Secondary Metabolites, including a New 5,6-Dihydropyran-2-One, Produced by the Fungus Diplodia corticola. Aphicidal Activity of the Main Metabolite, Sphaeropsidin A
Abstract
:1. Introduction
2. Results
2.1. Secondary Metabolites from Cultures of Diplodia corticola B305
2.2. Oral Toxicity of Sphaeropsidin A on Acyrthosiphon pisum
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Fungal Strain and Cultures Production
4.3. Extraction and Purification Processes of Metabolites 1–11
4.4. Insects Rearing and Oral Toxicity Bioassay
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Phillips, A.J.L.; Lopes, J.; Abdollahzadeh, J.; Bobev, S.; Alves, A. Resolving the Diplodia complex on apple and other Rosaceae hosts. Pers. Mol. Phylogeny Evol. Fungi 2012, 29, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. Rev. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- Evidente, A.; Masi, M.; Linaldeddu, B.T.; Franceschini, A.; Scanu, B.; Cimmino, A.; Andolfi, A.; Motta, A.; Maddau, L. Afritoxinones A and B, dihydrofuropyran-2-ones produced by Diplodia africana the causal agent of branch dieback on Juniperus phoenicea. Phytochemistry 2012, 77, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Reveglia, P.; Savocchia, S.; Billones-Baaijens, R.; Masi, M.; Cimmino, A.; Evidente, A. Phytotoxic metabolites by nine species of Botryosphaeriaceae involved in grapevine dieback in Australia and identification of those produced by Diplodia mutila, Diplodia seriata, Neofusicoccum australe and Neofusicoccum luteum. Nat. Prod. Res. 2019, 33, 2223–2229. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, X.L.; Shen, T.; Ren, D.M.; Lou, H.X.; Wang, X.N. Two new triterpenoids from the fungus Diplodia cupressi. Nat. Prod. Res. 2020, 34, 2179–2185. [Google Scholar] [CrossRef]
- Tuzi, A.; Andolfi, A.; Maddau, L.; Masi, M.; Evidente, A. Structure and stereochemical assignment of spheropsidone, a phytotoxin from Diplodia cupressi. J. Struct. Chem. 2012, 53, 786–792. [Google Scholar] [CrossRef]
- Masi, M.; Maddau, L.; Linaldeddu, B.T.; Cimmino, A.; D’Amico, W.; Scanu, B.; Evidente, M.; Tuzi, A.; Evidente, A. Bioactive secondary metabolites produced by the oak pathogen Diplodia corticola. J. Agric. Food Chem. 2016, 64, 217–225. [Google Scholar] [CrossRef]
- Di Lecce, R.; Masi, M.; Linaldeddu, B.T.; Pescitelli, G.; Maddau, L.; Evidente, A. Bioactive secondary metabolites produced by the emerging pathogen Diplodia olivarum. Phytopathol. Mediterr. 2021, 60, 129–138. [Google Scholar] [CrossRef]
- Cimmino, A.; Maddau, L.; Masi, M.; Evidente, M.; Linaldeddu, B.T.; Evidente, A. Further secondary metabolites produced by Diplodia corticola, a fungal pathogen involved in cork oak decline. Tetrahedron 2016, 72, 6788–6793. [Google Scholar] [CrossRef]
- Evidente, A.; Andolfi, A.; Fiore, M.; Spanu, E.; Franceschini, A.; Maddau, L. Diplobifuranylones A and B, 5′-monosubstituted tetrahydro-2H-bifuranyl-5-ones produced by Diplodia corticola, a fungus pathogen of cork oak. J. Nat. Prod. 2006, 69, 671–674. [Google Scholar] [CrossRef]
- Mahamedi, A.E.; Phillips, A.J.L.; Lopes, A.; Djellid, Y.; Arkam, M.; Eichmeier, A.; Zitouni, A.; Alves, A.; Berraf-Tebbal, A. Diversity, distribution and host association of Botryosphaeriaceae species causing oak decline across different forest ecosystems in Algeria. Eur. J. Plant Pathol. 2020, 158, 745–765. [Google Scholar] [CrossRef]
- Félix, C.; Pinto, G.; Amaral, J.; Fernandes, I.; Alves, A.; Esteves, A.C. Strain-related pathogenicity in Diplodia corticola. For. Pathol. 2017, 47, e12366. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Maddau, L.; Franceschini, A. First report of Diplodia corticola causing canker and dieback of Quercus ilex, Q. petraea, and Q. suber in Corsica (France). Plant Dis. 2017, 101, 256. [Google Scholar] [CrossRef]
- Masi, M.; Evidente, A. Sphaeropsidin A: A pimarane diterpene with interesting biological activities and promising practical applications. ChemBioChem 2021, 22, 3263–3269. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Andolfi, A.; Avolio, F.; Ali, A.; Tabanca, N.; Khan, I.A.; Evidente, A. Cyclopaldic acid, seiridin, and sphaeropsidin A as fungal phytotoxins, and larvicidal and biting deterrents against Aedes aegypti (Diptera: Culicidae): Structure-activity relationships. Chem. Biodivers. 2013, 10, 1239. [Google Scholar] [CrossRef]
- Evidente, A.; Venturi, V.; Masi, M.; Degrassi, G.; Cimmino, A.; Maddau, L.; Andolfi, A. In vitro antibacterial activity of sphaeropsidins and chemical derivatives toward Xanthomonas oryzae pv. oryzae, the causal agent of rice bacterial blight. J. Nat. Prod. 2011, 74, 2520–2525. [Google Scholar] [CrossRef]
- Padhi, S.; Masi, M.; Cimmino, A.; Tuzi, A.; Jena, S.; Tayung, K.; Evidente, A. Funiculosone, a substituted dihydroxanthene-1,9-dione with two of its analogues produced by an endolichenic fungus Talaromyces funiculosus and their antimicrobial activity. Phytochemistry 2019, 157, 175–183. [Google Scholar] [CrossRef]
- Di Lelio, I.; Salvatore, M.M.; DellaGreca, M.; Mahamedi, A.E.; Alves, A.; Berraf-Tebbal, A.; Volpe, G.; Russo, E.; Becchimanzi, A.; Nicoletti, R. Defensive mutualism of endophytic fungi: Effects of sphaeropsidin A against a model lepidopteran pest. Proceeding Pap. 2022. [Google Scholar] [CrossRef]
- Ellestad, G.A.; Kunstmann, M.P.; Mirando, P.; Morton, G.O. Structures of fungal diterpene antibiotics LL-S491/3 and -y. J. Am. Chem. Soc. 1972, 236, 6206–6208. [Google Scholar] [CrossRef]
- Evidente, A.; Sparapano, L.; Fierro, O.; Bruno, G.; Giordano, F.; Motta, A. Sphaeropsidins B and C, phytotoxic pimarane diterpenes from Sphaeropsis sapinea f. Sp. Cupressi Diplodia Mutila. Phytochemistry 1997, 45, 705–713. [Google Scholar] [CrossRef]
- Cabras, A.; Mannoni, M.A.; Serra, S.; Andolfi, A.; Fiore, M.; Evidente, A. Occurrence, isolation and biological activity of phytotoxic metabolites produced in vitro by Sphaeropsis sapinea, pathogenic fungus of Pinus radiata. Eur. J. Plant Pathol. 2006, 115, 187–193. [Google Scholar] [CrossRef]
- Evidente, A.; Sparapano, L.; Fierro, O.; Bruno, G.; Motta, A. Sapinofuranones A and B, two new 2(3H)-dihydrofuranones produced by Sphaeropsis sapinea, a common pathogen of conifers. J. Nat. Prod. 1999, 62, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Di Lecce, R.; Marsico, G.; Linaldeddu, B.T.; Maddau, L.; Superchi, S.; Evidente, A. Pinofuranoxins A and B, bioactive trisubstituted furanones produced by the invasive pathogen Diplodia sapinea. J. Nat. Prod. 2021, 84, 2600–2605. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Quintanilla, C.D.; Zhang, L. Total synthesis and structure revision of diplobifuranylone B. J. Org. Chem. 2019, 84, 11054–11060. [Google Scholar] [CrossRef] [PubMed]
- Félix, C.; Salvatore, M.M.; DellaGreca, M.; Ferreira, V.; Duarte, A.S.; Salvatore, F.; Naviglio, D.; Gallo, M.; Alves, A.; Esteves, A.C.; et al. Secondary metabolites produced by grapevine strains of Lasiodiplodia theobromae grown at two different temperatures. Mycologia 2019, 111, 466–476. [Google Scholar] [CrossRef]
- D’Annibale, A.; Ciaralli, L.; Bassetti, M.; Pasquini, C. Synthesis of alkyl-substituted six-membered lactones through ring-closing metathesis of homoallyl acrylates. An easy route to pyran-2-ones, constituents of tobacco flavor. J. Org. Chem. 2007, 72, 6067–6074. [Google Scholar] [CrossRef]
- Badertscher, M.; Bühlmann, P.; Pretsch, E. Structure Determination of Organic Compounds; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-93809-5. [Google Scholar]
- Salvatore, M.M.; Andolfi, A.; Nicoletti, R. The thin line between pathogenicity and endophytism: The case of Lasiodiplodia theobromae. Agriculture 2020, 10, 488. [Google Scholar] [CrossRef]
- Félix, C.; Salvatore, M.M.; Dellagreca, M.; Meneses, R.; Duarte, A.S.; Salvatore, F.; Naviglio, D.; Gallo, M.; Jorrín-Novo, J.V.; Alves, A.; et al. Production of toxic metabolites by two strains of Lasiodiplodia theobromae, isolated from a coconut tree and a human patient. Mycologia 2018, 110, 642–653. [Google Scholar] [CrossRef]
- Shabuer, G.; Ishida, K.; Pidot, S.J.; Roth, M.; Dahse, H.M.; Hertweck, C. Plant pathogenic anaerobic bacteria use aromatic polyketides to access aerobic territory. Science 2015, 350, 670–674. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Andolfi, A. Phytopathogenic fungi and toxicity. Toxins 2021, 13, 689. [Google Scholar] [CrossRef]
- Davies-Coleman, M.T.; Rivett, D.E.A. Naturally occurring 6-substituted 5, 6-dihydro-α-pyrones. In Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Springer: Vienna, Austria, 1998; pp. 181–209. ISBN 978-3-7091-9004-3. [Google Scholar]
- Dickinson, J.M. Microbial pyran-2-ones and dihydropyran-2-ones. Nat. Prod. Rep. 1991, 10, 71–98. [Google Scholar] [CrossRef] [PubMed]
- Sparapano, L.; Bruno, G.; Fierro, O.; Evidente, A. Studies on structure-activity relationship of sphaeropsidins A-F, phytotoxins produced by Sphaeropsis sapinea f. sp. cupressi. Phytochemistry 2004, 65, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Sparapano, L.; Motta, A.; Giordano, F.; Fierro, O.; Frisullo, S. A phytotoxic pimarane diterpene of Sphaeropsis sapinea f. sp. cupressi, the pathogen of a canker disease of cypress. Phytochemistry 1996, 42, 1541–1546. [Google Scholar] [CrossRef]
- Andolfi, A.; Maddau, L.; Basso, S.; Linaldeddu, B.T.; Cimmino, A.; Scanu, B.; Deidda, A.; Tuzi, A.; Evidente, A. Diplopimarane, a 20-nor-ent-pimarane produced by the oak pathogen Diplodia quercivora. J. Nat. Prod. 2014, 77, 2352–2360. [Google Scholar] [CrossRef]
- Evidente, A.; Sparapano, L.; Andolfi, A.; Bruno, G.; Giordano, F.; Motta, A. Chlorosphaeropsidone and epichlorosphaeropsidone, two new chlorinated dimedone methyl ethers isolated from liquid cultures of Sphaeropsis sapinea f. sp. cupressi. Phytopathol. Mediterr. 2000, 39, 299–330. [Google Scholar]
- Cimmino, A.; Maddau, L.; Masi, M.; Linaldeddu, B.T.; Pescitelli, G.; Evidente, A. Fraxitoxin, a new isochromanone isolated from Diplodia fraxini. Chem. Biodivers. 2017, 14, e1700325. [Google Scholar] [CrossRef] [Green Version]
- Saeed, A. Isocoumarins, miraculous natural products blessed with diverse pharmacological activities. Eur. J. Med. Chem. 2016, 116, 290–317. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Alves, A.; Andolfi, A. Secondary metabolites produced by Neofusicoccum species associated with plants: A review. Agriculture 2021, 11, 149. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Alves, A.; Andolfi, A. Secondary metabolites of Lasiodiplodia theobromae: Distribution, chemical diversity, bioactivity, and implications of their occurrence. Toxins 2020, 12, 457. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Félix, C.; Lima, F.; Ferreira, V.; Naviglio, D.; Salvatore, F.; Duarte, A.S.; Alves, A.; Andolfi, A.; Esteves, A.C. Secondary metabolites produced by Macrophomina phaseolina isolated from Eucalyptus globulus. Agriculture 2020, 10, 72. [Google Scholar] [CrossRef] [Green Version]
- Abou-Mansour, E.; Débieux, J.L.; Ramírez-Suero, M.; Bénard-Gellon, M.; Magnin-Robert, M.; Spagnolo, A.; Chong, J.; Farine, S.; Bertsch, C.; L’Haridon, F.; et al. Phytotoxic metabolites from Neofusicoccum parvum, a pathogen of Botryosphaeria dieback of grapevine. Phytochemistry 2015, 115, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.M.; DellaGreca, M.; Nicoletti, R.; Salvatore, F.; Tuzi, A.; De Tommaso, G.; Alves, A.; Mahamedi, A.E.; Berraf-Tebbal, A.; Andolfi, A. Mitidjospirone, a new spirodioxynaphthalene and GC-MS screening of secondary metabolites produced by strains of Lasiodiplodia mitidjana associated to Citrus sinensis dieback. Nat. Prod. Res. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Van Damme, E.J.M.; Smagghe, G. Evaluation of the susceptibility of the pea aphid, Acyrthosiphon pisum, to a selection of novel biorational insecticides using an artificial diet. J. Insect Sci. 2009, 9, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
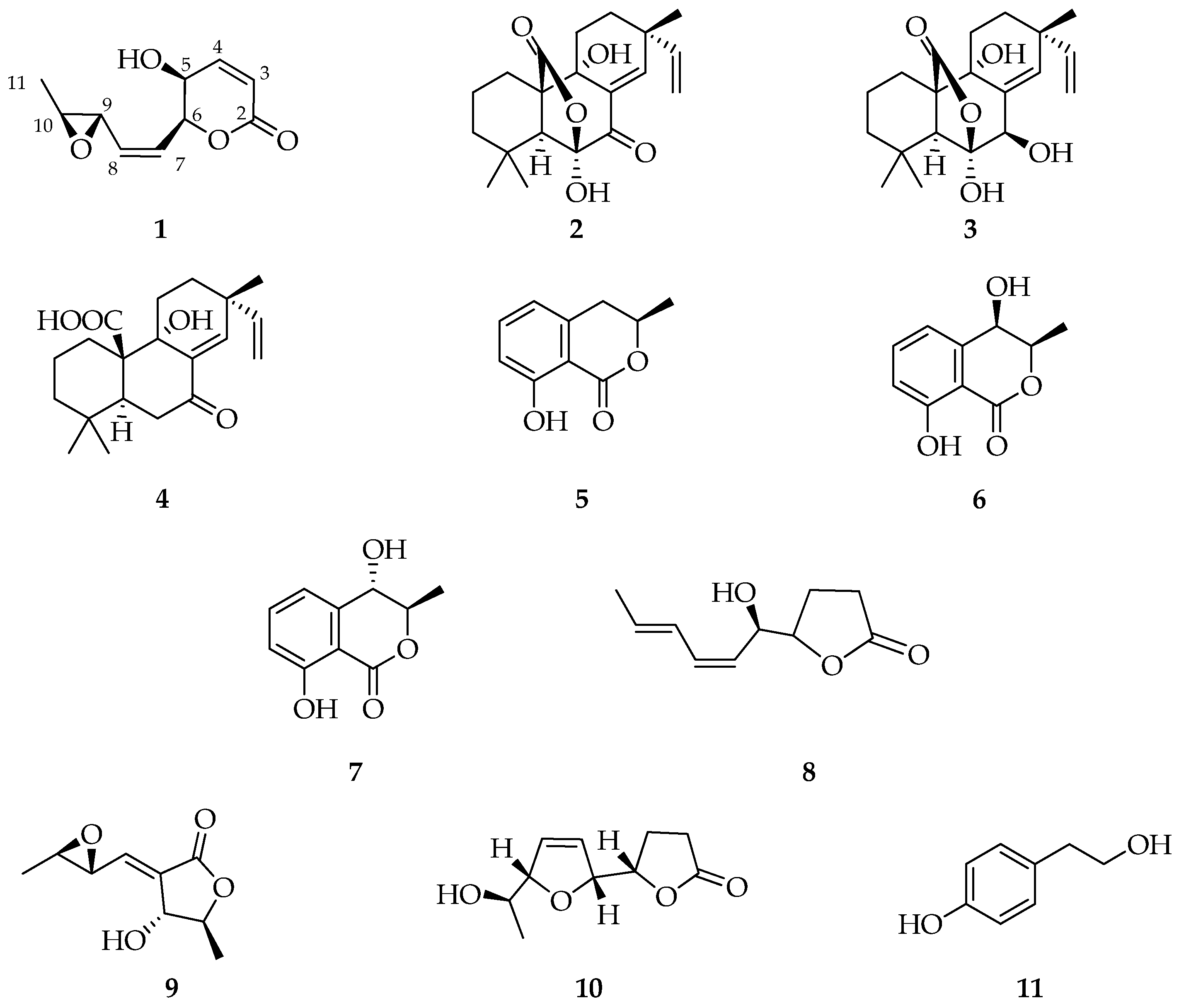
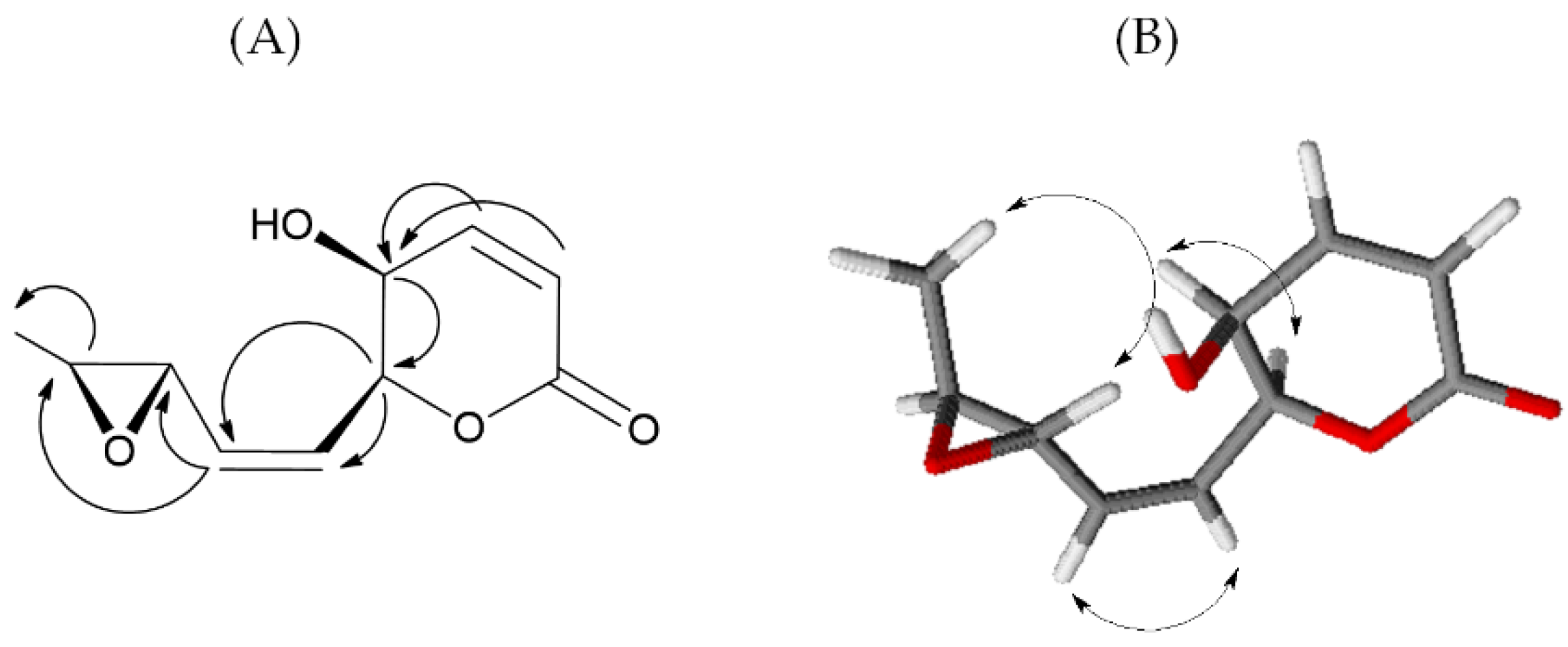


| No. | δC | δH (J in Hz) | HMBC |
|---|---|---|---|
| 1 | - | - | |
| 2 | 162.8 | - | |
| 3 | 122.9 | 6.17 (1H) br d (9.8) | C-2, C-5 |
| 4 | 144.3 | 7.03 (1H) dd (9.8, 5.4) | C-2, C-5, C-6 |
| 5 | 63.2 | 4.28 (1H) dd (5.4, 3.3) | C-3, C-4, C-6 |
| 6 | 76.9 | 5.40 (1H) dd (7.9, 3.3) | C-7, C-8 |
| 7 | 127.2 | 5.93 (1H) ddd (11.7, 7.9, 1.1) | C-9 |
| 8 | 132.4 | 5.63 (1H) ddd (11.7, 6.7, 1.1) | C-6, C-9, C-10 |
| 9 | 55.5 | 3.34 (1H) dd (6.7, 2.1) | C-8, C-7, C-10 |
| 10 | 56.7 | 3.00 (1H) dq (5.2, 2.1) | C-11 |
| 11 | 17.5 | 1.40 (3H) d (5.2) | C-9, C-8 |
| Code | Name | Source | Ref. |
|---|---|---|---|
| 2 | Sphaeropsidin A | D. corticola, D. sapinea, D. africana, D. quercivora | [3,10,34,35,36,37] |
| 3 | Sphaeropsidin B | D. corticola, D. sapinea | [10,34,37] |
| 4 | Sphaeropsidin C | D. corticola, D. sapinea, D. quercivora | [10,34,36,37] |
| 5 | (R)-mellein | D. africana, D. fraxini, D. mutila, D. seriata, D. sapinea | [3,4,21,38] |
| 6 | (3R,4R)-4-hydroxymellein | D. corticola, D. africana, D. sapinea | [3,10,21] |
| 7 | (3S,4R)-4-hydroxymellein | D. corticola, D. africana, D. sapinea | [3,10,21] |
| 8 | Sapinofuranone B | D. corticola | [10] |
| 9 | Pinofuranoxin A | D. sapinea | [23] |
| 10 | Diplobifuranylone B | D. corticola | [10] |
| 11 | Tyrosol | D. fraxini, D. mutila | [4,38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatore, M.M.; Di Lelio, I.; DellaGreca, M.; Nicoletti, R.; Salvatore, F.; Russo, E.; Volpe, G.; Becchimanzi, A.; Mahamedi, A.E.; Berraf-Tebbal, A.; et al. Secondary Metabolites, including a New 5,6-Dihydropyran-2-One, Produced by the Fungus Diplodia corticola. Aphicidal Activity of the Main Metabolite, Sphaeropsidin A. Molecules 2022, 27, 2327. https://doi.org/10.3390/molecules27072327
Salvatore MM, Di Lelio I, DellaGreca M, Nicoletti R, Salvatore F, Russo E, Volpe G, Becchimanzi A, Mahamedi AE, Berraf-Tebbal A, et al. Secondary Metabolites, including a New 5,6-Dihydropyran-2-One, Produced by the Fungus Diplodia corticola. Aphicidal Activity of the Main Metabolite, Sphaeropsidin A. Molecules. 2022; 27(7):2327. https://doi.org/10.3390/molecules27072327
Chicago/Turabian StyleSalvatore, Maria Michela, Ilaria Di Lelio, Marina DellaGreca, Rosario Nicoletti, Francesco Salvatore, Elia Russo, Gennaro Volpe, Andrea Becchimanzi, Alla Eddine Mahamedi, Akila Berraf-Tebbal, and et al. 2022. "Secondary Metabolites, including a New 5,6-Dihydropyran-2-One, Produced by the Fungus Diplodia corticola. Aphicidal Activity of the Main Metabolite, Sphaeropsidin A" Molecules 27, no. 7: 2327. https://doi.org/10.3390/molecules27072327
APA StyleSalvatore, M. M., Di Lelio, I., DellaGreca, M., Nicoletti, R., Salvatore, F., Russo, E., Volpe, G., Becchimanzi, A., Mahamedi, A. E., Berraf-Tebbal, A., & Andolfi, A. (2022). Secondary Metabolites, including a New 5,6-Dihydropyran-2-One, Produced by the Fungus Diplodia corticola. Aphicidal Activity of the Main Metabolite, Sphaeropsidin A. Molecules, 27(7), 2327. https://doi.org/10.3390/molecules27072327












