Secondary Metabolites from Hericium erinaceus and Their Anti-Inflammatory Activities
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Extraction and Isolation
3.4. Anti-Inflammatory Activity Assay
3.4.1. Cell Culture
3.4.2. Cell Viability
3.4.3. Pro-Inflammatory Cytokines (TNF-α and IL-6) Assay
3.4.4. Nitric Oxide (NO) Assay
3.4.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Friedman, M. Chemistry, nutrition, and health-promoting properties of Hericium erinaceus (lion’s mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef]
- Mizuno, T. Bioactive substances in Hericium erinaceus (Bull.: Fr.) Pers. and its medicinal utilization. Int. J. Med. Mushrooms 1999, 1, 105–119. [Google Scholar] [CrossRef]
- Nagano, M.; Shimizu, K.; Kondo, R.; Hayashi, C.; Sato, D.; Kitagawa, K.; Ohnuki, K. Reduction of depression and anxiety by 4 weeks Hercium erinaceus intake. Biomed. Res. 2010, 31, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Mori, K.; Inatomi, S.; Ouchi, K.; Azumi, Y.; Tuchida, T. Improving effects of the mushroom Yamabushitake (Hercium ericaceus) on mild cognitive impairment: A double-blind placebo-controlled clinical trial. Phytother. Res. 2009, 23, 367–372. [Google Scholar] [CrossRef]
- Tang, H.Y.; Yin, X.; Zhang, C.C.; Jia, Q.; Gao, J.M. Structure diversity, synthesis, and biological activity of cyathane diterpenoids in higher fungi. Curr. Med. Chem. 2015, 22, 2375–2391. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y.N.; Zhou, W.; Shim, S.H.; Kim, Y.H. Erinacene D, a new aromatic compound from Hericium erinaceum. J. Antibiot. 2014, 67, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Takahashi, T.; Horibe, I.; Ishikawa, R. Two new aromatic compounds and a new D-arabinitol ester from the mushroom Hericium erinaceum. Tetrahedron 2012, 68, 2007–2010. [Google Scholar] [CrossRef]
- Chen, B.; Han, J.; Bao, L.; Wang, W.; Ma, K.; Liu, H. Identification and α-glucosidase inhibitory activity of meroterpenoids from Hericium erinaceus. Planta Med. 2020, 86, 571–578. [Google Scholar] [CrossRef]
- Li, W.; Zhou, W.; Kim, E.J.; Shim, S.H.; Kang, H.K.; Kim, Y.H. Isolation and identification of aromatic compounds in Lion’s Mane mushroom and their anticancer activities. Food Chem. 2015, 170, 336–342. [Google Scholar] [CrossRef]
- Ma, B.J.; Yu, H.Y.; Shen, J.W.; Ruan, Y.; Zhao, X.; Zhou, H.; Wu, T.T. Cytotoxic aromatic compounds from Hericium erinaceus. J. Antibiot. 2010, 63, 713–715. [Google Scholar] [CrossRef]
- Li, W.; Zhou, W.; Song, S.B.; Shim, S.H.; Kim, Y.H. Sterol fatty acid esters from the mushroom Hericium erinaceum and their PPAR transactivational effects. J. Nat. Prod. 2014, 77, 2611–2618. [Google Scholar] [CrossRef]
- Li, W.; Zhou, W.; Cha, J.Y.; Kwon, S.U.; Baek, K.H.; Shim, S.H.; Lee, Y.M.; Kim, Y.H. Sterols from Hericium erinaceum and their inhibition of TNF-α and NO production in lipopolysaccharide-indeced RAW 264.7 cells. Phytochemistry 2015, 115, 231–238. [Google Scholar] [CrossRef]
- Hou, Y.; Ding, X.; Hou, W. Composition and antioxidant activity of water-soluble oligosaccharide from Hericium erinaceus. Mol. Med. Rep. 2015, 11, 3794–3799. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.Z.; Wu, D.; Chen, X.; Zhou, S.; Liu, Y.; Yang, Y.; Cui, F. Chemical compositions and macrophage activation of polysaccharides from Lion’s Mane culinary-medicinal mushroom Hericium erinaceus (higher Basidiomycetes) in different maturation stages. Int. J. Med. Mushrooms 2015, 17, 443–452. [Google Scholar] [CrossRef]
- Cui, F.J.; Li, Y.H.; Zan, X.Y.; Yang, Y.; Sun, W.J.; Qian, J.Y.; Zhou, Q.; Yu, S.L. Purification and partial characterization of a novel hemagglutinating glycoprotein from the cultured mycelia of Hericium erinaceus. Process Biochem. 2014, 49, 1362–1369. [Google Scholar] [CrossRef]
- Yu, R.; Sun, M.; Meng, Z.; Zhao, J.; Qin, T.; Ren, Z. Immunomodulatory effects of polysaccharides enzymatic hydrolysis from Hericium erinaceus on the MODE-K/DCs co-culture model. Int. J. Biol. Macromol. 2021, 187, 272–280. [Google Scholar] [CrossRef]
- Sheu, S.C.; Lyu, Y.; Lee, M.S.; Cheng, J.H. Immunomodulatory effects of polysaccharides isolated from Hericium erinaceus on dendritic cells. Process Biochem. 2013, 48, 1402–1408. [Google Scholar] [CrossRef]
- Kawagishi, H.; Ando, M.; Sakamoto, H.; Yoshida, S.; Ojima, F.; Ishiguro, Y.; Ukai, N.; Furukawa, S. Hericenones C, D and E, stimulators of nerve growth factor (NGF) synthesis, from the mushroom Hericium erinaceum. Tetrahedron Lett. 1991, 32, 4561–4564. [Google Scholar] [CrossRef]
- Ma, B.J.; Shen, J.W.; Yu, H.Y.; Ruan, Y.; Wu, T.T.; Zhao, X. Hericenones and erinacines: Stimulators of nerve growth factor (NGF) biosynthesis in Hericium erinaceus. Mycology 2010, 1, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Tu, X.; Tang, L.; Xie, G.; Deng, K.; Xie, L. Chemical composition of aromas and lipophilic extracts from black morel (Morchella importuna) grown in China. Mycobiology 2021, 49, 78–85. [Google Scholar] [CrossRef]
- Tu, X.M.; Xie, G.B.; Tang, L.; Deng, K.J.; Xie, L.Y. Chemical composition of Morchella sextelata (Pezizales, Ascomycota). Mycosystema 2021, 40, 2134–2147. [Google Scholar]
- Deng, K.; Lan, X.; Fang, Q.; Li, M.; Xie, G.; Xie, L. Untargeted metabolomics reveals alterations in the primary metabolites and potential pathways in the vegetative growth of Morchella sextelata. Front. Mol. Biosci. 2021, 8, 632341. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Lan, X.; Chen, Y.; Wang, T.; Li, M.; Xu, Y.; Cao, X.; Xie, G.; Xie, L. Integration of transcriptomics and metabolomics for understanding the different vegetative growth in Morchella sextelata. Front. Genet. 2022, 12, 829379. [Google Scholar] [CrossRef]
- Takaishi, Y.; Uda, M.; Ohashi, T.; Nakano, K.; Murakami, K.; Tomimatsu, T. Glycosides of ergosterol derivatives from Hericum erinacens. Phytochemistry 1991, 30, 4117–4120. [Google Scholar] [CrossRef]
- Lin, C.N.; Tome, W.P. Novel cytotoxic principles of Formosan Ganoderma lucidum. J. Nat. Prod. 1991, 54, 998–1002. [Google Scholar] [CrossRef]
- Huh, S.; Kim, Y.S.; Jung, E.; Lim, J.; Jung, K.S.; Kim, M.O.; Lee, J.; Park, D. Melanogenesis inhibitory effect of fatty acid alkyl esters isolated from Oxalis triangularis. Biol. Pharm. Bull. 2010, 33, 1242–1245. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Kim, H.M.; Ryu, B.; Lee, W.S.; Shin, J.S.; Lee, K.T.; Jang, D.S. Constituents of PG201 (Layla®), a multi-component phytopharmaceutical, with inhibitory activity on LPS-induced nitric oxide and prostaglandin E2 productions in macrophages. Arch. Pharm. Res. 2016, 39, 231–239. [Google Scholar] [CrossRef]
- Gan, K.H.; Kuo, S.H.; Lin, C.N. Steroidal constituents of Ganoderma applanatum and Ganoderma neo-japonicum. J. Nat. Prod. 1998, 61, 1421–1422. [Google Scholar] [CrossRef]
- Kawagishi, H.; Ando, M.; Shinba, K.; Sakamoto, H.; Yoshida, S.; Ojima, F.; Ishiguro, Y.; Ukai, N.; Furukawa, S. Chromans, hericenones F, G and H from the mushroom Hericium erinaceum. Phytochemistry 1993, 32, 175–178. [Google Scholar] [CrossRef]
- Shirane, N.; Takenaka, H.; Ueda, K.; Hashimoto, Y.; Katoh, K.; Ishii, H. Sterol analysis of DMI-resistant and -sensitive strains of Venturia inaequalis. Phytochemistry 1996, 41, 1301–1308. [Google Scholar] [CrossRef]
- Hybelbauerová, S.; Sejbal, J.; Dračínský, M.; Hahnová, A.; Koutek, B. Chemical constituents of Stereum subtomentosum and two other birch-associated Basidiomycetes: An interspecies comparative study. Chem. Biodivers. 2008, 5, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Hong, K.; Zhang, X.; Liu, H.W.; Wang, N.L.; Zhuang, L.; Yao, X.S. New steryl esters of fatty acids from the mangrove fungus Aspergillus awamori. Helv. Chim. Acta 2007, 90, 1165–1178. [Google Scholar] [CrossRef]
- Bazzoni, F.; Beutler, B. Seminars in medicine of the Beth Israel hospital, Boston: The tumor necrosis factor ligand and receptor families. N. Engl. J. Med. 1996, 334, 1717–1725. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; AI-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Perrone, A.; Plaza, A.; Ercolino, S.F.; Hamed, A.I.; Parente, L.; Pizza, C.; Piacente, S. 14,15-secopregnane derivatives from the leaves of Solenostemma argel. J. Nat. Prod. 2006, 69, 50–54. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Oh, Y.C.; Cho, W.K.; Lee, B.; Ma, J.Y. Anti-inflammatory effects of melandrii herba ethanol extract via inhibition of NF-κB and MAPK signaling pathways and induction of HO-1 in RAW 264.7 cells and mouse primary macrophages. Molecules 2016, 21, 818. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yang, X.; Zhang, Y.; Chen, R.; Cui, Y.; Wang, Q. Anti-inflammatory chalcone-isoflavone dimers and chalcone dimers from Caragana jubata. J. Nat. Prod. 2019, 82, 2761–2767. [Google Scholar] [CrossRef]
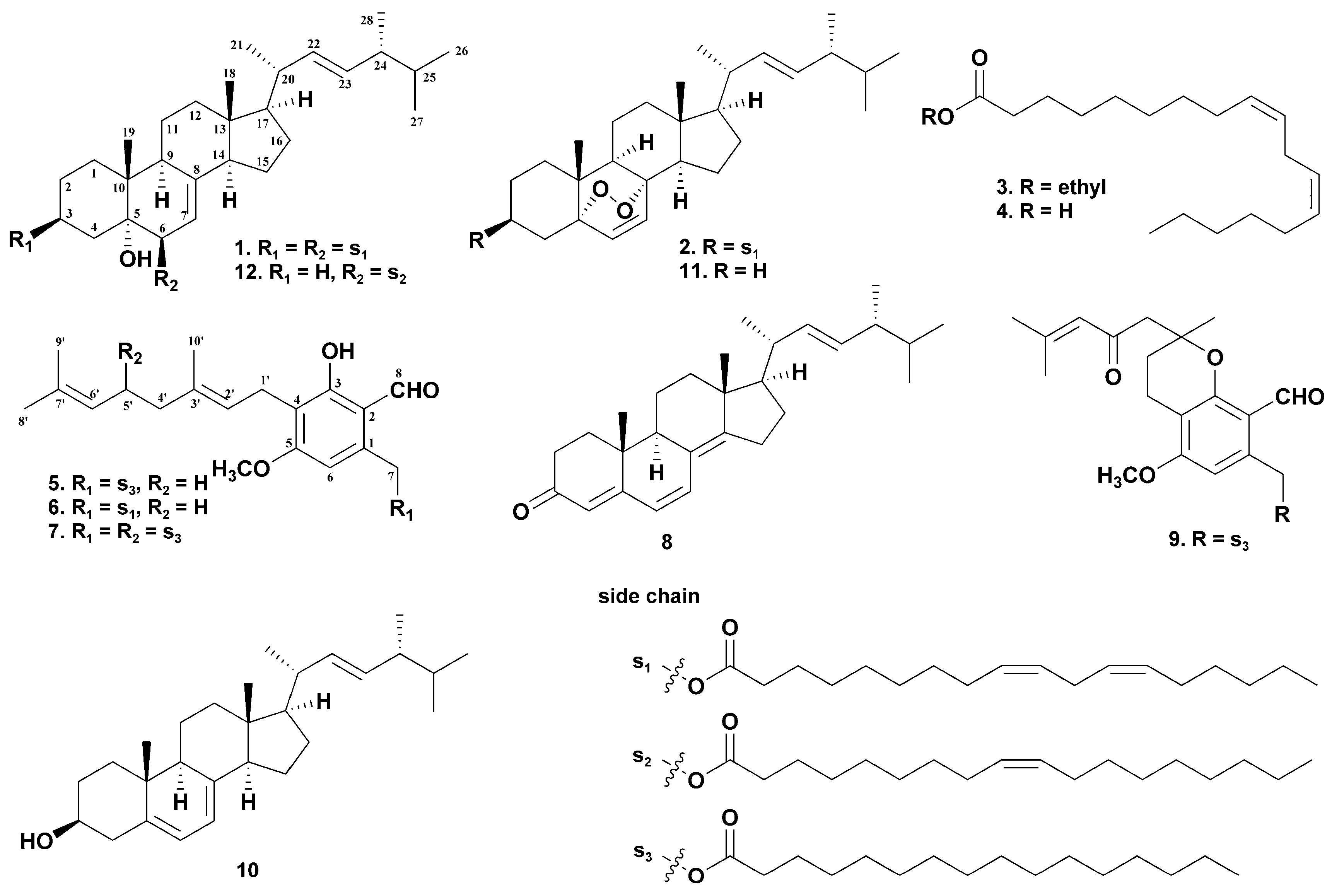

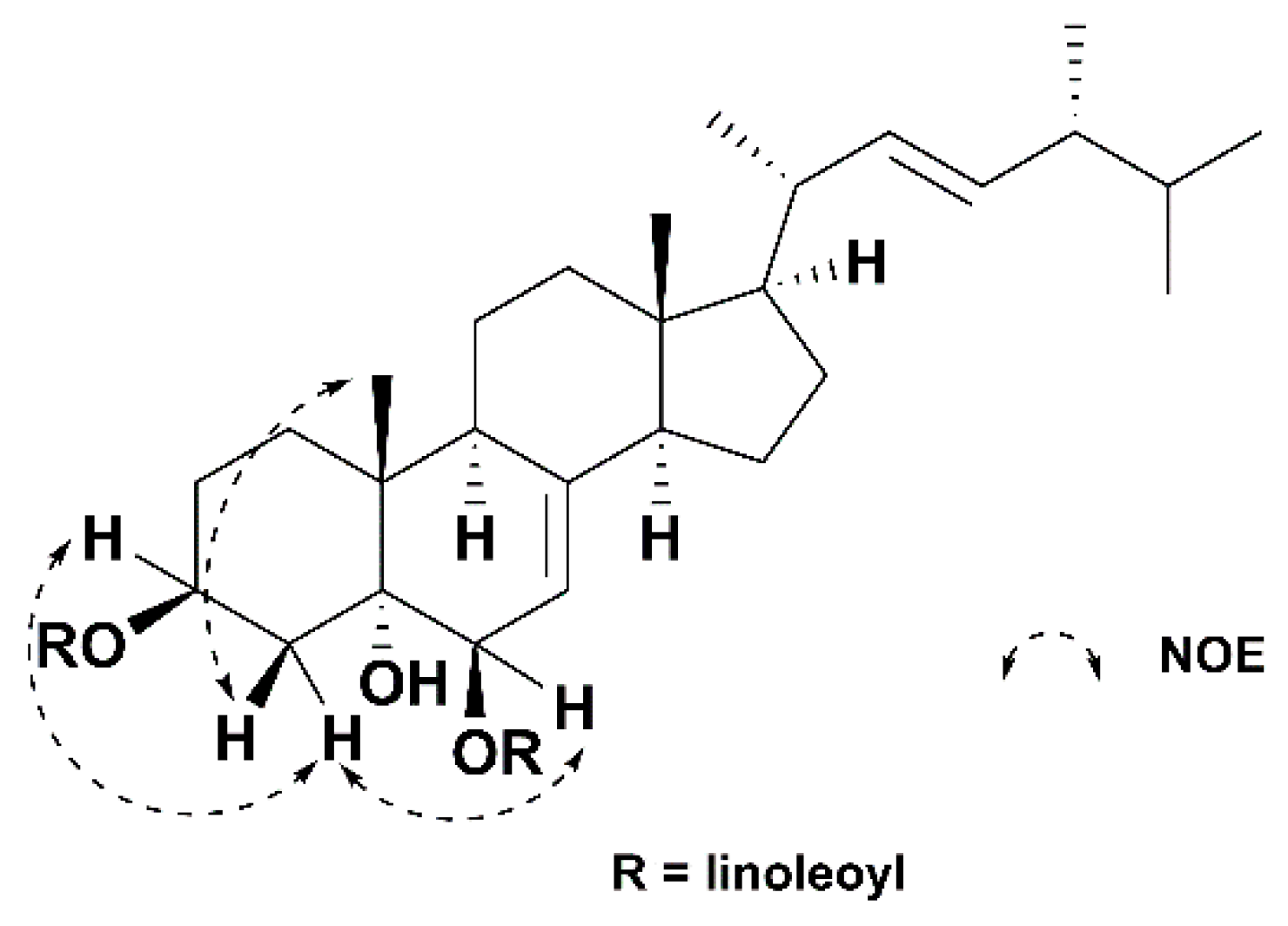


| Position | δC | δH (J in Hz) | Position | δC | δH (J in Hz) |
|---|---|---|---|---|---|
| 1 | 32.2 | 1.55 (m); 1.70 (m) | 24 | 42.8 | 1.84 (m) |
| 2 | 26.9 | 1.49 (m); 1.89 (m) | 25 | 33.0 | 1.46 (m) |
| 3 | 70.4 | 5.14 (m) | 26 | 19.9 | 0.83 (d, J = 6.48) |
| 4 | 35.7 | 1.71 (m); 1.96 (m) | 27 | 19.6 | 0.82 (d, J = 6.44) |
| 5 | 74.9 | - | 28 | 17.6 | 0.91 (d, J = 6.80) |
| 6 | 73.4 | 4.80 (d, J = 4.7) | 1′/1″ | 173.3, 173.0 | - |
| 7 | 114.0 | 5.28 (m) | 2′/2″ | 34.6, 34.6 | 2.25 (t, J = 7.5); 2.30 (t, J = 7.2) |
| 8 | 145.6 | - | 3′/3″ | 24.9, 25.0 | 1.60 (m) |
| 9 | 43.2 | 2.02 (m) | 4′/4″ | 29.1–29.7 | 1.23–1.35 (m) |
| 10 | 37.3 | - | 5′/5″ | 29.1–29.7 | 1.23–1.35 (m) |
| 11 | 21.9 | 1.57 (m) | 6′/6″ | 29.1–29.7 | 1.23–1.35 (m) |
| 12 | 39.1 | 1.32 (m); 2.05 (m) | 7′/7″ | 29.1–29.7 | 1.23–1.35 (m) |
| 13 | 43.7 | - | 8′/8″ | 27.2, 27.2 | 2.03 (m) |
| 14 | 54.8 | 1.91 (m) | 9′/9″ | 127.8, 127.9 | 5.30–5.40 (m) |
| 15 | 22.8 | 1.39 (m); 1.42 (m) | 10′/10″ | 128.0, 128.0 | 5.30–5.40 (m) |
| 16 | 27.8 | 1.72 (m) | 11′/11″ | 25.6, 25.6 | 2.77 (t, J = 6.3) |
| 17 | 55.9 | 1.27 (m) | 12′/12″ | 130.0, 130.0 | 5.30–5.40 (m) |
| 18 | 12.3 | 0.57 (s) | 13′/13″ | 130.2, 130.2, | 5.30–5.40 (m) |
| 19 | 18.2 | 1.06 (s) | 14′/14″ | 27.2, 27.2 | 2.03 (m) |
| 20 | 40.4 | 2.01 (m) | 15′/15″ | 29.1–29.7 | 1.23–1.35 (m) |
| 21 | 21.1 | 1.02 (d, J = 6.56) | 16′/16″ | 31.5, 31.9 | 1.26 (m) |
| 22 | 135.3 | 5.19 (m) | 17′/17″ | 22.5. 22.7 | 1.29 (m) |
| 23 | 132.1 | 5.21 (m) | 18′/18″ | 14.0, 14.1 | 0.88 (m) |
| Compounds | TNF-α | IL-6 | NO |
|---|---|---|---|
| 5 | 78.50 ± 3.72 | 56.33 ± 6.81 | 87.31 ± 8.77 |
| 6 | 298.50 ± 18.77 | - | - |
| 7 | 168.30 ± 9.69 | - | - |
| 9 | 62.46 ± 3.18 | 48.50 ± 6.54 | 76.16 ± 9.11 |
| Aspirin b | 27.08 ± 1.86 | 28.43 ± 4.46 | 51.82 ± 8.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, G.; Tang, L.; Xie, Y.; Xie, L. Secondary Metabolites from Hericium erinaceus and Their Anti-Inflammatory Activities. Molecules 2022, 27, 2157. https://doi.org/10.3390/molecules27072157
Xie G, Tang L, Xie Y, Xie L. Secondary Metabolites from Hericium erinaceus and Their Anti-Inflammatory Activities. Molecules. 2022; 27(7):2157. https://doi.org/10.3390/molecules27072157
Chicago/Turabian StyleXie, Guangbo, Lan Tang, Yu Xie, and Liyuan Xie. 2022. "Secondary Metabolites from Hericium erinaceus and Their Anti-Inflammatory Activities" Molecules 27, no. 7: 2157. https://doi.org/10.3390/molecules27072157
APA StyleXie, G., Tang, L., Xie, Y., & Xie, L. (2022). Secondary Metabolites from Hericium erinaceus and Their Anti-Inflammatory Activities. Molecules, 27(7), 2157. https://doi.org/10.3390/molecules27072157






