acids in Encapsulated Fish Oils and Fish Oil Methyl and Ethyl Esters; AOAC International: Rockville, MD, USA, 2000; Chapter 41; pp. 26–28. [Google Scholar]
- Hatano, T.; Edamatsu, R.; Mori, A.; Fujita, Y.; Yasuhara, T.; Yoshida, T.; Okuda, T. Effects of the interaction of tannins with co-existing substances. VI. Effects of tannins and related polyphenols on superoxide anion radical, and on 1,1-diphenyl-picrygydrazyl radical. Chem. Pharm. Bull. 1989, 37, 2016–2021. [Google Scholar] [CrossRef] [Green Version]




| Sample/Lipid Content | HSHE | HSWH | HSCA | HSHU | HSCP 1 |
|---|---|---|---|---|---|
| Total lipid (%) 2 | 54.7 ± 2.3 | 48.0 ± 2.8 | 13.1 ± 0.1 | 17.5 ± 0.1 | n/a |
| Oil (%) | 45.9 | 36.4 | 7.4 | 12.1 | n/a |
| Neutral lipids (%) | 99.4 | 97.3 | 97.8 | 97.1 | 98.9 |
| Glycolipids (%) | 0.3 | 1.4 | 1.5 | 2.2 | 0.7 |
| Phospholipids (%) | 0.3 | 1.3 | 0.7 | 0.7 | 0.4 |
| HSHE | HSWH | HSCA | HSHU | HSCP | RT (min) | Measured (m/z) | Calculated (m/z) | Error (ppm) | C:DB | TAG Identity |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.35 | 888.70819 | 888.70756 | 0.71 | 54:10 | 18:4/18:3/18:3 | |||||
| 1.45 | 890.72369 | 890.72321 | 0.54 | 54:9 | 18:4/18:3/18:2 | |||||
| 1.70 | 962.81750 | 962.81711 | 0.41 | 59:8 | NI | |||||
| 1.70 | 892.74231 | 892.73886 | 3.86 | 54:8 | 18:3/18:3/18:2 | |||||
| 1.70 | 866.72357 | 866.72321 | 0.42 | 52:7 | 18:4/18:3/16:0 | |||||
| 1.95 | 894.75446 | 894.75451 | 0.06 | 54:7 | 18:3/18:2/18:2 | |||||
| 1.95 | 868.74005 | 868.73886 | 1.37 | 52:6 | 18:3/18:3/16:0 | |||||
| 1.95 | 922.78534 | 922.78581 | 0.51 | 56:7 | 20:0/18:4/18:0 | |||||
| 2.35 | 896.76996 | 896.77016 | 0.22 | 54:6 | 18:3/18:2/18:1 | |||||
| 2.35 | 870.75519 | 870.75451 | 0.78 | 52:5 | 18:3/18:2/16:0 | |||||
| 2.35 | 844.73926 | 844.73886 | 0.47 | 50:4 | NI | |||||
| 2.35 | 966.84924 | 966.84841 | 0.86 | 59:6 | NI | |||||
| 2.56 | 872.76996 | 872.77016 | 0.23 | 52:4 | 18:2/18:2/16:0 | |||||
| 2.70 | 898.78320 | 898.78581 | 2.90 | 54:5 | 18:2/18:2/18:1; 18:3/18:2/18:0 | |||||
| 2.70 | 924.80255 | 924.80146 | 1.18 | 56:6 | 20:1/18:3/18:2 | |||||
| 2.91 | 886.78650 | 886.78581 | 0.78 | 53:4 | 18:2/18:2/17:0 | |||||
| 3.15 | 900.80005 | 900.80146 | 1.57 | 54:4 | 18:2/18:2/18:0; 18:2/18:1/18:1 | |||||
| 3.15 | 874.78625 | 874.78581 | 0.50 | 52:3 | 18:2/18:1/16:0 | |||||
| 3.15 | 848.77167 | 848.77016 | 1.78 | 50:2 | 18:2/16:0/16:0 | |||||
| 3.15 | 926.81543 | 926.81711 | 1.81 | 56:5 | 20:1/18:2/18:2; 20:0/18:3/18:2 | |||||
| 3.40 | 928.83148 | 928.83276 | 1.38 | 56:4 | 20:0/18:2/18:2 | |||||
| 3.40 | 876.80115 | 876.80146 | 0.35 | 52:2 | 18:2/18:0/16:0; 18:1/18:1/16:0 | |||||
| 3.40 | 902.81738 | 902.81711 | 0.30 | 54:3 | 18:2/18:1/18:0 | |||||
| 3.40 | 954.84802 | 954.84841 | 0.41 | 58:5 | 22:0/18:3/18:2 | |||||
| 3.70 | 980.86426 | 980.86406 | 0.20 | 60:6 | NI | |||||
| 3.70 | 968.86395 | 968.86406 | 0.11 | 59:5 | 23:0/18:3/18:2 | |||||
| 3.70 | 904.83282 | 904.83276 | 0.07 | 54:2 | 20:0/18:2/16:0 | |||||
| 3.85 | 930.84845 | 930.84841 | 0.04 | 56:3 | 20:0/18:2/18:1 | |||||
| 3.95 | 956.86414 | 956.86406 | 0.08 | 58:4 | 22:0/18:2/18:2 | |||||
| 3.95 | 982.87933 | 982.87971 | 0.39 | 60:5 | 24:0/18:3/18:2 | |||||
| 4.17 | 970.88013 | 970.87971 | 0.43 | 59:4 | 23:0/18:2/18:2 | |||||
| 4.21 | 932.86420 | 932.86406 | 0.15 | 56:2 | 22:0/18:2/16:0; 20:0/18:1/18:1; 20:0/18:2/18:0 | |||||
| 4.21 | 958.87958 | 958.87971 | 0.14 | 58:3 | 22:0/18:2/18:1 | |||||
| 4.21 | 984.89496 | 984.89536 | 0.41 | 60:4 | 24:0/18:2/18:2 | |||||
| 4.35 | 958.87994 | 958.87971 | 0.24 | 58:3 | 20:3/20:0/18:0 | |||||
| 4.35 | 932.86432 | 932.86406 | 0.28 | 56:2 | 20:0/18:1/18:1 | |||||
| 4.84 | 960.89569 | 960.89536 | 0.34 | 58:2 | 22:0/18:1/18:1 | |||||
| 4.84 | 986.91125 | 986.911 | 0.25 | 60:3 | 24:0/18:2/18:1 | |||||
| 5.00 | 988.92682 | 988.92666 | 0.16 | 60:2 | 24:0/18:1/18:1 | |||||
| 5.00 | 896.77002 | 896.77016 | 0.16 | 54:6 | 18:3/18:2/18:1 | |||||
| 5.00 | 1014.94234 | 1014.94231 | 0.03 | 62:3 | NI |
 High Abundance.
High Abundance.| Fatty Acid (FA) | HSHE | HSWH | HSCA | HSHU | HSCP |
|---|---|---|---|---|---|
| Myristic acid (C14:0) | - | - | - | - | - |
| Myristoleic acid (C14:1) | - | - | - | - | - |
| Pentadecanoic acid (C15:0) | - | - | - | - | - |
| cis-10-Pentadecenoic acid (C15:1) | - | - | - | - | - |
| Palmitic acid (C16:0) | 47.2 ± 12.0 (5.8) | 45.6 ± 4.3 (6.1) | 55.2 ± 2.9 (6.4) | 55.1 ± 0.6 (6.8) | 49.0 ± 0.4 (5.7) |
| Palmitoleic acid (C16:1 n-7) | - | - | - | - | - |
| C16:2 n-4 | - | - | - | - | - |
| C17:0 (Heptadecanoic acid) | 0.3 ± 0.2 (0.0) | 0.1 ± 0.2 (0.0) | - | 0.6 ± 0.0 (0.1) | - |
| C16:3 n-4 | - | - | - | - | - |
| cis-10-Heptadecenoic acid (C17:1) | - | - | - | - | - |
| C16:4 n-1 | - | - | - | - | - |
| Stearic acid (C18:0) | 21.1 ± 0.5 (2.6) | 18.4 ± 1.8 (2.5) | 20.7 ± 1.1 (2.4) | 18.8 ± 0.2 (2.3) | 20.8 ± 0.2 (2.4) |
| Oleic acid (C18:1 n-9) | 33.0 ± 57.2 (11.9) | 80.3 ± 7.8 (10.7) | 86.4 ± 5.1 (10.0) | 79.3 ± 0.8 (9.7) | 81.2 ± 0.6 (9.5) |
| cis-Vaccenic acid(C18:1 n-7) | 5.7 ± 0.2 (0.7) | 6.3 ± 0.6 (0.8) | 7.9 ± 0.4 (0.9) | 7.5 ± 0.1 (0.9) | 6.8 ± 0.1 (0.8) |
| Linoleic acid (C18:2 n-6) | 455.9 ± 10.9 (56.9) | 417.8 ± 41.2 (55.9) | 481.5 ± 28.0 (55.7) | 447.8 ± 4.7 (54.9) | 480.1 ± 3.7 (55.9) |
| γ-linolenic acid (C18:3 n-6) | 27.1 ± 0.7 (3.3) | 30.0 ± 3.0 (4.0) | 27.1 ± 1.6 (3.1) | 34.4 ± 0.4 (4.2) | 36.8 ± 0.3 (4.3) |
| α-linolenic acid (C18:3 n-3) | 136.2 ± 3.3 (16.7) | 125.1 ± 12.9 (16.7) | 158.6 ± 9.1 (18.4) | 143.5 ± 1.5 (17.6) | 155.6 ± 1.2 (18.1) |
| Stearidonic acid (C18:4 n-3) | 9.6 ± 0.2 (1.2) | 9.7 ± 1.0 (1.3) | 9.4 ± 0.6 (1.1) | 10.4 ± 0.1 (1.3) | 12.8 ± 0.1 (1.5) |
| Arachidic acid (C20:0) | 7.3 ± 0.2 (0.9) | 7.0 ± 0.7 (0.9) | 7.5 ± 0.4 (0.9) | 7.9 ± 0.1 (1.0) | 7.7 ± 0.1 (0.9) |
| cis-11-Eicosenoic acid (C20:1 n-9) | 3.6 ± 0.1 (0.4) | 3.3 ± 0.3 (0.4) | 3.0 ± 0.2 (0.5) | 3.8 ± 0.1 (0.5) | 3.7 ± 0.1 (0.4) |
| cis-11,14-Eicosadienoic acid (C20:2) | 0.4 ± 0.3 (0.0) | 1.1 ± 0.8 (0.1) | 1.6 ± 1.9 (0.2) | 0.7 ± 0.0 (0.1) | 0.2 ± 0.4 (0.0) |
| cis-8,11,14-Eicosatrienoic acid (C20:3 n-6) | - | - | - | - | - |
| Henicosanoic acid (C21:0) | - | - | - | - | - |
| cis-8,11,14-Eicosatrienoic acid (C20:3 n-3) | - | - | - | - | - |
| Arachidonic acid (C20:4 n-6) | - | - | - | - | - |
| Eicosapentaenoic acid (C20:5 n-3) | - | - | - | - | - |
| Behenic acid (C22:0) | 2.7 ± 0.1 (0.3) | 0.7 ± 0.1 (0.1) | 3.3 ± 0.1 (0.4) | 3.7 ± 0.0 (0.5) | 3.1 ± 0.0 (0.4) |
| Erucic acid (C22:1 n-9) | - | 1.0 ± 0.1 (0.1) | - | - | - |
| Docosadienoic acid (C22:2 n-3) | - | - | - | - | - |
| Lignoceric acid (C24:0) | 1.1 ± 0.0 (0.1) | 1.3 ± 0.1 (0.4) | - | 1.9 ± 0.0 (0.2) | 1.4 ± 0.0 (0.2) |
| Docosahexaenoic acid (C22:6 n-3) | - | - | - | - | - |
| Others | 1.1 ± 0.4 (0.1) | 1.3 ± 0.4 (0.5) | 0.8 ± 0.0 (0.1) | 1.0 ± 0.0 (0.1) | 1.3 ± 0.4 (0.2) |
| Total | 814.9 (100) | 747.7 (100) | 863.9 (100) | 815.4 (100) | 859.2 (100) |
| ∑ SFA | 79.7 (9.8) | 73.1 (9.8) | 86.7 (10.0) | 88 (10.8) | 82.0 (9.5) |
| ∑ MUFA | 106.0 (13.0) | 89.9 (12.0) | 98.2 (11.4) | 90.6 (11.1) | 91.7 (10.7) |
| ∑ n-6 PUFA | 483.0 (59.3) | 447.8 (59.9) | 508.6 (58.9) | 482.2 (59.1) | 516.9 (60.2) |
| ∑ n-3 PUFA | 145.8 (17.9) | 134.8 (18.0) | 168 (19.4) | 153.9 (18.9) | 168.4 (19.6) |
| ω-6:ω-3 ratio | 3.3 | 3.3 | 3.0 | 3.1 | 3.1 |
| Terpene/Sample | HSHE | HSWH | HSCA | HSHU | HSCP |
|---|---|---|---|---|---|
| Lutein | 0.001 | 0.002 | 0.125 | 0.011 | 0.026 |
| α-Carotene | - | - | 0.024 | 0.001 | 0.029 |
| β-Carotene | - | - | 0.017 | 0.002 | 0.008 |
| Cannabidiol (CBD) | - | + | + | + | + |
| Cannabidiolic acid (CBDA) | - | + | 0.027 | 0.039 | + |
| α-Pinene | + | + | + | + | + |
| β-Myrcene | + | - | + | - | + |
| β-Pinene | + | + | + | + | + |
| δ-3-Carene | - | + | + | - | - |
| Limonene | - | + | + | - | + |
| p-Cymene | - | + | - | - | - |
| Terpinolene | + | + | + | + | + |
| Isopulegol | - | + | - | - | - |
| Geraniol | - | + | + | - | + |
| β-Carophyllene | + | + | + | + | + |
| α-Humulene | + | + | + | + | + |
| Guaiol | - | + | + | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banskota, A.H.; Jones, A.; Hui, J.P.M.; Stefanova, R. Triacylglycerols and Other Lipids Profiling of Hemp By-Products. Molecules 2022, 27, 2339. https://doi.org/10.3390/molecules27072339
Banskota AH, Jones A, Hui JPM, Stefanova R. Triacylglycerols and Other Lipids Profiling of Hemp By-Products. Molecules. 2022; 27(7):2339. https://doi.org/10.3390/molecules27072339
Chicago/Turabian StyleBanskota, Arjun H., Alysson Jones, Joseph P. M. Hui, and Roumiana Stefanova. 2022. "Triacylglycerols and Other Lipids Profiling of Hemp By-Products" Molecules 27, no. 7: 2339. https://doi.org/10.3390/molecules27072339
APA StyleBanskota, A. H., Jones, A., Hui, J. P. M., & Stefanova, R. (2022). Triacylglycerols and Other Lipids Profiling of Hemp By-Products. Molecules, 27(7), 2339. https://doi.org/10.3390/molecules27072339







