Effects of Abelmoschus manihot Flower Extract on Enhancing Sexual Arousal and Reproductive Performance in Zebrafish
Abstract
:1. Introduction
2. Results
2.1. Effects on Sexual Performance
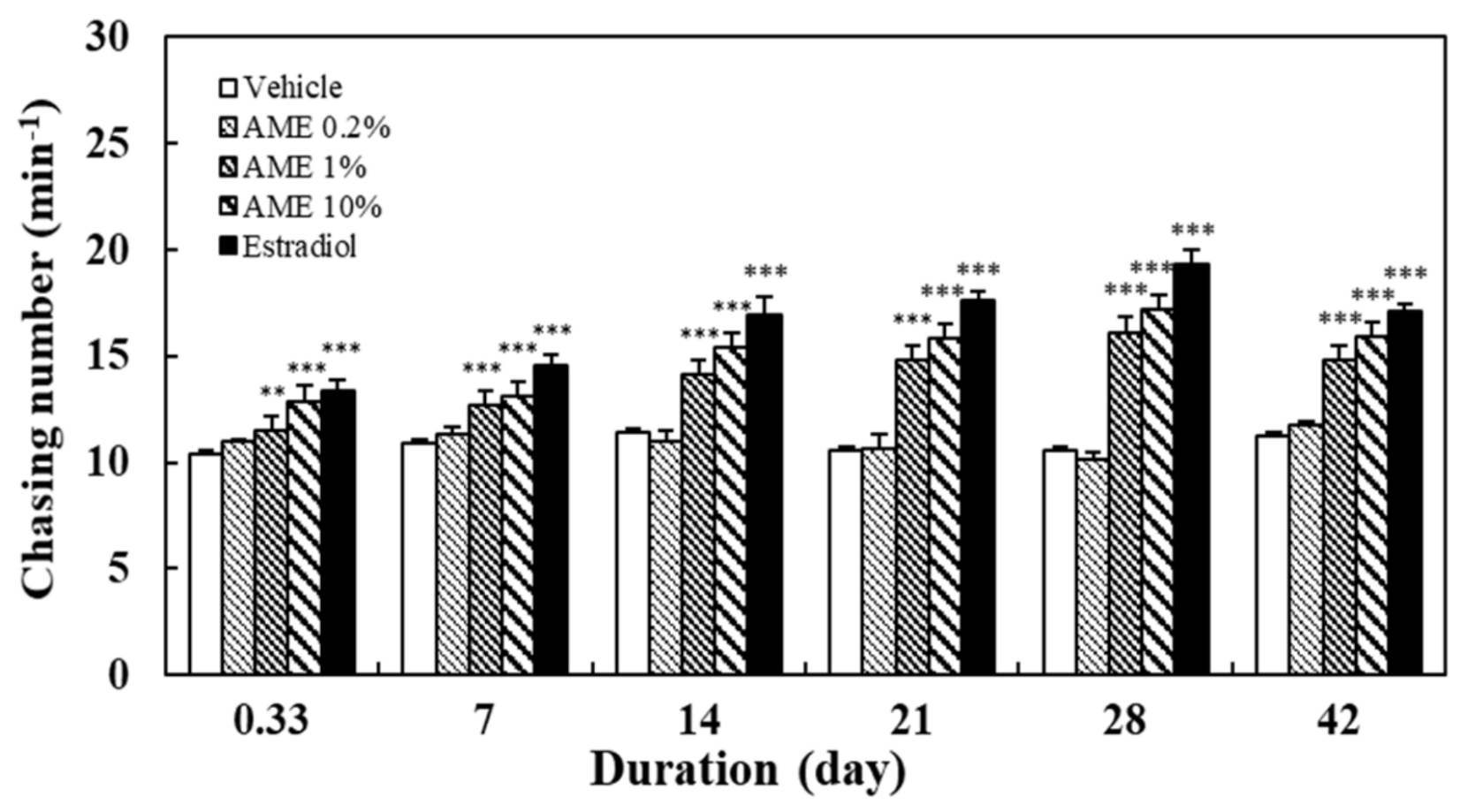
2.1.1. Chasing Behavior
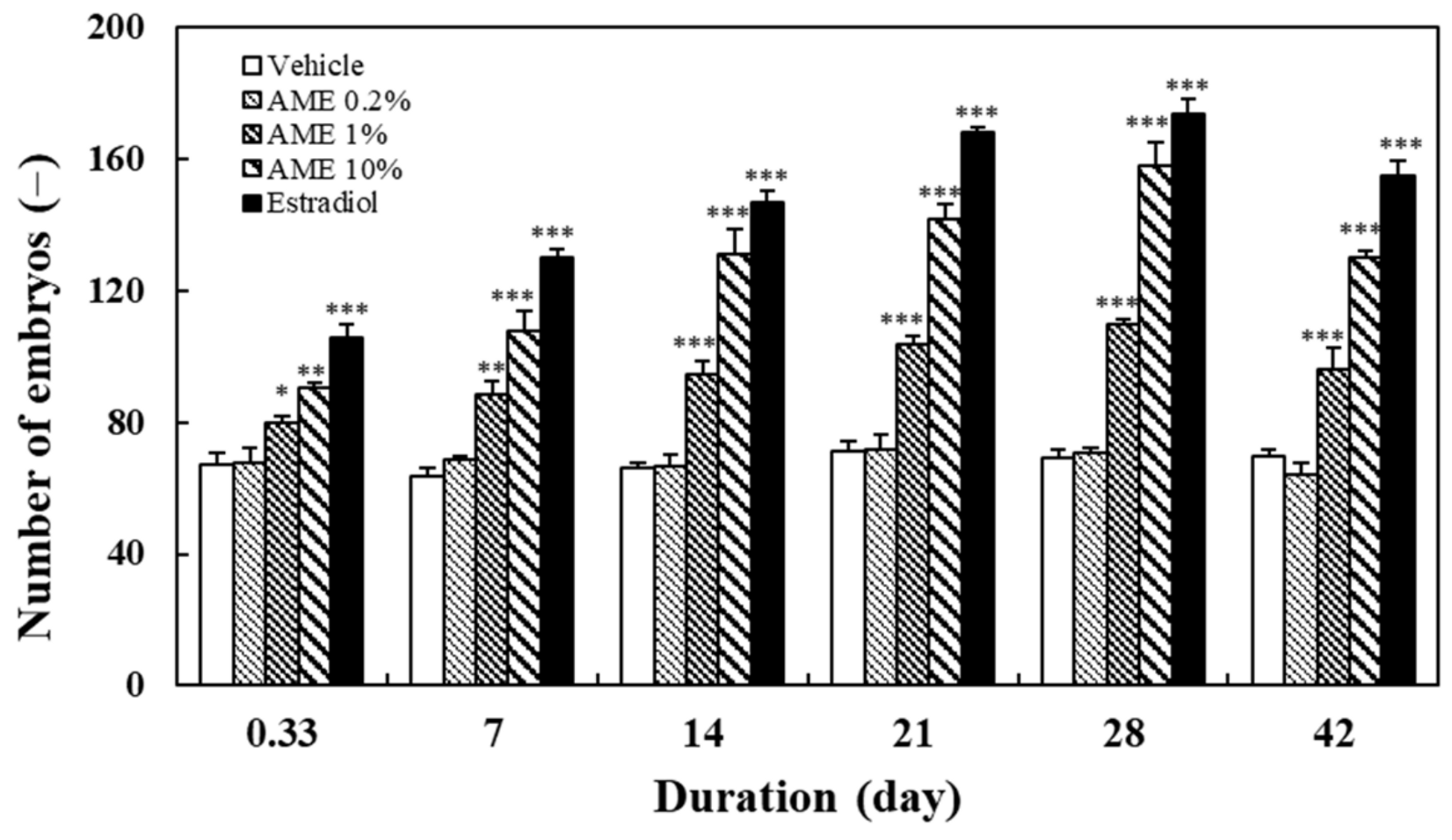
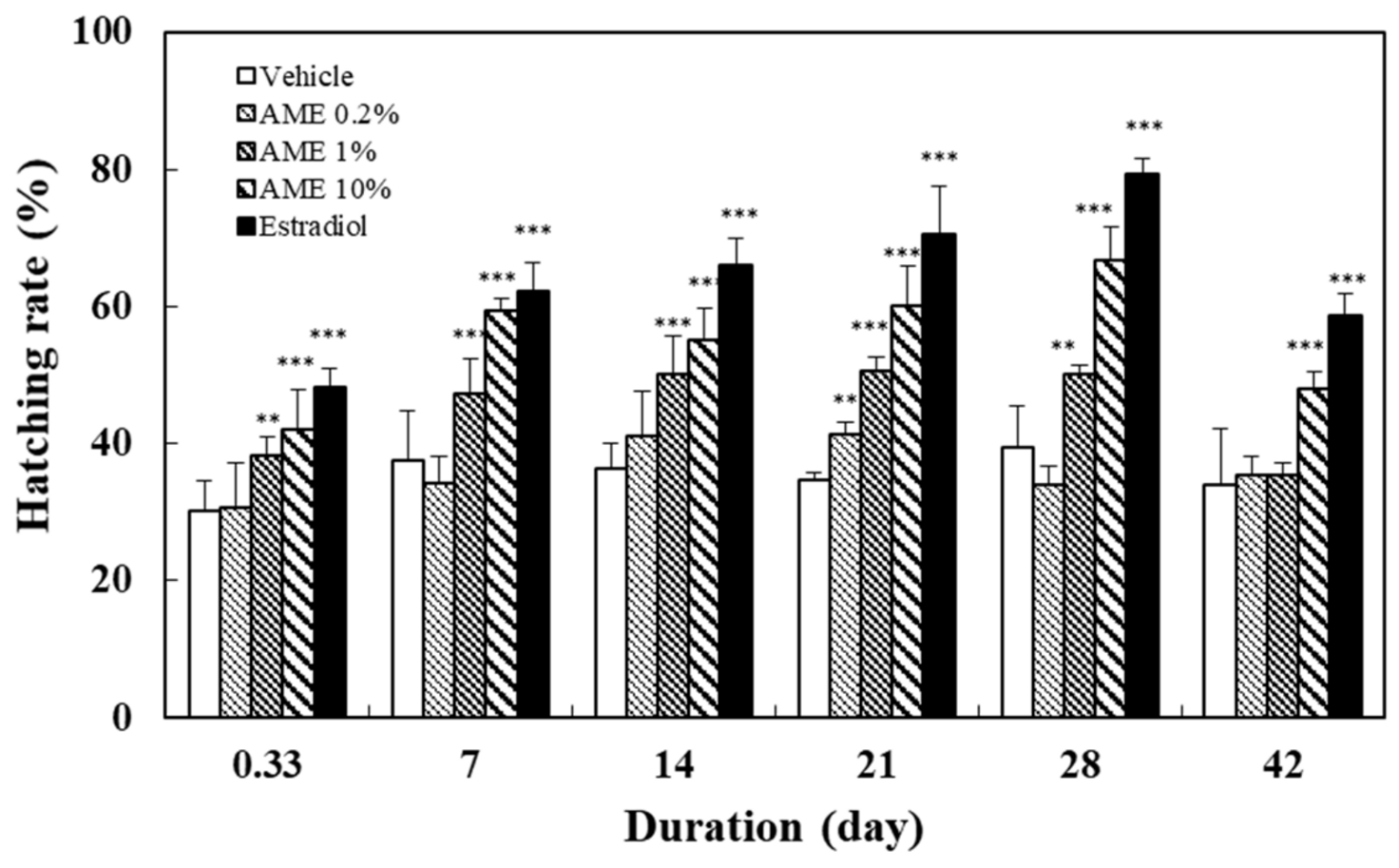
2.1.2. Spawning Outcome
2.1.3. Correlations between Mating and Spawning Outcomes
2.1.4. Body Weight
2.1.5. Lasting Effect of AME
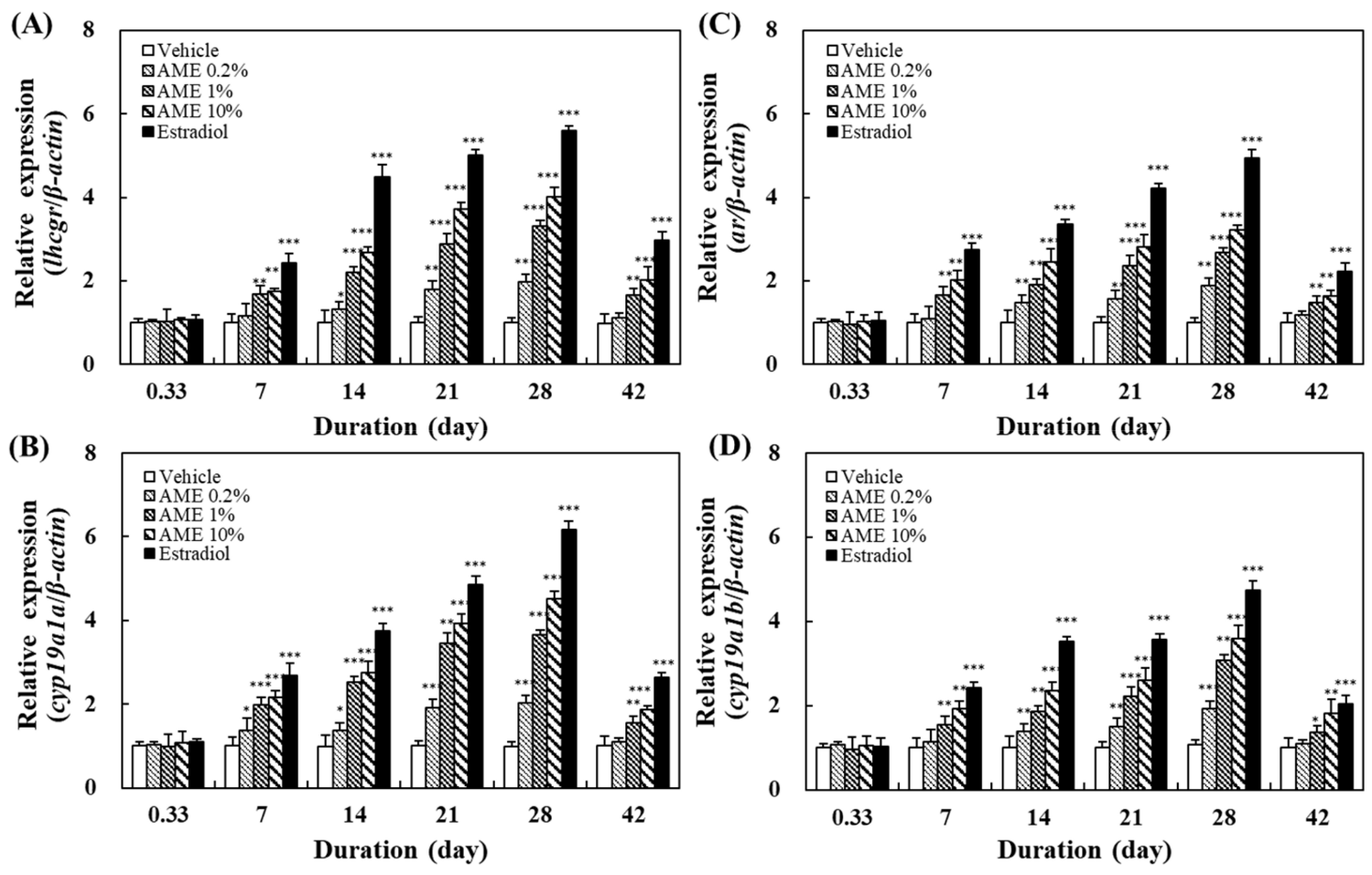
2.2. Effects on the Expression of Related Genes
2.3. Chemical Composition of AME
3. Discussion
4. Materials and Methods
4.1. Preparation of AME
4.2. Fish Husbandry
4.3. Sexual Behavior Evaluation
4.4. Gene Expression Analysis with Quantitative RT-PCR
4.5. Analysis of Chemical Compositions by HPLC
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Quinn-Nilas, C.; Milhausen, R.R.; McKay, A.; Holzapfel, S. Prevalence and predictors of sexual problems among midlife Canadian adults: Results from a national survey. J. Sex. Med. 2018, 15, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S.; Soler, E.; Ramírez, M.; Lilue, M.; Khorsandi, D.; Losa, F. Effect of a multi-ingredient based food supplement on sexual function in women with low sexual desire. BMC Women’s Health 2019, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.D., Jr.; Glasser, D.B.; Gingell, C. Sexual activity, sexual dysfunction and associated help-seeking behaviours in middle-aged and older adults in Spain: A population survey. World J. Urol. 2005, 23, 422–429. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.P.; Sharlip, I.D.; Lewis, L.; Atalla, E.; Balon, R.; Fisher, A.D.; Laumann, E.; Lee, S.W.; Segraves, R.T. Incidence and prevalence of sexual dysfunction in women and men: A consensus statement from the fourth international consultation on sexual medicine 2015. J. Sex. Med. 2016, 13, 144–152. [Google Scholar] [CrossRef]
- Shifren, J.L.; Monz, B.U.; Russo, P.A.; Segreti, A.; Johannes, C.B. Sexual problems and distress in United States women: Prevalence and correlates. Obstet. Gynecol. 2008, 112, 970–978. [Google Scholar] [CrossRef] [Green Version]
- Faubion, S.S.; Rullo, J.E. Sexual dysfunction in women: A practical approach. Am. Fam. Physician 2015, 92, 281–288. [Google Scholar]
- Santoro, N.; Torrens, J.; Crawford, S.; Allsworth, J.E.; Finkelstein, J.S.; Gold, E.B.; Korenman, S.; Lasley, W.L.; Luborsky, J.L.; McConnell, D.; et al. Correlates of circulating androgens in mid-life women: The study of women’s health across the nation. J. Clin. Endocrinol. Metab. 2005, 90, 4836–4845. [Google Scholar] [CrossRef]
- Turna, B.; Apaydin, E.; Semerci, B.; Altay, B.; Cikili, N.; Nazli, O. Women with low libido: Correlation of decreased androgen levels with female sexual function index. Int. J. Impot. Res. 2005, 17, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Davis, S.R.; McCloud, P.; Strauss, B.J.; Burger, H. Testosterone enhances estradiol’s effects on postmenopausal bone density and sexuality. Maturitas 2008, 61, 17–26. [Google Scholar] [CrossRef]
- Nathorst-Böös, J.; Wiklund, I.; Mattsson, L.A.; Sandin, K.; von Schoultz, B. Is sexual life influenced by transdermal estrogen therapy? A double blind placebo controlled study in postmenopausal women. Acta Obstet. Gynecol. Scand. 1993, 72, 656–660. [Google Scholar] [CrossRef]
- Silva, T.; Jesus, M.; Cagigal, C.; Silva, C. Food with influence in the sexual and reproductive health. Curr. Pharm. Biotechnol. 2019, 20, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Eplov, L.; Giraldi, A.; Davidsen, M.; Garde, K.; Kamper-Jørgensen, F. Sexual desire in a nationally representative Danish population. J. Sex. Med. 2007, 4, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J. The endocrinology of sexual arousal. J. Endocrinol. 2005, 186, 411–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho, E.M.; Huhtaniemi, I.T.; O’Neill, T.W.; Finn, J.D.; Pye, S.R.; Lee, D.M.; Tajar, A.; Bartfai, G.; Boonen, S.; Casanueva, F.F.; et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: Longitudinal results from the European Male Ageing Study. Eur. J. Endocrinol. 2013, 168, 445–455. [Google Scholar] [CrossRef]
- Meissner, V.H.; Schroeter, L.; Köhn, F.M.; Kron, M.; Zitzmann, M.; Arsov, C.; Imkamp, F.; Hadaschik, B.; Gschwend, J.E.; Herkommer, K. Factors associated with low sexual desire in 45-year-old men: Findings from the German Male Sex-Study. J. Sex. Med. 2019, 16, 981–991. [Google Scholar] [CrossRef]
- Carvalheira, A.; Traeen, B.; Stulhofer, A. Correlates of men’s sexual interest: A cross-cultural study. J. Sex. Med. 2014, 11, 154–164. [Google Scholar] [CrossRef]
- Graham, C.A.; Mercer, C.H.; Tanton, C.; Jones, K.G.; Johnson, A.M.; Wellings, K.; Mitchell, K.R. What factors are associated with reporting lacking interest in sex and how do these vary by gender? Findings from the third British national survey of sexual attitudes and lifestyles. BMJ Open 2017, 7, e016942. [Google Scholar] [CrossRef] [Green Version]
- Malviya, N.; Jain, S.; Gupta, V.B.; Vyas, S. Recent studies on aphrodisiac herbs for the management of male sexual dysfunction—A review. Acta Pol. Pharm. 2011, 68, 3–8. [Google Scholar]
- Gill, M.; Rai, A.; Kinra, M.; Sumalatha, S.; Rao, C.M.; Cheruku, S.P.; Devkar, R.; Kumar, N. Chemically characterised extract of Saraca asoca improves the sexual function in male Wistar rats. Andrologia 2018, 50, e13037. [Google Scholar] [CrossRef]
- Koloko, B.L.; Bushra, I.; Wankeu-Nya, M.; Njila, M.I.N.; Kenmogne, H.; Nzeubang, D.C.N.; Nzangueu, C.B.; Dimo, T.; Dongmo, A.B.; Lembe, D.M. In vivo effects of Rauvolfia vomitoria ethanolic extract on sexual performance and reproductive activity in male rats. Andrologia 2020, 52, e13414. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, G.; Sun, X.; Chen, X. Treatment of chronic kidney disease using a traditional Chinese medicine, Flos Abelmoschus Manihot (Linnaeus) Medicus (Malvaceae). Clin. Exp. Pharmacol. Physiol. 2016, 43, 145–148. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; He, W.; Xia, P.; Sun, W.; Shi, M.; Zhou, Y.; Zhu, W.; Zhang, L.; Liu, B.; Zhu, J.; et al. Total extracts of Abelmoschus manihot L. attenuates adriamycin-induced renal tubule injury via suppression of ROS-ERK1/2-mediated NLRP3 inflammasome activation. Front. Pharmacol. 2019, 10, 567. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Bian, Y.; Zhu, Y.; Guo, M.; Yang, Y.; Guo, J.; Gu, C.; Duan, J.A. HUANGKUISIWUFANG inhibits pyruvate dehydrogenase to improve glomerular injury in anti-Thy1 nephritis model. J. Ethnopharmacol. 2020, 253, 112682. [Google Scholar] [CrossRef]
- Liu, S.; Ye, L.; Tao, J.; Ge, C.; Huang, L.; Yu, J. Total flavones of Abelmoschus manihot improve diabetic nephropathy by inhibiting the iRhom2/TACE signalling pathway activity in rats. Pharm. Biol. 2017, 56, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Hu, W.; Han, W.B.; Liu, Y.L.; Tu, Y.; Yang, H.M.; Fang, Q.J.; Zhou, M.Y.; Wan, Z.Y.; Tang, R.M.; et al. Inhibition of Akt/mTOR/p70S6K signaling activity with Huangkui capsule alleviates the early glomerular pathological changes in diabetic nephropathy. Front. Pharmacol. 2018, 9, 443. [Google Scholar] [CrossRef]
- Shi, L.; Feng, L.; Zhang, M.; Li, X.; Yang, Y.; Zhang, Y.; Ni, Q. Abelmoschus manihot for diabetic nephropathy: A systematic review and meta-analysis. Evid. Based Complement. Altern. Med. 2019, 2019, 9679234. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.L.; Yang, X.B.; Huang, Z.M.; Liu, H.Z.; Wu, G.X. In vivo and in vitro antiviral activity of hyperoside extracted from Abelmoschus manihot (L) medik. Acta Pharmacol. Sin. 2007, 28, 404–409. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, Q.H.; Hao, J.L.; Zhou, L.L. Protective effect of total flavones of Abelmoschus manihot L. Medic against poststroke depression injury in mice and its action mechanism. Anat. Rec. 2009, 292, 412–422. [Google Scholar] [CrossRef]
- Guo, J.; Xue, C.; Duan, J.A.; Qian, D.; Tang, Y.; You, Y. Anticonvulsant, antidepressant-like activity of Abelmoschus manihot ethanol extract and its potential active components in vivo. Phytomedicine 2011, 18, 1250–1254. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Wang, M. Extraction of flavonoids from the flowers of Abelmoschus manihot (L.) Medic by modified supercritical CO₂ extraction and determination of antioxidant and anti-adipogenic activity. Molecules 2016, 21, 810. [Google Scholar]
- Qiu, Y.; Ai, P.F.; Song, J.J.; Liu, C.; Li, Z.W. Total flavonoid extract from Abelmoschus manihot (L.) Medic flowers attenuates d-galactose-induced oxidative stress in mouse liver through the Nrf2 pathway. J. Med. Food 2017, 20, 557–567. [Google Scholar] [CrossRef]
- Ai, G.; Liu, Q.; Hua, W.; Huang, Z.; Wang, D. Hepatoprotective evaluation of the total flavonoids extracted from flowers of Abelmoschus manihot (L.) Medic: In vitro and in vivo studies. J. Ethnopharmacol. 2013, 146, 794–802. [Google Scholar] [CrossRef]
- Tang, L.; Pan, W.; Zhu, G.; Liu, Z.; Lv, D.; Jiang, M. Total flavones of Abelmoschus manihot enhances angiogenic ability both in vitro and in vivo. Oncotarget 2017, 8, 69768–69778. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.S.; Tang, L.Y.; Lv, D.L.; Jiang, M. Total flavones of Abelmoschus manihot exhibits pro-angiogenic activity by activating the VEGF-A/VEGFR2-PI3K/Akt signaling axis. Am. J. Chin. Med. 2018, 46, 567–583. [Google Scholar] [CrossRef]
- An, Y.; Zhang, Y.; Li, C.; Qian, Q.; He, W.; Wang, T. Inhibitory effects of flavonoids from Abelmoschus manihot flowers on triglyceride accumulation in 3T3-L1 adipocytes. Fitoterapia 2011, 82, 595–600. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, C.; Han, Q.; Chen, Y.; Guo, J.; Wu, Q.; Zhu, B.; Shan, J.; Shi, L. Flos Abelmoschus manihot extract attenuates DSS-induced colitis by regulating gut microbiota and Th17/Treg balance. Biomed. Pharmacother. 2019, 117, 109162. [Google Scholar] [CrossRef]
- Wen, J.Y.; Chen, Z.W. Protective effect of pharmacological preconditioning of total flavones of Abelmoschus manihot on cerebral ischemic reperfusion injury in rats. Am. J. Chin. Med. 2007, 35, 653–661. [Google Scholar] [CrossRef] [Green Version]
- Lv, D.; Cheng, X.; Tang, L.; Jiang, M. The cardioprotective effect of total flavonoids on myocardial ischemia/reperfusion in rats. Biomed. Pharmacother. 2017, 88, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, P.; Liu, Y.; Shu, Y.; Zhou, J.Y.; Jiang, F.; Chen, T.; Yang, B.L.; Chen, Y.G. Total flavone of Abelmoschus manihot ameliorates Crohn’s disease by regulating the NF-κB and MAPK signaling pathways. Int. J. Mol. Med. 2019, 44, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Puel, C.; Mathey, J.; Kati-Coulibaly, S.; Davicco, M.J.; Lebecque, P.; Chanteranne, B.; Horcajada, M.N.; Coxam, V. Preventive effect of Abelmoschus manihot (L.) Medik. on bone loss in the ovariectomised rats. J. Ethnopharmacol. 2005, 99, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Qian, J.; Li, Z.; Gong, A.; Zhong, S.; Qiao, L.; Qian, S.; Zhang, Y.; Dou, R.; Li, R.; et al. Bioactive compounds from Abelmoschus manihot L. alleviate the progression of multiple myeloma in mouse model and improve bone marrow microenvironment. OncoTargets Ther. 2020, 13, 959–973. [Google Scholar] [CrossRef] [Green Version]
- Hoo, J.Y.; Kumari, Y.; Shaikh, M.F.; Hue, S.M.; Goh, B.H. Zebrafish: A versatile animal model for fertility research. BioMed Res. Int. 2016, 2016, 9732780. [Google Scholar] [CrossRef] [Green Version]
- Zon, L.I.; Peterson, R.T. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef]
- Pitchai, A.; Rajaretinam, R.K.; Freeman, J.L. Zebrafish as an emerging model for bioassay-guided natural product drug discovery for neurological disorders. Medicines 2019, 6, 61. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.; Zhou, R.; Wang, X. Comparison of enhanced male mice sexual function among three medicinal materials. Andrologia 2018, 50, e13087. [Google Scholar] [CrossRef]
- Li, D.; Ren, J.; He, L.; Sun, J.; Liu, P.; Li, Y. Combined effects of oligopeptides isolated from Panax ginseng C.A. Meyer and Ostrea gigas Thunberg on sexual function in male mice. Int. J. Environ. Res. Public Health 2021, 18, 2349. [Google Scholar] [CrossRef]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. 2008, 83, 13–34. [Google Scholar] [CrossRef]
- Norton, W.; Bally-Cuif, L. Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci. 2010, 11, 90. [Google Scholar] [CrossRef] [Green Version]
- Yabuki, Y.; Koide, T.; Miyasaka, N.; Wakisaka, N.; Masuda, M.; Ohkura, M.; Nakai, J.; Tsuge, K.; Tsuchiya, S.; Sugimoto, Y.; et al. Olfactory receptor for prostaglandin F2α mediates male fish courtship behavior. Nat. Neurosci. 2016, 19, 897–904. [Google Scholar] [CrossRef]
- Li, X.; Guo, J.Y.; Li, X.; Zhou, H.J.; Zhang, S.H.; Liu, X.D.; Chen, D.Y.; Fang, Y.C.; Feng, X.Z. Behavioural effect of low-dose BPA on male zebrafish: Tuning of male mating competition and female mating preference during courtship process. Chemosphere 2017, 169, 40–52. [Google Scholar] [CrossRef]
- Reolon, G.K.; de Melo, G.M.; da Rosa, J.G.D.S.; Barcellos, L.J.G.; Bonan, C.D. Sex and the housing: Effects on behavior, cortisol levels and weight in zebrafish. Behav. Brain Res. 2018, 336, 85–92. [Google Scholar] [CrossRef]
- Luan, F.; Wu, Q.; Yang, Y.; Lv, H.; Liu, D.; Gan, Z.; Zeng, N. Traditional uses, chemical constituents, biological properties, clinical settings, and toxicities of Abelmoschus manihot L.: A comprehensive review. Front. Pharmacol. 2020, 11, 1068. [Google Scholar] [CrossRef]
- Li, N.; Tang, H.; Wu, L.; Ge, H.; Wang, Y.; Yu, H.; Zhang, X.; Ma, J.; Gu, H.F. Chemical constituents, clinical efficacy and molecular mechanisms of the ethanol extract of Abelmoschus manihot flowers in treatment of kidney diseases. Phytother. Res. 2021, 35, 198–206. [Google Scholar] [CrossRef]
- Hosseini, B.; Eslamian, G. Association of dietary factors with male and female infertility: Review of current evidence. Thrita 2014, 3, e20953. [Google Scholar] [CrossRef]
- Holbech, H.; Schröder, K.D.; Nielsen, M.L.; Brande-Lavridsen, N.; Holbech, B.F.; Bjerregaard, P. Estrogenic effect of the phytoestrogen biochanin A in zebrafish, Danio rerio, and brown trout, Salmo trutta. Aquat. Toxicol. 2013, 144–145, 19–25. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, G.; Peng, L.; Ni, W.; Wang, X.; Zhang, J.; Wu, K. Effect of 2,2’,4,4’-tetrabromodiphenyl ether (BDE-47) on sexual behaviors and reproductive function in male zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2015, 111, 102–108. [Google Scholar] [CrossRef]
- Pradhan, A.; Olsson, P.E. Zebrafish sexual behavior: Role of sex steroid hormones and prostaglandins. Behav. Brain Funct. 2015, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Shu, T.; Zhai, G.; Pradhan, A.; Olsson, P.E.; Yin, Z. Zebrafish cyp17a1 knockout reveals that androgen-mediated signaling is important for male brain sex differentiation. Gen. Comp. Endocrinol. 2020, 295, 113490. [Google Scholar] [CrossRef] [PubMed]
- Bassi, I.; André, V.; Marelli, F.; Marelli, F.; Vezzoli, V.; Merlo, G.R.; Cariboni, A.; Persani, L.; Gothilf, Y.; Bonomi, M. The zebrafish: An emerging animal model for investigating the hypothalamic regulation of reproduction. Minerva Endocrinol. 2016, 41, 250–265. [Google Scholar] [PubMed]
- Li, L.; Wojtowicz, L.J.; Malin, J.H.; Huang, T.; Lee, E.B.; Chen, Z. GnRH-mediated olfactory and visual inputs promote mating-like behaviors in male zebrafish. PLoS ONE 2017, 12, e0174143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Verdugo, C.; Sun, G.J.; Fawcett, C.H.; Zhu, P.; Fishman, M.C. Mating suppresses alarm response in zebrafish. Curr. Biol. 2019, 29, 2541–2546. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.D. The role of androgens in male gender role behavior. Endocr. Rev. 1999, 20, 726–737. [Google Scholar] [CrossRef]
- Matsumoto, T.; Sakari, M.; Okada, M.; Yokoyama, A.; Takahashi, S.; Kouzmenko, A.; Kato, S. The androgen receptor in health and disease. Annu. Rev. Physiol. 2013, 75, 201–224. [Google Scholar] [CrossRef]
- Narayan, P. Genetic models for the study of luteinizing hormone receptor function. Front. Endocrinol. 2015, 6, 152. [Google Scholar] [CrossRef] [Green Version]
- Qiao, J.; Han, B. Diseases caused by mutations in luteinizing hormone/chorionic gonadotropin receptor. Prog. Mol. Biol. Transl. Sci. 2019, 161, 69–89. [Google Scholar]
- Schlinger, B.A.; Greco, C.; Bass, A.H. Aromatase activity in hindbrain vocal control region of a teleost fish: Divergence among males with alternative reproductive tactics. Proc. R. Soc. Lond. 1999, 266, 131–136. [Google Scholar] [CrossRef]
- Gonzalez, A.; Piferrer, F. Aromatase activity in the European sea bass (Dicentrarchus labrax L.) brain. Distribution and changes in relation to age, sex, and the annual reproductive cycle. Gen. Comp. Endocrinol. 2003, 132, 223–230. [Google Scholar] [CrossRef]
- Gupta, S.; Moulik, S.R.; Pal, P.; Majumder, S.; Das, S.; Guha, P.; Juin, S.K.; Panigrahi, A.K.; Mukherjee, D. Estrogen-regulated expression of cyp19a1a and cyp19a1b genes in swim-up fry of Labeo rohita. Gen. Comp. Endocrinol. 2017, 251, 85–93. [Google Scholar] [CrossRef]
- Risalde, M.A.; Molina, A.M.; Lora, A.J.; Ayala, N.; Gómez-Villamandos, J.C.; Moyano, M.R. Immunohistochemical expression of aromatase cyp19a1a and cyp19a1b in the ovary and brain of zebrafish (Danio rerio) exposed to different concentrations of bisphenol A. Aquat. Toxicol. 2021, 237, 105876. [Google Scholar] [CrossRef]
- Lai, X.; Liang, H.; Zhao, Y.; Wang, B. Simultaneous determination of seven active flavonols in the flowers of Abelmoschus manihot by HPLC. J. Chromatogr. Sci. 2009, 47, 206–210. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.M.; Lu, Y.W.; Shang, E.X.; Li, T.; Liu, Y.; Duan, J.A.; Qian, D.W.; Tang, Y. Metabolite identification strategy of non-targeted metabolomics and its application for the identification of components in Chinese multicomponent medicine Abelmoschus manihot L. Phytomedicine 2015, 22, 579–587. [Google Scholar] [CrossRef]
- Yamagata, K.; Tagami, M.; Yamori, Y. Dietary polyphenols regulate endothelial function and prevent cardiovascular disease. Nutrition 2015, 31, 28–37. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and quercetin: Promising flavonoids with chemopreventive potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef] [Green Version]
- Calis, Z.; Mogulkoc, R.; Baltaci, A.K. The roles of flavonols/flavonoids in neurodegeneration and neuroinflammation. Mini Rev. Med. Chem. 2020, 20, 1475–1488. [Google Scholar] [CrossRef]
- Nagai, M.; Conney, A.H.; Zhu, B.T. Strong inhibitory effects of common tea catechins and bioflavonoids on the O-methylation of catechol estrogens catalyzed by human liver cytosolic catechol-O-methyltransferase. Drug Metab. Dispos. 2004, 32, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Wang, C.Y.; Lambert, J.D.; Ai, N.; Welsh, W.J.; Yang, C.S. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: Structure–activity relationship and molecular-modeling studies. Biochem. Pharmacol. 2005, 69, 1523–1531. [Google Scholar] [CrossRef]
- Hou, W.C.; Lin, R.D.; Chen, C.T.; Lee, M.H. Monoamine oxidase B (MAO-B) inhibition by active principles from Uncaria rhynchophylla. J. Ethnopharmacol. 2005, 100, 216–220. [Google Scholar] [CrossRef]
- Rashid, K.; Wachira, F.N.; Nyabuga, J.N.; Wanyonyi, B.; Murilla, G.; Isaac, A.O. Kenyan purple tea anthocyanins ability to cross the blood brain barrier and reinforce brain antioxidant capacity in mice. Nutr. Neurosci. 2014, 17, 178–185. [Google Scholar] [CrossRef]
- Cheruku, S.P.; Ramalingayya, G.V.; Chamallamudi, M.R.; Biswas, S.; Nandakumar, K.; Nampoothiri, M.; Gourishetti, K.; Kumar, N. Catechin ameliorates doxorubicin-induced neuronal cytotoxicity in in vitro and episodic memory deficit in in vivo in Wistar rats. Cytotechnology 2018, 70, 245–259. [Google Scholar] [CrossRef]
- Cocuzza, M.; Sikka, S.C.; Athayde, K.S.; Agarwal, A. Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: An evidence-based analysis. Int. Braz. J. Urol. 2007, 33, 603–621. [Google Scholar] [CrossRef]
- Tsai, Y.D.; Hsu, H.F.; Chen, Z.H.; Wang, Y.T.; Huang, S.H.; Chen, H.J.; Wang, P.W.; Wang, S.W.; Chang, C.C.; Houng, J.Y. Antioxidant, anti-inflammatory, and anti-proliferative activities of extracts from different parts of farmed and wild Glossogyne tenuifolia. Ind. Crop. Prod. 2014, 57, 98–105. [Google Scholar] [CrossRef]

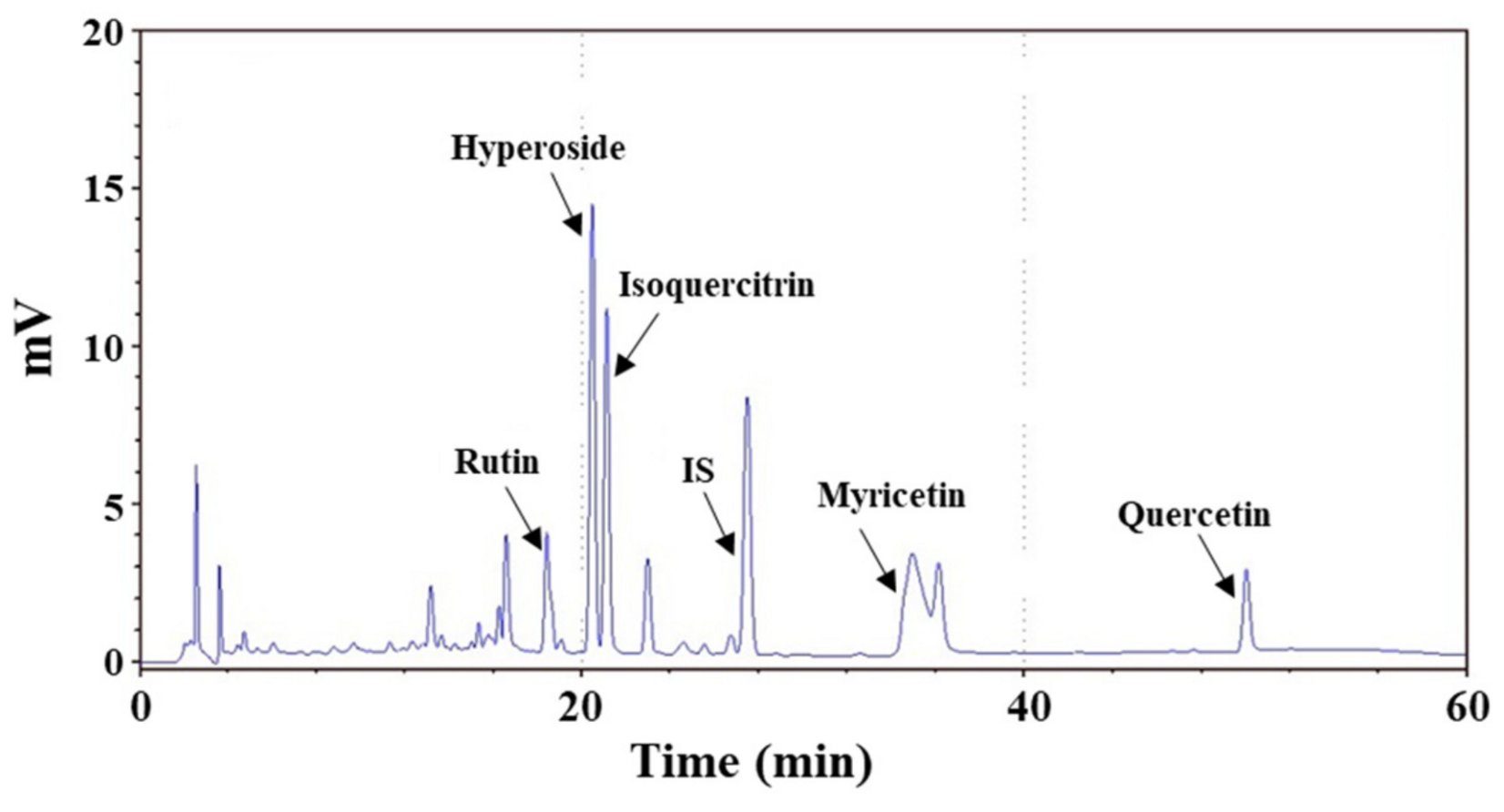
| Chemical Composition | Concentration (mg/g Extract) |
|---|---|
| Rutin | 10.0 ± 0.4 |
| Hyperoside | 37.8 ± 2.1 |
| Isoquercitrin | 20.6 ± 1.7 |
| Myricetin | 36.9 ± 1.4 |
| Quercetin | 2.6 ± 0.1 |
| Total polyphenols content | 120.8 ± 0.8 |
| Total flavonoids content | 57.0 ± 1.0 |
| Primer | Sequence |
|---|---|
| β-Actin | 5′-CACCATGAAGATCAAGATCA-3′ |
| 5′-TTTATTCAAGATGGAGCCACCGATCC-3′ | |
| lhcgr | 5′-GGCTGACCTGTCTGCAATCT-3′ |
| 5′-GAAATAGGCGCCATGCACAG-3′ | |
| ar | 5′-CGTAGGATGCACGTCTCCAG-3′ |
| 5′-AGTCCATCAGTCGTGTCAGC-3′ | |
| cyp19a1a | 5′-GGCACACGCAGAGAAACTTG-3′ |
| 5′-GCTGGAAGAAACGACTCGGA-3′ | |
| cyp19a1b | 5′-TTTCAGTACCTGGCAGACGG-3′ |
| 5′-GTCAGCCGACTCTACGTCTC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-C.; Houng, J.-Y.; Peng, W.-H.; Yeh, T.-W.; Wang, Y.-Y.; Chen, Y.-L.; Chang, T.-H.; Hung, W.-C.; Yu, T.-H. Effects of Abelmoschus manihot Flower Extract on Enhancing Sexual Arousal and Reproductive Performance in Zebrafish. Molecules 2022, 27, 2218. https://doi.org/10.3390/molecules27072218
Chang C-C, Houng J-Y, Peng W-H, Yeh T-W, Wang Y-Y, Chen Y-L, Chang T-H, Hung W-C, Yu T-H. Effects of Abelmoschus manihot Flower Extract on Enhancing Sexual Arousal and Reproductive Performance in Zebrafish. Molecules. 2022; 27(7):2218. https://doi.org/10.3390/molecules27072218
Chicago/Turabian StyleChang, Chi-Chang, Jer-Yiing Houng, Wei-Hao Peng, Tien-Wei Yeh, Yun-Ya Wang, Ya-Ling Chen, Tzu-Hsien Chang, Wei-Chin Hung, and Teng-Hung Yu. 2022. "Effects of Abelmoschus manihot Flower Extract on Enhancing Sexual Arousal and Reproductive Performance in Zebrafish" Molecules 27, no. 7: 2218. https://doi.org/10.3390/molecules27072218
APA StyleChang, C.-C., Houng, J.-Y., Peng, W.-H., Yeh, T.-W., Wang, Y.-Y., Chen, Y.-L., Chang, T.-H., Hung, W.-C., & Yu, T.-H. (2022). Effects of Abelmoschus manihot Flower Extract on Enhancing Sexual Arousal and Reproductive Performance in Zebrafish. Molecules, 27(7), 2218. https://doi.org/10.3390/molecules27072218







