UHPLC-ESI-QqTOF Analysis and In Vitro Rumen Fermentation for Exploiting Fagus sylvatica Leaf in Ruminant Diet
Abstract
:1. Introduction
2. Results and Discussion
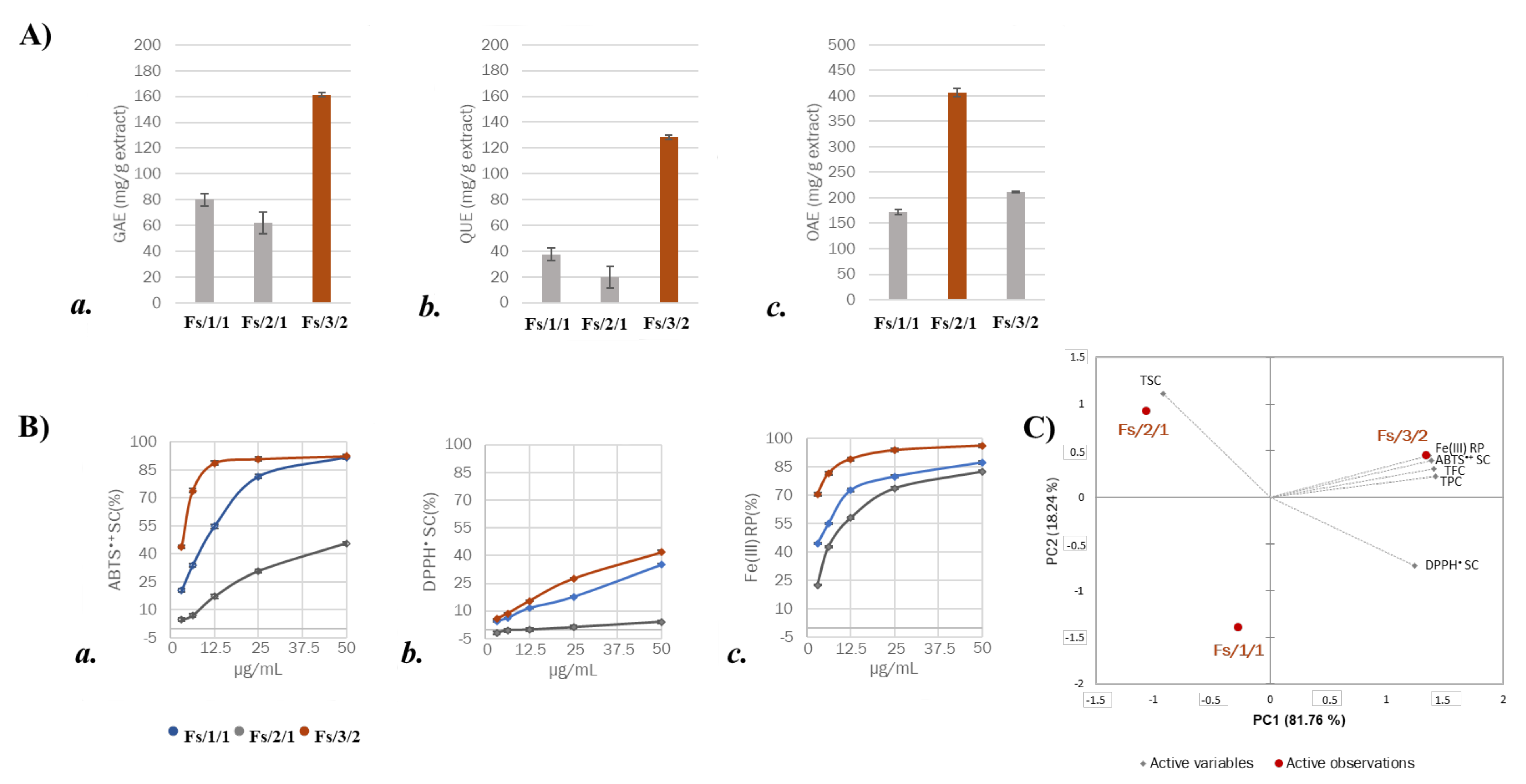
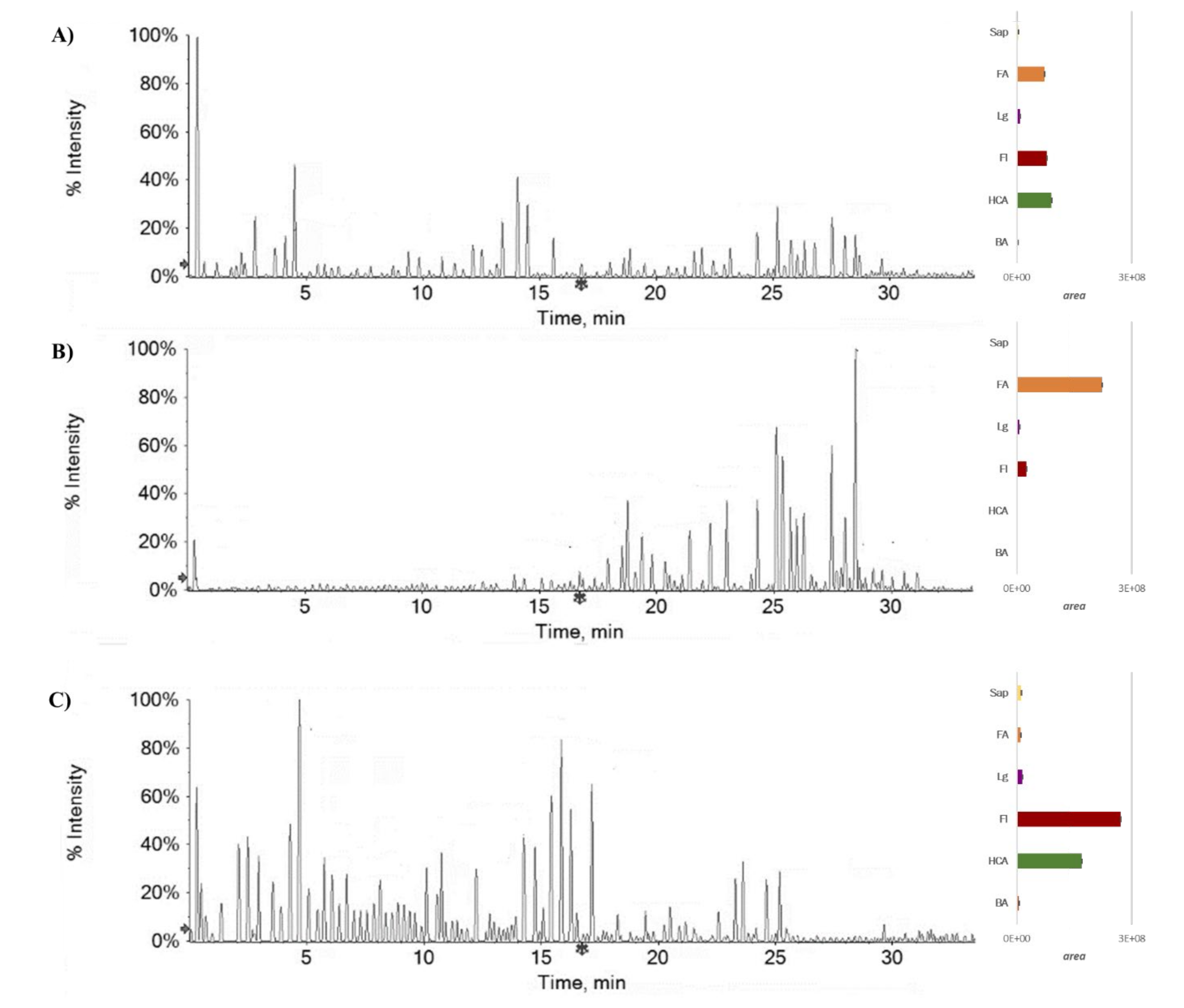
2.1. Chemical Investigation on Fs/2/1 and Fs/3/2 Fractions
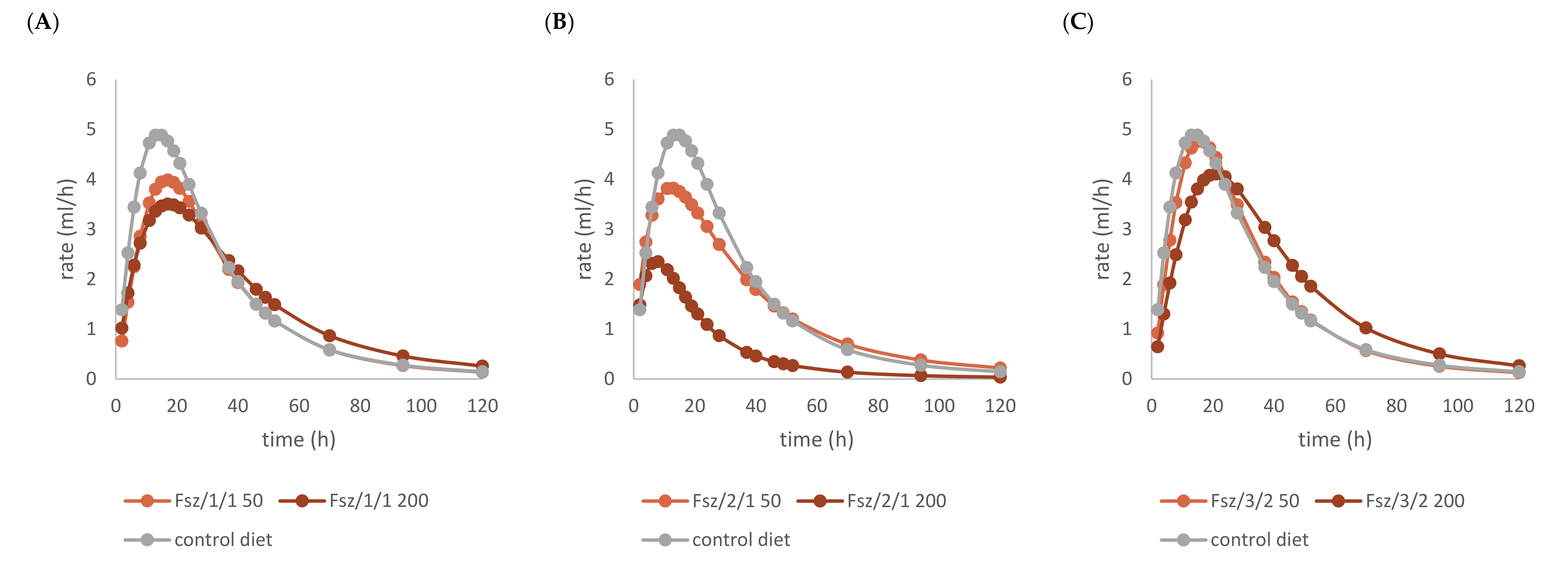
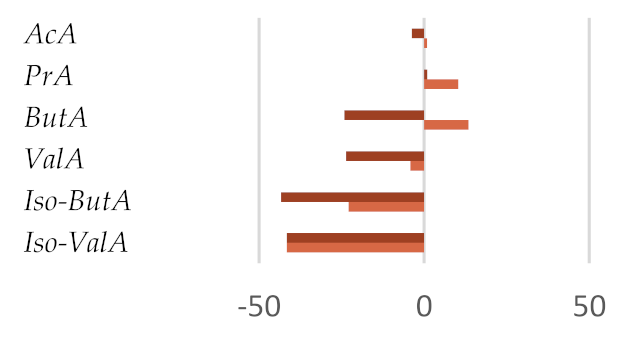
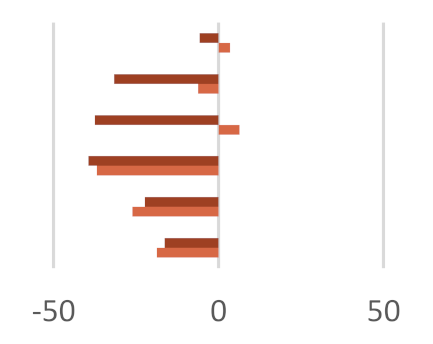
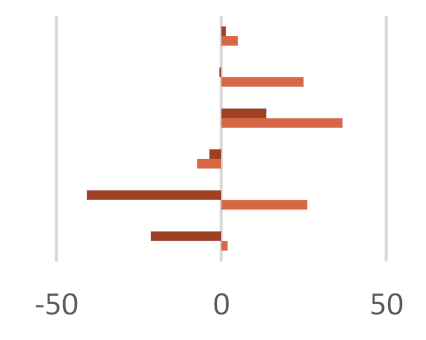
2.2. Effects of Beech Leaf Alcoholic Extract and Its Fractions on Fermentative Parameters
3. Materials and Methods
3.1. Plant Collection and Fractionation
3.2. UHPLC-HRMS and MS/MS Parameters and UV-Vis Analyses
3.3. Radical Scavenging Capacity: DPPH and ABTS Tests
3.4. Fe (III) Reducing Power
3.5. Determination of Total Phenolic Content
3.6. Determination of Total Flavonoidic Content
3.7. Determination of Total Lipidic Content
3.8. In Vitro Fermentation
3.9. Fermentation End Products Assessment
3.10. Data Processing and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Koneswaran, G.; Nierenberg, D. Global Farm Animal Production and Global Warming: Impacting and Mitigating Climate Change. Environ. Health Perspect. 2008, 116, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Ripple, W.J.; Smith, P.; Haberl, H.; Montzka, S.A.; McAlpine, C.; Boucher, D.H. Ruminants, climate change and climate policy. Nat. Clim. Chang. 2014, 4, 2–5. [Google Scholar] [CrossRef]
- Henry, B.K.; Eckard, R.J.; Beauchemin, K.A. Review: Adaptation of ruminant livestock production systems to climate changes. Animal 2018, 12, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Bodas, R.; López, S.; Fernández, M.; García-González, R.; Rodríguez, A.B.; Wallace, R.J.; González, J.S. In vitro screening of the potential of numerous plant species as antimethanogenic feed additives for ruminants. Anim. Feed Sci. Technol. 2008, 145, 245–258. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Cuchillo-Hilario, M.; León-Ortiz, L.; Ramírez-Rodríguez, A.; Cabiddu, A.; Navarro-Ocaña, A.; Morales-Romero, A.M.; Medina-Campos, O.N.; Pedraza-Chaverri, J. Goats’ Feeding Supplementation with Acacia farnesiana Pods and Their Relationship with Milk Composition: Fatty acids, polyphenols, and antioxidant activity. Animals 2019, 9, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can Agro-Industrial By-Products Rich in Polyphenols be Advantageously Used in the Feeding and Nutrition of Dairy Small Ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, G.; Hao, H.; Xie, S.; Wang, X.; Dai, M.; Huang, L.; Yuan, Z. Antibiotic alternatives: The substitution of antibiotics in animal husbandry? Front. Microbiol. 2014, 5, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballou, M.A.; Davis, E.M.; Kasl, B.A. Nutraceuticals: An Alternative Strategy for the Use of Antimicrobials. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 507–534. [Google Scholar] [CrossRef]
- Vastolo, A.; Calabrò, S.; Cutrignelli, M.I. A review on the use of agro-industrial coproducts in animals’ diets. Ital. J. Anim. Sci. 2022, 21, 577–594. [Google Scholar] [CrossRef]
- Mhlongo, G.; Mnisi, C.M.; Mlambo, V. Cultivating oyster mushrooms on red grape pomace waste enhances potential nutritional value of the spent substrate for ruminants. PLoS ONE 2021, 16, e0246992. [Google Scholar] [CrossRef]
- Tzamaloukas, O.; Neofytou, M.C.; Simitzis, P.E. Application of olive by-products in livestock with emphasis on small ruminants: Implications on rumen function, growth performance, milk and meat quality. Animals 2021, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Bambidis, V.A.; Robinson, P.H. Citrus by-product as ruminant feeds: A review. Anim. Feed Sci. Technol. 2006, 128, 175–217. [Google Scholar] [CrossRef]
- Boussaada, A.; Arhab, R.; Calabrò, S.; Grazioli, R.; Ferrara, M.; Musco, N.; Thlidjane, M.; Cutrignelli, M.I. Effect of Eucalyptus globulus leaves extracts on in vitro rumen fermentation, methanogenesis, degradability and protozoa population. Ann. Anim. Sci. 2018, 18, 753–767. [Google Scholar] [CrossRef] [Green Version]
- Kholif, A.E.; Olafadehan, O.A. Essential oils and phytogenic feed additives in ruminant diet: Chemistry, ruminal microbiota and fermentation, feed utilization and productive performance. Phytochem. Rev. 2021, 20, 1087–1108. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2017, 4, 137–150. [Google Scholar] [CrossRef]
- Das, T.; Banerjee, D.; Chakraborty, D.; Pakhira, M.; Shrivastava, B.; Kuhad, R. Saponin: Role in Animal system. Vet. World 2012, 5, 248–254. [Google Scholar] [CrossRef]
- Kim, E.T.; Guan, L.L.; Lee, S.J.; Lee, S.M.; Lee, S.S.; Lee, I.D.; Lee, S.K.; Lee, S.S. Effects of flavonoid-rich plant extracts on in vitro ruminal methanogenesis, microbial populations and fermentation characteristics. Asian Australas. J. Anim. Sci. 2015, 28, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Cieslak, A.; Szumacher-Strabel, M.; Stochmal, A.; Oleszek, W. Plant components with specific activities against rumen methanogens. Animal 2013, 7, 253–265. [Google Scholar] [CrossRef]
- Haulisah, N.A.; Hassan, L.; Bejo, S.K.; Jajere, S.M.; Ahmad, N.I. High levels of antibiotic resistance in isolates from diseased livestock. Front. Vet. Sci. 2021, 8, 652351. [Google Scholar] [CrossRef]
- Kalantar, M. The Importance of Flavonoids in Ruminant Nutrition. Arch. Anim. Husb. Dairy Sci. 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Owens, F.N.; Basalan, M. Ruminal fermentation. In Rumenology; Millen, D.D., Arrigoni, M.D.B., Pacheco, R.D.L., Eds.; Springer: Cham, Switzerland, 2016; pp. 63–102. [Google Scholar]
- Siwach, R.; Tokas, J.; Seth, R. Use of lycopene as a natural antioxidant in extending the shelf-life of anhydrous cow milk fat. Food Chem. 2016, 199, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Yildirim-Elikoglu, S.; Erdem, Y.K. Interactions between milk proteins and polyphenols: Binding mechanisms, related changes, and the future trends in the dairy industry. Food Rev. Int. 2017, 34, 665–697. [Google Scholar] [CrossRef]
- Bryszak, M.; Szumacher-Strabel, M.; El-Sherbiny, M.; Stochmal, A.; Oleszek, W.; Roj, E.; Patra, A.K.; Cieslak, A. Effects of berry seed residues on ruminal fermentation, methane concentration, milk production, and fatty acid proportions in the rumen and milk of dairy cows. Int. J. Dairy Sci. 2019, 102, 1257–1273. [Google Scholar] [CrossRef] [Green Version]
- Formato, M.; Piccolella, S.; Zidorn, C.; Pacifico, S. UHPLC-HRMS analysis of Fagus sylvatica (Fagaceae) leaves: A renewable source of antioxidant polyphenols. Antioxidants 2021, 10, 1140. [Google Scholar] [CrossRef]
- Matzke, K.; Riederer, M. A comparative study into the chemical constitution of cutins and suberins from Picea abies (L.) Karst, Quercus robur L., and Fagus sylvatica L. Planta 1991, 185, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Anschau, A.; Caruso, C.S.; Kuhn, R.C.; Franco, T.T. Validation of the sulfo-phospho-vanillin (SPV) method for the determination of lipid content in oleaginous microorganisms bioprocess engineering. Braz. J. Chem. Eng. 2017, 35, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Antonopoulou, I.; Enman, J.; Rova, U.; Christkopoulos, P.; Matsakas, L. Lipids detection and quantification in oleaginous microorganisms: An overview of the current state of the art. BMC Chem. Eng. 2019, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Pacifico, S.; Piccolella, S.; Nocera, P.; Tranquillo, E.; Dal Poggetto, F.; Catauro, M. New insights into phenol and polyphenol composition of Stevia rebaudiana leaves. J. Pharm. Biomed. Anal. 2019, 163, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Renault, H.; Alber, A.; Horst, N.A.; Basilio Lopes, A.; Fich, E.A.; Kriegshauser, M.A.; Wiedemann, G.; Ullmann, P.; Herrgott, L.; Erhardt, M.; et al. A phenol-enriched cuticle is ancestral to lignin evolution in land plants. Nat. Comm. 2013, 8, 14713. [Google Scholar] [CrossRef]
- Hahn, R.; Nahrstedt, A. Hydroxycinnamic acid derivatives, caffeoylmalic and new caffeoylaldonic acid esters, from Chelidonium majus. Planta Med. 1993, 59, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Nishida, R.; Kuwahara, Y. Oviposition stimulant for a Rutaceae-feeding swallowtail butterfly, Papilio bianor (Lepidoptera: Papilionidae): Hydroxycinnamic acid derivative from Orixa japonica. Appl. Entomol. Zool. 2000, 35, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Schrader, K.K.; Hamann, M.T.; McChesney, J.D.; Rodenburg, D.L.; Ibrahim, M.A. Antibacterial activities of metabolites from Platanus occidentalis (American sycamore) against fish pathogenic bacteria. J. Aquac. Res. Dev. 2015, 6, 364. [Google Scholar] [CrossRef] [Green Version]
- Holler, J.G.; Christensen, S.B.; Slotved, H.C.; Rasmussen, H.B.; Gúzman, A.; Olsen, C.E.; Petersen, B.; Mølgaard, P. Novel inhibitory activity of the Staphylococcus aureus NorA efflux pump by a kaempferol rhamnoside isolated from Persea lingue Nees. J. Antimicrob. Chemother. 2012, 67, 1138–1144. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.C.; Chen, C.M.; Row, L.C. Flavonoids and benzene derivatives from the flowers and fruit of Tetrapanax papyriferus. J. Nat. Prod. 2005, 68, 1773–1775. [Google Scholar] [CrossRef] [PubMed]
- Krauze-Baranowska, M.; Sowiński, P.; Kawiak, A.; Sparzak, A.B. Flavonoids from Pseudotsuga menziesii. J. Biosci. 2013, 68, 87–96. [Google Scholar]
- Akbaribazm, M.; Rasoul Khazaei, M.; Khazaei, M. Phytochemicals and antioxidant activity of alcoholic/hydroalcoholic extract of Trifolium pratense. Chin. Herb. Med. 2020, 12, 326–335. [Google Scholar] [CrossRef]
- Üstünes, L.; Claeys, M.; Laekeman, G.; Herman, A.G.; Vlietinck, A.J.; Özer, A. Isolation and identification of two isomeric trihydroxy octadecenoic acids with prostaglandin E-like activity from onion bulbs. Prostaglandins 1985, 29, 847–865. [Google Scholar] [CrossRef]
- Zhu, J.H.; Yu, R.M.; Yang, L.; Li, W.M. Two new compounds from transgenic Panax quinquefolium. Fitoterapia 2010, 81, 339–342. [Google Scholar] [CrossRef]
- Xia, C.; Deng, J.; Pan, Y.; Lin, C.; Zhu, Y.; Xiang, Z.; Li, W. Comprehensive Profiling of Macamides and Fatty Acid Derivatives in Maca with Different Postharvest Drying Processes Using UPLC-QTOF-MS. ACS Omega 2021, 6, 24484–24492. [Google Scholar] [CrossRef]
- Focant, M.; Froidmont, E.; Archambeau, Q.; Dang Van, Q.C.; Larondelle, Y. The effect of oak tannin (Quercus robur) and hops (Humulus lupulus) on dietary nitrogen efficiency, methane emission, and milk fatty acid composition of dairy cows fed a low-protein diet including linseed. Int. J. Dairy Sci. 2019, 102, 1144–1159. [Google Scholar] [CrossRef] [Green Version]
- Kilic, U.; Boga, M.; Guven, I. Chemical Composition and Nutritive Value of Oak (Quercus robur) Nut and Leaves. J. Appl. Anim. Res. 2010, 38, 101–104. [Google Scholar] [CrossRef]
- Śliwiński, B.J.; Soliva, C.R.; Machmüller, A.; Kreuzer, M. Efficacy of plant extracts rich in secondary constituents to modify rumen fermentation. Anim. Feed Sci. Technol. 2002, 101, 101–114. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, S.; Li, Y.; Zhang, S.; Qi, X.; Guo, K.; Guo, Y.; Fortina, R. Pilot Study of the effects of polyphenols from chestnut involucre on methane production, volatile fatty acids, and ammonia concentration during in vitro rumen fermentation. Animals 2021, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Odeyinka, S.M.; Hector, B.L.; Ørskov, E.R. Nutritive evaluation of some trees and browse species from Scotland. Eur. J. Sci. Res. 2006, 14, 311–318. [Google Scholar]
- Vastolo, A.; Calabrò, S.; Pacifico, S.; Koura, B.I.; Cutrignelli, M.I. Chemical and nutritional characteristics of Cannabis sativa L. co-products. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1–9. [Google Scholar] [CrossRef]
- Krebs, G.L.; De Rosa, D.W.; White, D.M.; Blake, B.L.; Dods, K.C.; May, C.D.; Tai, Z.X.; Clayton, E.H.; Lynch, E.E. Intake, nutrient digestibility, rumen parameters, growth rate, carcase characteristics and cannabinoid residues of sheep fed pelleted rations containing hemp (Cannabis sativa L.) stubble. Transl. Anim. Sci. 2021, 5, txab213. [Google Scholar] [CrossRef]
- Oskoueian, E.; Abdullah, N.; Oskoueian, A. Effects of Flavonoids on Rumen Fermentation Activity, Methane Production, and Microbial Population. Biomed Res. Int. 2013, 2013, 349129. [Google Scholar] [CrossRef] [Green Version]
- Firkins, J.L.; Yu, Z.; Morrison, M. Ruminal Nitrogen Metabolism: Perspectives for integration of microbiology and nutrition for dairy. J. Dairy Sci. 2007, 90, E1–E16. [Google Scholar] [CrossRef]
- Calabrò, S.; Cutrignelli, M.I.; Lo Presti, V.; Tudisco, R.; Chiofalo, V.; Grossi, M.; Infascelli, F.; Chiofalo, B. Characterization and effect of year of harvest on the nutritional properties of three varieties of white lupine (Lupinus albus L.). J. Sci. Food Agric. 2015, 95, 3127–3136. [Google Scholar] [CrossRef]
- Fievez, V.; Colman, E.; Castro-Montoya, J.M.; Stefanov, I.; Vlaeminck, B. Milk odd- and branched-chain fatty acids as biomarkers of rumen function—An update. Anim. Feed Sci. Technol. 2012, 172, 51–65. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Fievez, V.; Tamminga, S.; Dewhurst, R.J.; van Vuuren, A.; De Brabander, D.; Demeyer, D. Milk odd- and branched-chain fatty acids in relation to the rumen fermentation pattern. J. Dairy Sci. 2006, 89, 3954–3964. [Google Scholar] [CrossRef] [Green Version]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Hu, Z.; Zhang, S.; Cheng, G.; Hou, Q.; Wang, Y.; Yan, Z.; Shi, K.; Wang, Z. A Study on the Mechanism Regulating Acetate to Propionate Ratio in Rumen Fermentation by Dietary Carbohydrate Type. Adv. Biosci. Biotechnol. 2020, 11, 369–390. [Google Scholar] [CrossRef]
- Aderao, G.N.; Sahoo, A.; Bhatt, R.S.; Kumawat, P.K.; Soni, L. In vitro rumen fermentation kinetics, metabolite production, methane and substrate degradability of polyphenol rich plant leaves and their component complete feed blocks. J. Anim. Sci. Technol. 2018, 60, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Yang, C.; Dong, L.; Diao, Q.; Si, B.; Ma, J.; Tu, Y. Rumen fermentation characteristics in pre- and post-weaning calves upon feeding with mulberry leaf flavonoids and Candida tropicalis individually or in combination as a supplement. Animals 2019, 9, 990. [Google Scholar] [CrossRef] [Green Version]
- De Paula, E.M.; Samensari, R.B.; Machado, E.; Pereira, L.M.; Maia, F.J.; Yoshimura, E.H.; Franzolin, R.; Faciola, A.P.; Zeoula, L.M. Effects of phenolic compounds on ruminal protozoa population, ruminal fermentation, and digestion in water buffaloes. Livest. Sci. 2016, 185, 136–141. [Google Scholar] [CrossRef]
- Lengowski, M.B.; Zuber, K.H.R.; Witzig, M.; Möhring, J.; Boguhn, J.; Rodehutscord, M. Changes in Rumen Microbial Community Composition during Adaption to an in vitro System and the Impact of Different Forages. PLoS ONE 2016, 11, e0150115. [Google Scholar] [CrossRef] [Green Version]
- Paula, E.M.; Broderick, G.A.; Danes, M.A.C.; Lobos, N.E.; Zanton, G.I.; Faciola, A.P. Effects of replacing soybean meal with canola meal or treated canola meal on ruminal digestion, omasal nutrient flow, and performance in lactating dairy cows. J. Dairy Sci. 2018, 101, 328–339. [Google Scholar] [CrossRef]
- Zahedifar, M.; Castro, F.B.; Ørskov, E.R. Effect of hydrolytic lignin on formation of protein–lignin complexes and protein degradation by rumen microbes. Anim. Feed Sci. Technol. 2002, 95, 83–92. [Google Scholar] [CrossRef]
- Sinz, S.; Kunz, C.; Liesegang, A.; Braun, U.; Marquardt, S.; Soliva, C.R.; Kreuzer, M. In vitro bioactivity of various pure flavonoids in ruminal fermentation, with special reference to methane formation. Czech J. Anim. Sci. 2018, 63, 293–304. [Google Scholar] [CrossRef]
- Marcos, C.N.; Garcìa-Rebollar, P.; de Blas, C.; Carro, M.D. Variability in the Chemical Composition and In vitro Ruminal Fermentation of Olive Cake By-Products. Animals 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorka, P.; Kowalski, Z.M.; Pietrzak, P.; Kotunia, A.; Kiljanczyk, R.; Flaga, J.; Holst, J.J.; Guilloteau, P.; Zabielski, R. Effect of sodium butyrate supplementation in milk replacer and starter diet on rumen development in calves. J. Physiol. Pharmacol. 2009, 60, 47–53. [Google Scholar]
- Pacifico, S.; Piccolella, S.; Galasso, S.; Fiorentino, A.; Kretschmer, N.; Pan, S.P.; Nocera, P.; Lettieri, A.; Bauer, R.; Monaco, P. Influence of harvest season on chemical composition and bioactivity of wild rue plant hydroalcoholic extracts. Food Chem. Toxicol. 2016, 90, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; Galasso, S.; Piccolella, S.; Kretschmer, N.; Pan, S.P.; Nocera, P.; Lettieri, A.; Bauer, R.; Monaco, P. Winter wild fennel leaves as a source of anti-inflammatory and antioxidant polyphenols. Arab. J. Chem. 2018, 11, 513–524. [Google Scholar] [CrossRef] [Green Version]
- Piccolella, S.; Fiorentino, A.; Pacifico, S.; D’Abrosca, B.; Uzzo, P.; Monaco, P. Antioxidant properties of sour cherries (Prunus cerasus L.): Role of colorless phytochemicals from the methanolic extract of ripe fruits. J. Agric. Food Chem. 2008, 56, 1928–1935. [Google Scholar] [CrossRef]
- Murakami, A.N.; Amboni, R.D.; Prudêncio, E.S.; Amante, E.R.; Fritzen-Freire, C.B.; Boaventura, B.C.; Muñoz Ide, B.; Branco Cdos, S.; Salvador, M.; Maraschin, M. Concentration of biologically active compounds extracted from Ilex paraguariensis St. Hil. by nanofiltration. Food Chem. 2013, 141, 60–65. [Google Scholar] [CrossRef] [Green Version]
- EC Council. Regulation 882/2004 on Official controls performed to ensure verification of compliance with feed and food law, animal health and animal welfare rules. Off. J. Eur. Union L191/1 2004, 1–52. [Google Scholar]
- Searle, P.L. The Berthelot or indophenol reaction and its use in the analytical chemistry of nitrogen. A Review. Analyst 1984, 109, 549–568. [Google Scholar] [CrossRef]
- Groot, J.C.J.; Cone, J.W.; Williams, B.A.; Debersaques, F.M.A.; Lantinga, E.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feedstuff. J. Food Sci. Technol. 1996, 64, 77–89. [Google Scholar] [CrossRef]
- Bauer, E.; Williams, B.A.; Voigt, C.; Mosenthin, R.; Verstegen, M.W.A. Microbial activities of faeces from unweaned and adult pigs, in relation to selected fermentable carbohydrates. J. Anim. Sci. 2001, 73, 313–322. [Google Scholar] [CrossRef]


 detected.
detected.
 detected.
detected.| Peak | Rt (min) | Tentative Assignment | Formula | [M-H]− Found (m/z) | [M-H]− Calc. (m/z) | Error (ppm) | RDB | MS/MS Fragment Ions (m/z) and Relative Intensity | Fs/1/1 | Fs/2/1 | Fs/3/2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.397 | Quinic acid | C7H12O6 | 191.0557 | 191.0561 | −2.2 | 2 | 191.0557;129.0192; 111.0096; 87.0098; 85.0307 | |||
| 2 | 0.454 | Citric acid | C6H8O7 | 191.0203 | 191.0197 | 3 | 3 | 111.0089; 87.0089 | |||
| 3 | 0.769 | Galloyl hexose | C13H16O10 | 331.0667 | 331.0671 | −1.1 | 6 | 331.0662; 211.0236; 169.0139; 151.0033 | |||
| 4 | 1.311 | Dihydroxybenzoic acid hexoside | C13H16O9 | 315.0720 | 315.719 | 0.1 | 6 | 315.0722, 271.0396, 227.0528, 195.0287, 153.0199; 152.0114 | |||
| 5 | 1.574 | Hydroxybenzoyl hexose | C13H16O8 | 299.0770 | 299.0772 | −0.8 | 6 | 299.0770; 239.0557; 179.0345; 151.0394; 137.0237 | |||
| 6 | 1.890 | Hydroxyphenylacetic acid hexoside | C14H18O8 | 313.0939 | 313.0929 | 3.2 | 6 | 313.0910; 151.0390 | |||
| 7 | 2.038 | 3-O-Caffeoyl quinic acid (cis) | C16H18O9 | 353.0885 | 353.0878 | 2 | 8 | 353.0888; 191.0565; 179.0353;135.0451 | |||
| 8 | 2.235 | 3-O-Caffeoyl quinic acid (trans) | C16H18O9 | 353.0888 | 353.0878 | 2.2 | 8 | 353.0888; 191.0565; 179.0353;135.0451 | |||
| 9 | 2.444 | Caffeoyl threonic acid (1) | C13H14O8 | 297.0614 | 297.0622 | 2 | 7 | 179.0340; 161.0245; 135.0310; 117.0192; 89.0248 | |||
| 10 | 2.603 | Caffeoyl acid hexoside | C15H18O9 | 341.0874 | 341.0878 | 7 | −1.2 | 179.0344; 161.0244; 135.0444; 134.0296 | |||
| 11 | 2.822 | Caffeoyl threonic acid (2) | C13H14O8 | 297.0618 | 297.0616 | 0.7 | 7 | 179.0344; 135.0301 | |||
| 12 | 2.839 | 3-O-p-Coumaroyl quinic acid | C16H18O8 | 337.0926 | 337.0929 | −0.9 | 8 | 191.0560; 163.0401; 119.0499 | |||
| 13 | 3.210 | 3-O-p-Coumaroyl quinic acid | C16H18O8 | 337.0926 | 337.0929 | −0.9 | 8 | 191.0556; 163.0340; 119.0502 | |||
| 14 | 3.444 | Procyanidin | C30H26O12 | 577.1353 | 577.1352 | 0.3 | 18 | 577.1393, 451.1046, 425.0883, 407.0781, 289.0711, 245.0463, 125.0236 | |||
| 15 | 3.523 | p-Coumaroyl acid hexoside | C15H18O8 | 325.0926 | 325.0929 | −0.9 | 7 | 163.0393; 119.0500 | |||
| 16 | 3.676 | p-Coumaroyl threonic acid (1) | C13H14O7 | 281.0662 | 281.0666 | −1.7 | 7 | 163.0396; 135.0298; 119.0502 | |||
| 17 | 3.950 | Caffeic acid | C9H8O4 | 179.0357 | 179.0350 | 4.0 | 6 | 135.0454; 134.0377;117.0348; 107.0508; 89.0403 | |||
| 18 | 4.259 | p-Coumaroyl threonic acid (2) | C13H14O7 | 281.0666 | 281.0666 | 0 | 7 | 177.0570; 163.0399; 145.0293; 135.0298; 119.0503; 117.0196; 87.0089 | |||
| 19 | 4.624 | 5-O-Caffeoyl quinic acid (trans) | C16H18O9 | 353.0879 | 353.0878 | 0.3 | 8 | 191.0571; 85.0306 | |||
| 20 | 5.665 | Caffeoyl threonic acid (3) | C13H14O8 | 297.0616 | 297.0612 | −1.3 | 7 | 179.0343; 161.0242; 135.0242 | |||
| 21 | 5.693 | Caffeoyl propionic acid | C12H12O6 | 253.0718 | 253.0718 | 0.2 | 6 | 253.0705; 179.0344; 161.0243; 135.0450; 134.0375; 133.0375 | |||
| 22 | 5.759 | 5-O-Caffeoyl quinic acid (cis) | C16H18O9 | 353.0874 | 353.0878 | −1.1 | 8 | 191.0557; 161.0240;85.0293 | |||
| 23 | 6.090 | Eriodictyol 7-O-hexoside | C21H22O11 | 449.1092 | 449.1089 | −0.3 | 11 | 449.1093; 421.11444; 313.0705; 287.0553; 283.0602; 259.0607; 243.0665; 215.0702; 178.9980; 125.0242 | |||
| 24 | 6.645 | Tuberonic acid | C18H28O9 | 387.1662 | 387.1661 | 0.4 | 5 | 387.1655; 207.1024; 163.1130; 89.0243 | |||
| 25 | 6.981 | 5-O-p-Coumaroyl quinic acid | C16H18O8 | 337.0930 | 337.0929 | 0.3 | 8 | 191.0559 | |||
| 26 | 7.312 | p-O-Coumaroylmalic acid (I) | C13H12O7 | 279.0508 | 279.0510 | −0.1 | 8 | 279.0506; 179.0352; 161.0248; 133.0301 | |||
| 27 | 7.871 | Caffeoylshikimic Acid | C16H16O8 | 335.0766 | 335.0772 | −1.9 | 9 | 335.0766; 179.0355; 161.0246; 135.0450 | |||
| 28 | 8.101 | 5-O-p-Coumaroyl quinic acid | C16H18O8 | 337.0925 | 337.0929 | −1.2 | 8 | 191.0560; 173.0461; 93.0347; 85.0296 | |||
| 29 | 8.314 | Caffeoylshikimic Acid | C16H16O8 | 335.0770 | 335.0772 | −0.7 | 9 | 179.0350; 161.0243; 135.0453 | |||
| 30 | 9.288 | p-O-Coumaroylmalic acid (II) | C13H12O7 | 279.0508 | 279.0510 | −0.1 | 8 | 179.0348; 135.0451 | |||
| 31 | 10.091 | Naringenin-C-hexoside (1) | C21H22O10 | 433.1145 | 433.1140 | 1.1 | 11 | 433.1153; 415.1027; 343.0826; 325.0715; 313.0715; 271.0611; 223.0245; 193.0143; 119.0506 | |||
| 32 | 10.712 | Naringenin-C-hexoside (2) | C21H22O10 | 433.1154 | 433.1140 | 1.6 | 11 | 433.1151; 415.1043; 343.0826; 325.0710; 313.0718; 283.0610; 271.0611; 223.0246; 193.0142; 151.0040 | |||
| 33 | 11.960 | Myricetin-3-O-hexoside | C21H20O13 | 479.0839 | 479.0831 | 1.6 | 12 | 479.0841; 317.0286; 316.0221; 287.0193; 271.0239 | |||
| 34 | 14.293 | Quercetin-3-O-hexoside (1) | C21H20O12 | 463.0893 | 463.0882 | 2.4 | 12 | 463.0898; 301.0355; 300.0270; 271.0246; 255.0298; 178.9989; 151.0035 | |||
| 35 | 14.445 | Quercetin-3-O-hexuronide | C21H18O13 | 477.0679 | 477.0675 | 0.9 | 13 | 477.0688; 301.0347; 178.9979; 151.0033 | |||
| 36 | 14.728 | Quercetin-3-O-hexoside (2) | C21H20O12 | 463.0891 | 463.0882 | 1.9 | 12 | 463.0899; 301.0355; 300.0271; 271.0247; 255.0295; 243.0296; 178.9985; 151.0035 | |||
| 37 | 14.739 | Kaempferol-3-O-hexuronide (1) | C21H18O12 | 461.0733 | 461.0725 | 1.6 | 13 | 285.0398 | |||
| 38 | 14.940 | Naringenin-C-hexoside (3) | C21H22O10 | 433.1145 | 433.1140 | 1.1 | 11 | 433.1146; 415.1026; 343.0827; 325.0731; 313.0713; 283.0602; 271.0605; 223.0236; 193.0138; 119.0503 | |||
| 39 | 15.083 | Neolignan (I) | C25H34O11 | 509.2033 | 509.2028 | 0.9 | 9 | 509.2033; 491.1937; 461.1823; 367.1396; 313.1288; 179.0711; 167.0708; 149.0605; 147.0446; 134.0372 | |||
| 40 | 15.218 | Neolignan (II) | C25H34O11 | 509.2043 | 509.2028 | 2.9 | 9 | 509.2052; 491.1949; 473.1834: 461.1832; 367.1406; 313.1301; 179.0717; 149.0608 | |||
| 41 | 15.338 | Quercetin-3-O-pentoside | C20H18O11 | 433.0783 | 433.0776 | 1.5 | 12 | 433.0795; 301.0359; 300.0277; 271.0250; 255.0299; 243.0294; 178.9986 | |||
| 42 | 15.423 | Kaempferol-3-O-hexoside (1) | C21H20O11 | 447.0944 | 447.0933 | 2.5 | 12 | 447.0945; 285.0398; 284.0318; 255.0292; 227.0340; 151.0031 | |||
| 43 | 15.517 | Quercetin-3-O-(acetyl)hexoside | C23H22O13 | 505.1003 | 505.0988 | 3.0 | 13 | 505.1018; 463; 0897; 447.0945; 301.0357; 300.0277; 271.0238; 255.0296 | |||
| 44 | 15.743 | Kaempferol-3-O-hexuronide (2) | C21H18O12 | 461.0741 | 461.0725 | 3.4 | 13 | 461.0739; 285.0406; 257.0460; 229.0511; 113.0248 | |||
| 45 | 15.903 | Kaempferol-3-O-hexoside (2) | C21H20O11 | 447.0951 | 447.0933 | 4.1 | 12 | 447.0944; 327.0504; 285.0397; 284.0317; 255.0295; 227.0345; 151.0040 | |||
| 46 | 16.237 | Kaempferol-3-O-pentoside (1) | C20H18O10 | 417.0843 | 417.0827 | 3.8 | 12 | 417.0836; 285.0399; 284.0323; 255.0295; 227.0345; 151.0036 | |||
| 47 | 16.456 | Kaempferol-3-O-pentoside (2) | C20H18O10 | 417.0833 | 417.0827 | 1.4 | 12 | 417.0841; 285.0406; 284.0330; 255.0300; 227.0349 | |||
| 48 | 16.748 | Kaempferol-3-O-(acetyl)hexoside | C23H22O12 | 489.1049 | 489.1039 | 2.1 | 13 | 489.1069; 429.0800; 369.0996; 285.0412; 284.0333; 255.0301; 227.0350; 151.0034 | |||
| 49 | 17.073 | Kaempferol-3-O-deoxyhexoside | C21H20O10 | 431.0991 | 431.0984 | 1.7 | 12 | 431.1002; 285.0408; 284.0332; 255.0303; 227.0352; 229.0506 | |||
| 50 | 18.232 | Neolignan (III) | C27H38O12 | 553.2313 | 553.2291 | 4.1 | 9 | 553.2330; 343.1403; 328.1162; 183.0655 | |||
| 51 | 19.380 | Kaempferol p-coumaroylhexoside | C30H26O13 | 593.1325 | 593.1320 | 3.3 | 18 | 593.1322; 447.0935; 307.0813; 285.0387; 284.0323; 255.0294 | |||
| 52 | 19.623 | Kaempferol | C15H10O6 | 285.0404 | 285.0405 | −0.2 | 11 | 285.0400; 257.0445; 255.0293; 229.0495; 211.0393; 187.0393; 151.0034 | |||
| 53 | 18.438 | Dodecenedioic acid | C12H20O4 | 227.1289 | 227.1300 | 4.0 | 3 | 183.1394; 165.1278 | |||
| 54 | 18.867 | 9,12,13-trihydroxy-10,15-octadecadienoic acid | C18H32O5 | 327.2191 | 327.2177 | 0.9 | 3 | 309.2076; 291.1970; 239.1288; 229.1446; 221.1179; 211.1338; 191.1236; 183.1391; 171.1022; 137.0966 | |||
| 55 | 21.080 | Neolignan (IV) | C38H52O16 | 763.3187 | 763.3183 | 0.6 | 13 | 763.3225; 343.1403; 328.1154; 183.0654 | |||
| 56 | 19.695 | 9,12,13-trihydroxy-10-octadecenoic acid | C18H34O5 | 329.2337 | 329.2340 | 2.0 | 2 | 329.2343; 229.1448; 211.1344; 183.1393; 171.1030 | |||
| 57 | 20.297 | Dihydroxyhexadecanoic acid | C16H32O4 | 287.2228 | 287.2240 | 4.2 | 1 | 287.2235; 269.2127 | |||
| 58 | 20.558 | 9,16-diidrossi-octadeca-10, 12,14-trienoic acid (1) | C18H30O4 | 309.2072 | 309.2071 | 0.2 | 4 | 309.2078; 291.1972; 273.1841; 251.1654; 239.1650; 221.1544; 197.1162; 183.1026; 171.1032; 107.0865 | |||
| 59 | 20.846 | 16-idrossi-9-ossooctadeca-12,14-dienoic acid | C18H30O4 | 309.2071 | 309.2071 | −0.1 | 4 | 309.2059; 291.1962; 251.1655; 171.1028; 125.0971 | |||
| 60 | 21.053 | 9,16-diidrossi-octadeca-10, 12,14-trienoic acid (2) | C18H30O4 | 309.2071 | 309.2071 | 0.2 | 4 | 309.2079; 291.1967; 251.1649; 185.1180; 171.1021; 137.0971 | |||
| 61 | 21.350 | 16-idrossi-9-ossooctadeca-6,12,14-trienoic acid | C18H28O4 | 307.1915 | 307.1925 | 3.3 | 5 | 289.1793; 249.1492; 235.1342; 211.1340; 185.1187; 125.0976; 121.0659 | |||
| 62 | 21.350 | Dihydroxyoctadecenoic quinic acid | C25H42O9 | 485.2776 | 485.2756 | 4.1 | 5 | 485.2791; 311.2238; 223.1706; 191.0568 | |||
| 63 | 22.220 | 16-idrossi-9-ossooctadeca-6,12,14,16-tetraenoic acid | C18H26O4 | 305.1758 | 305.1760 | 0.5 | 6 | 305.1744; 249.1498; 205.1587; 135.0817 | |||
| 64 | 22.435 | Kaempferol p-coumaroyldeoxyhexoside (1) | C30H26O12 | 577.1368 | 577.1352 | 2.9 | 18 | 577.1389; 431.0988; 285.0404; 284.0316; 257.0452; 299.0495 | |||
| 65 | 22.553 | Kaempferol p-coumaroyldeoxyhexoside (2) | C30H26O12 | 577.1357 | 577.1352 | 1.0 | 18 | 577.1382; 285.0399; 284.0328 | |||
| 66 | 22.842 | Kaempferol di-p-coumaroylpentoside | C38H30O14 | 709.1463 | 709.1590 | 3.8 | 24 | 709.1641; 563.1244; 423.1116; 285.0416; 284.0332; 145.0290 | |||
| 67 | 23.154 | 15,16-dihydroxy-9Z,12Z- octadecadienoic acid | C18H32O4 | 311.2242 | 311.2228 | 1 | 3 | 311.2238; 293.2112; 275.2011; 235.1708; 223.1705; 201.1135; 87.0454 | |||
| 68 | 23.310 | 3-O-(dihexosyl)hexuronidyl oleanonic acid | C48H76O19 | 955.4928 | 955.4908 | 2.1 | 11 | 955.4984; 793.4427; 731.4414; 613.3786; 569.3871; 523.3830 | |||
| 69 | 23.582 | 3-O-(dihexosyl)hexuronidyl oleanonic acid | C48H76O19 | 955.4923 | 955.4908 | 1.6 | 11 | 955.4972; 793.4423; 569.3868 | |||
| 70 | 24.029 | Dihydroxyoctadecenoic acid | C18H34O4 | 313.2382 | 313.2384 | −0.7 | 2 | 313.2377; 295.2270; 277.2163; 183.1384; 129.0916 | |||
| 71 | 24.050 | 3-O-(hexosylpentosyl)hexuronidyl oleanonic acid | C47H74O18 | 925.4801 | 925.4802 | −0.2 | 11 | 925.4840; 763.4277 | |||
| 72 | 24.128 | 3-O-(hexosyl)hexuronidyl oleanonic acid | C42H66O14 | 793.4395 | 793.4380 | 0.9 | 10 | 793.4407; 631.3873; 569.3854 | |||
| 73 | 24.457 | Kaempferol di-p-coumaroyldeoxyhexoside | C39H32O14 | 723.1720 | 723.1719 | 0.1 | 24 | 723.1784; 577.1396; 559.1250; 437.1265; 285.0409; 284.0332; 187.0391; 163.0401; 145.0294 | |||
| 74 | 24.483 | Kaempferol p-coumaroyl feruloyl pentoside | C40H34O15 | 753.1825 | 753.1823 | −0.3 | 24 | 753.1884; 607.1502; 589.1358; 467.1378; 285.0407; 284.0320; 193.0495 | |||
| 75 | 25.032 | 9-oxooctadeca-10,12-dienoic acid | C18H30O3 | 293.2122 | 293.2130 | 2.7 | 4 | 293.2126; 275.2019; 235.1708; 211.1340; 183.1392; 171.1025; 121.1026 | |||
| 76 | 25.302 | 9-oxooctadeca-10,12,15-trienoic acid | C18H28O3 | 291.1971 | 291.1966 | 1.8 | 5 | 291.1969; 273.1851; 247.2069; 223.1701; 195.1382 | |||
| 77 | 25.611 | 13-hydroxyoctadeca-9,15-dienoic acid | C18H32O3 | 295.2283 | 295.2279 | 1.5 | 3 | 295.2284; 277.2176; 183.1389 | |||
| 78 | 25.783 | 13-hydroxyoctadeca-9,11-dienoic acid | C18H32O3 | 295.2286 | 295.2279 | 1.8 | 3 | 295.2272; 277.2166; 195.1387; 183.1023; 155.1065; 113.0971 | |||
| 79 | 25.937 | 13-oxo-9Z,11E-octadecadienoic acid | C18H30O3 | 293.2121 | 293.2122 | 2.0 | 4 | 293.2119; 275.2022; 249.2220; 195.1390; 185.1179; 153.1287; 113.0974 | |||
| 80 | 26.267 | 9-hydroxyoctadec-12-enoic acid | C18H34O3 | 297.2444 | 297.2435 | 3.0 | 2 | 297.2439; 279.2331; 171.1027; 155.1075 | |||
| 81 | 27.520 | α-Linolenic acid | C18H30O2 | 277.2180 | 277.2173 | 2.5 | 4 | 277.2181 | |||
| 82 | 28.089 | Linoleic acid | C18H32O3 | 279.2338 | 279.2330 | 3.0 | 3 | 279.2347; 259.2098 | |||
| 83 | 28.552 | Palmitic acid | C16H32O2 | 255.2336 | 255.2330 | 2.5 | 1 | 255.2334; 237.2220; 201.8341 | |||
| 84 | 28.638 | Oleic acid | C18H34O2 | 281.2491 | 281.2486 | 1.8 | 2 | 281.2493 | |||
| 85 | 29.311 | Stearic acid | C18H36O2 | 283.2656 | 283.2643 | 4.8 | 1 | 283.2652; 265.2517 |
| (A) | (B) | |||
| Parameter | control diet | Fs/1/1 | ||
| 50 mg | 200 mg | 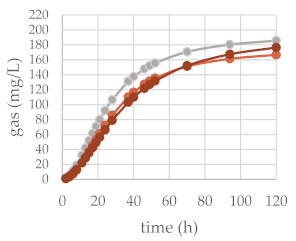 | ||
| OMD (%) | 77.43 | 75.96 | 71.98 | |
| OMCV (mL/g) | 225.59 | 210.02 | 216.75 | |
| A (mL/g) | 194.83 | 175.61 * | 195.34 | |
| B (h) | 25.42 | 28.64 | 34.80 ** | |
| C | 1.93 | 2.04 | 1.80 | |
| Tmax (h) | 14.37 | 16.88 | 17.34 | |
| Rmax (mL/h) | 4.87 | 4.01 | 3.62 | |
| control diet | Fs/2/1 | |||
| 50 mg | 200 mg | 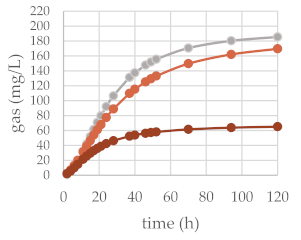 | ||
| OMD (%) | 77.43 | 74.01 | 45.37 ** | |
| OMCV (mL/g) | 225.59 | 212.13 | 83.37 ** | |
| A (mL/g) | 194.83 | 187.71 | 67.91 ** | |
| B (h) | 25.42 | 29.93 | 17.64 ** | |
| C | 1.93 | 1.61 ** | 1.64 * | |
| Tmax (h) | 14.37 | 13.47 ** | 7.39 ** | |
| Rmax (mL/h) | 4.87 | 4.58 | 2.36 ** | |
| control diet | Fs/3/2 | |||
| 50 mg | 200 mg | 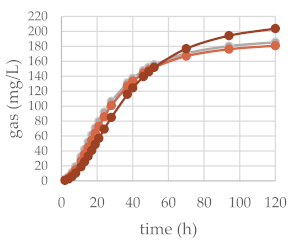 | ||
| OMD (%) | 77.43 | 76.63 | 74.40 | |
| OMCV (mL/g) | 225.59 | 217.17 | 241.98 | |
| A (mL/g) | 194.83 | 188.47 | 220.53 ** | |
| B (h) | 25.42 | 26.19 | 35.24 ** | |
| C | 1.93 | 2.08 | 2.04 | |
| Tmax (h) | 14.37 | 15.83 | 20.82 | |
| Rmax (mL/h) | 4.87 | 4.77 | 4.12 | |
| Control Diet | Fs/1/1 | Fs/2/1 | Fs/3/2 | ||||
|---|---|---|---|---|---|---|---|
| 50 mg | 200 mg | 50 mg | 200 mg | 50 mg | 200 mg | ||
| pH | 6.99 ± 0.02 | 6.95 ± 0.01 | 6.96 ± 0.02 | 7.03 ± 0.07 | 7.09 ± 0.04 * | 7.01 ± 0.04 | 6.98 ± 0.03 |
| NH3-N (mmol/g) | 5.32 ± 0.04 | 5.15 ± 0.05 * | 5.15 ± 0.05 * | 5.08 ± 0.04 ** | 5.06 ± 0.02 ** | 5.26 ± 0.07 | 5.15 ± 0.05 * |
| Total VFA (mmol/g) | 122.42 ± 2.40 | 92.15 ± 2.64 ** | 80.52 ± 3.85 ** | 92.86 ± 10.7 ** | 49.54 ± 0.46 ** | 137.53 ± 0.03 ǂ | 124.65 ± 2.99 |
| BCFA (%VFA) | 3.32 ± 0.13 | 3.56 ± 0.19 | 2.96 ± 0.18 | 3.70 ± 0.33 | 6.20 ± 0.26 ** | 3.47 ± 0.11 | 2.30 ± 0.17 ** |
| A/P | 3.08 ± 0.10 | 2.88 ± 0.06 | 2.95 ± 0.29 | 3.42 ± 0.27 | 4.26 ± 0.25 ** | 2.44 ± 0.30 ǂ | 3.15 ± 0.17 |
| % VFA | Control Diet | Fs/1/1 | Fs/2/1 | Fs/3/2 | |||
|---|---|---|---|---|---|---|---|
| 50 mg | 200 mg | 50 mg | 200 mg | 50 mg | 200 mg | ||
| AcA | 65.33 ± 0.53 | 65.89 ± 0.06 | 62.90 ± 2.47 ǂ | 67.63 ± 1.35 ǂ | 61.59 ± 1.23 | 68.62 ± 1.38 | 66.24 ± 0.47 |
| PrA | 21.18 ± 0.60 | 23.38 ± 0.69 | 21.39 ± 1.30 | 19.86 ± 1.18 | 14.47 ± 1.24 ** | 28.47 ± 2.99 ** | 21.04 ± 1.00 |
| ButA | 7.37 ± 0.15 | 8.36 ± 0.22 | 5.59 ± 0.45 | 7.84 ± 0.75 | 4.61 ± 2.58 | 10.08 ± 0.04 | 8.38 ± 0.41 |
| ValA | 1.90 ± 0.05 | 1.82 ± 0.22 | 1.45 ± 0.06 ǂ | 1.20 ± 0.05 ** | 1.15 ± 0.05 ** | 1.76 ± 0.14 | 1.83 ± 0.09 |
| iso-ButA | 1.57 ± 0.07 | 1.21 ± 0.04 | 0.89 ± 0.11 * | 1.16 ± 0.02 ǂ | 1.22 ± 0.01 | 1.98 ± 0.42 ǂ | 0.93 ± 0.03 * |
| iso-ValA | 2.57 ± 0.02 | 2.07 ± 0.16 ǂ | 1.50 ± 0.15 ** | 2.09 ± 0.16 ǂ | 2.15 ± 0.32 | 2.62 ± 0.23 * | 2.02 ± 0.10 |
 |  |  | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formato, M.; Piccolella, S.; Zidorn, C.; Vastolo, A.; Calabrò, S.; Cutrignelli, M.I.; Pacifico, S. UHPLC-ESI-QqTOF Analysis and In Vitro Rumen Fermentation for Exploiting Fagus sylvatica Leaf in Ruminant Diet. Molecules 2022, 27, 2217. https://doi.org/10.3390/molecules27072217
Formato M, Piccolella S, Zidorn C, Vastolo A, Calabrò S, Cutrignelli MI, Pacifico S. UHPLC-ESI-QqTOF Analysis and In Vitro Rumen Fermentation for Exploiting Fagus sylvatica Leaf in Ruminant Diet. Molecules. 2022; 27(7):2217. https://doi.org/10.3390/molecules27072217
Chicago/Turabian StyleFormato, Marialuisa, Simona Piccolella, Christian Zidorn, Alessandro Vastolo, Serena Calabrò, Monica Isabella Cutrignelli, and Severina Pacifico. 2022. "UHPLC-ESI-QqTOF Analysis and In Vitro Rumen Fermentation for Exploiting Fagus sylvatica Leaf in Ruminant Diet" Molecules 27, no. 7: 2217. https://doi.org/10.3390/molecules27072217
APA StyleFormato, M., Piccolella, S., Zidorn, C., Vastolo, A., Calabrò, S., Cutrignelli, M. I., & Pacifico, S. (2022). UHPLC-ESI-QqTOF Analysis and In Vitro Rumen Fermentation for Exploiting Fagus sylvatica Leaf in Ruminant Diet. Molecules, 27(7), 2217. https://doi.org/10.3390/molecules27072217











