Valorization of a Waste Product of Edible Flowers: Volatile Characterization of Leaves
Abstract
:1. Introduction
2. Results and Discussion
2.1. HS-SPME Analysis
2.1.1. Mentheae Tribe
2.1.2. Ocimeae Tribe
2.2. EO Analysis
2.2.1. Mentheae Tribe
2.2.2. Ocimeae Tribe
3. Materials and Methods
3.1. Plant Material and Cultivation
3.2. Phytochemical Survey
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Motti, R. Wild plants used as herbs and spices in Italy: An ethnobotanical review. Plants 2021, 10, 5633. [Google Scholar] [CrossRef] [PubMed]
- De Favari Tardivo, C.; Meru, G.M. Production of Edible Flowers in Florida. Edis 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. An Overview on the Market of Edible Flowers. Food Rev. Int. 2020, 36, 258–275. [Google Scholar] [CrossRef]
- Karpun, O. Conceptual model of floriculture supply chain management. Intellect. Logist. Supply Chain. Manag. 2020, 1, 41–52. [Google Scholar] [CrossRef]
- Shanaida, M.; Golembiovska, O.; Hudz, N.; Wieczorek, P.P. Phenolic compounds of herbal infusions obtained from some species of the Lamiaceae family. Curr. Issues Pharm. Med. Sci. 2018, 31, 194–199. [Google Scholar] [CrossRef] [Green Version]
- Frezza, C.; Venditti, A.; Serafini, M.; Bianco, A. Phytochemistry, Chemotaxonomy, Ethnopharmacology, and Nutraceutics of Lamiaceae. In Studies in Natural Products Chemistry; Nakanishi, K., Goto, T., Itô, S., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 62, pp. 125–178. ISBN 9780444641854. [Google Scholar] [CrossRef]
- Çelik, G.; Kılıç, G.; Kanbolat, Ş.; Özlem Şener, S.; Karaköse, M.; Yaylı, N.; Karaoğlu, Ş.A. Biological activity, and volatile and phenolic compounds from five Lamiaceae species. Flavour Fragr. J. 2021, 36, 223–232. [Google Scholar] [CrossRef]
- Rattray, R.D.; Van Wyk, B.-E. The Botanical, Chemical and Ethnobotanical Diversity of Southern African Lamiaceae. Molecules 2021, 26, 3712. [Google Scholar] [CrossRef]
- Khoury, M.; Stien, D.; Eparvier, V.; Ouaini, N.; El Beyrouthy, M. Report on the Medicinal Use of Eleven Lamiaceae Species in Lebanon and Rationalization of Their Antimicrobial Potential by Examination of the Chemical Composition and Antimicrobial Activity of Their Essential Oils. Evid.-Based Complement. Altern. Med. 2016, 2016, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciriello, M.; Formisano, L.; El-Nakhel, C.; Kyriacou, M.C.; Soteriou, G.A.; Pizzolongo, F.; Romano, R.; De Pascale, S.; Rouphael, Y. Genotype and Successive Harvests Interaction Affects Phenolic Acids and Aroma Profile of Genovese Basil for Pesto Sauce Production. Foods 2021, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Valussi, M.; Jugran, A.K.; Martorell, M.; Ramírez-Alarcón, K.; Stojanović-Radić, Z.Z.; Antolak, H.; Kręgiel, D.; Mileski, K.S.; Sharifi-Rad, M.; et al. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018, 80, 104–122. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Alves Borges Leal, A.L.; et al. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Henson, D.L. The Genus Monarda in Oklahoma. The American Midland Naturalist; The University of Notre Dame: South Bay, IN, USA, 1941; Volume 25, pp. 358–360. [Google Scholar]
- Matei, C.F.; Duda, M.M.; Ardelean, A.E.; Covaci, A.D.; Mǎdaş, M.N. The Importance An Usage of The Agastache foeniculum Species (Pursh) Kuntze. Hop Med. Plants 2010, 18, 49–52. [Google Scholar]
- Wilson, L.A.; Widrlechner, M.P.; Senechal, N.P. Headspace Analysis of the Volatile Oils of Agastache. J. Agric. Food Chem. 1992, 40, 1362–1366. [Google Scholar] [CrossRef] [Green Version]
- Yamani, H.; Mantri, N.; Morrison, P.D.; Pang, E. Analysis of the volatile organic compounds from leaves, flower spikes, and nectar of Australian grown Agastache Rugosa. BMC Complement. Altern. Med. 2014, 14, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Nestorović, J.; Mišić, D.; Šiler, B.; Soković, M.; Glamočlija, J.; Ćirić, A.; Maksimović, V.; Grubišić, D. Nepetalactone content in shoot cultures of three endemic Nepeta species and the evaluation of their antimicrobial activity. Fitoterapia 2010, 81, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, L.M.; Al-Jaber, H.I.; Abu Zarga, M.H. Volatile Organic Compounds and Essential Oil Composition of Selected Organs of Nepeta curviflora Collected from Two Regions in Jordan. Jordan J. Chem. 2017, 12, 101–112. [Google Scholar]
- Cozzolino, R.; Ramezani, S.; Martignetti, A.; Mari, A.; Piacente, S.; De Giulio, B. Determination of volatile organic compounds in the dried leaves of Salvia species by solid-phase microextraction coupled to gas chromatography mass spectrometry. Nat. Prod. Res. 2016, 30, 841–848. [Google Scholar] [CrossRef]
- Najar, B.; Pistelli, L.; Cervelli, C.; Fico, G.; Giuliani, C. Salvia broussonetii Benth.: Aroma profile and micromorphological analysis. Nat. Prod. Res. 2018, 32, 1660–1668. [Google Scholar] [CrossRef]
- Klimánková, E.; Holadová, K.; Hajšlová, J.; Čajka, T.; Poustka, J.; Koudela, M. Aroma profiles of five basil (Ocimum basilicum L.) cultivars grown under conventional and organic conditions. Food Chem. 2008, 107, 464–472. [Google Scholar] [CrossRef]
- Tarchoune, I.; Baâtour, O.; Harrathi, J.; Cioni, P.L.; Lachaâl, M.; Flamini, G.; Ouerghi, Z. Essential oil and volatile emissions of basil (Ocimum basilicum) leaves exposed to NaCl or Na2SO4 salinity. J. Plant Nutr. Soil Sci. 2013, 176, 748–755. [Google Scholar] [CrossRef]
- Ronga, D.; Pellati, F.; Brighenti, V.; Laudicella, K.; Laviano, L.; Fedailaine, M.; Benvenuti, S.; Pecchioni, N.; Francia, E. Testing the influence of digestate from biogas on growth and volatile compounds of basil (Ocimum basilicum L.) and peppermint (Mentha x piperita L.) in hydroponics. J. Appl. Res. Med. Aromat. Plants 2018, 11, 18–26. [Google Scholar] [CrossRef]
- Matłok, N.; Gorzelany, J.; Stępień, A.E.; Figiel, A.; Balawejder, M. Effect of Fertilization in Selected Phytometric Features and Contents of Bioactive Compounds in Dry Matter of Two Varieties of Basil (Ocimum basilicum L.). Sustainability 2019, 11, 6590. [Google Scholar] [CrossRef] [Green Version]
- Khairun Fadila, S.; Chun Hui, A.; Sook Mei, K.; Cheng Hock, C. Chemical constituents and antioxidant capacity of Ocimum basilicum and Ocimum sanctum. Iran. J. Chem. Chem. Eng. 2019, 38, 139–152. [Google Scholar]
- Açıkgöz, M.A. Establishment of cell suspension cultures of Ocimum basilicum L. and enhanced production of pharmaceutical active ingredients. Ind. Crops Prod. 2020, 148. [Google Scholar] [CrossRef]
- Tirillini, B.; Maggi, F. Volatile organic compounds of the glandular trichomes of Ocimum basilicum and artifacts during the distillation of the leaves. Appl. Sci. 2021, 11, 7312. [Google Scholar] [CrossRef]
- Al-Kateb, H.; Mottram, D.S. The relationship between growth stages and aroma composition of lemon basil Ocimum citriodorum Vis. Food Chem. 2014, 152, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Haiyan, G.; Lijuan, H.; Shaoyu, L.; Chen, Z.; Ashraf, M.A. Antimicrobial, antibiofilm and antitumor activities of essential oil of Agastache rugosa from Xinjiang, China. Saudi J. Biol. Sci. 2016, 23, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, S.; Dąbrowska, M.; Kozłowska, W.; Kalemba, D.; Abel, R.; Dryś, A.; Szumny, A.; Matkowski, A. Ontogenetic and trans-generational variation of essential oil composition in Agastache rugosa. Ind. Crops Prod. 2017, 97, 612–619. [Google Scholar] [CrossRef]
- Wesolowska, A. Influence of Distillation Time on the Content and Composition of Essential Oils Isolated from Different Parts of Agastache astromontana ‘Pink Pop’. J. Essent. Oil Bear. Plants 2019, 22, 311–323. [Google Scholar] [CrossRef]
- Juárez, Z.N.; Hernández, L.R.; Bach, H.; Sánchez-Arreola, E.; Bach, H. Antifungal activity of essential oils extracted from Agastache mexicana ssp. xolocotziana and Porophyllum linaria against post-harvest pathogens. Ind. Crops Prod. 2015, 74, 178–182. [Google Scholar] [CrossRef]
- Wróblewska, K.; Szumny, A.; Żarowska, B.; Kromer, K.; Dębicz, R.; Fabian, S. Impact of mulching on growth essential oil composition and its biological activity in Monarda didyma L. Ind. Crops Prod. 2019, 129, 299–308. [Google Scholar] [CrossRef]
- Gontar, Ł.; Herman, A.; Osińska, E. Monarda essential oils as natural cosmetic preservative systems. Nat. Volatiles Essent. Oils 2021, 8, 29–38. [Google Scholar] [CrossRef]
- Bojinescu, F.N.; Pop, G. Features of the Species Monarda didyma Cultivated Under the Pedoclimatic Conditions of Didactic-Experimental Station in Timisoara, Romania. Res. J. Agric. Sci. 2020, 52, 31–36. [Google Scholar]
- Radulović, N.; Blagojević, P.D.; Rabbitt, K.; De Sousa Menezes, F. Essential oil of Nepeta x faassenii Bergmans ex Stearn (N. mussinii Spreng. x N. nepetella L.): A comparison study. Nat. Prod. Commun. 2011, 6, 1015–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jianu, C.; Moleriu, R.; Stoin, D.; Cocan, I.; Bujancă, G.; Pop, G.; Lukinich-Gruia, A.T.; Muntean, D.; Rusu, L.C.; Horhat, D.I. Antioxidant and antibacterial activity of Nepeta × faassenii bergmans ex stearn essential oil. Appl. Sci. 2021, 11, 442. [Google Scholar] [CrossRef]
- Satyal, P.; Calderon, C.; Setzer, W.N. Seasonal variation in the essential oil composition of Salvia fruticosa Mill. cultivated in Portugal. Am. J. Essent. Oils Nat. Prod. 2020, 8, 6–10. [Google Scholar]
- Chouit, H.; Touafek, O.; Brada, M.; Benssouici, C.; Fauconnier, M.-L.; El Hattab, M. GC-MS Analysis and Biological Activities of Algerian Salvia microphylla Essential Oils. J. Mex. Chem. Soc. 2021, 65, 582–601. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Satyal, P.; Setzer, W.N.; Wink, M. Chemical compositions of the essential oils of three Salvia species cultivated in Germany. Am. J. Essent. Oils Nat. Prod. 2015, 3, 26–29. [Google Scholar]
- Najar, B.; Pistelli, L.; Venturi, F.; Ferroni, G.; Giovanelli, S.; Cervelli, C.; Bedini, S.; Conti, B. Salvia spp. Essential oils against the arboviruses vector aedes albopictus (diptera: Culicidae): Bioactivity, composition, and sensorial profile—stage 1. Biology 2020, 9, 206. [Google Scholar] [CrossRef] [PubMed]
- Lal, R.; Shasany, A.; Singh, V.; Gupta, A.; Singh, S.; Sarkar, S.; Chanotiya, C.; Dhawan, O.; Kumar, B.; Dhawan, S.S.; et al. Registration of high citral rich essential oil yielding variety-CIM Jyoti of Ocimum africanum. J. Med. Aromat. Plant Sci. 2017, 39, 135–138. [Google Scholar]
- Bunwijit, J.; Sripanidkulchai, B.; Pannangrong, W.; Junlatat, J.; Sripanidkulchai, K. Gastroprotective effect of hydroalcoholic extract of Ocimum africanum leaves. Songklanakarin J. Sci. Technol. 2017, 39, 539–547. [Google Scholar] [CrossRef]
- Aburjai, T.A.; Mansi, K.; Azzam, H.; Alqudah, D.A.; Alshaer, W.; Abuirjei, M. Chemical compositions and anticancer potential of essential oil from greenhouse-cultivated Ocimum basilicum leaves. Indian J. Pharm. Sci. 2020, 82, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Majdi, C.; Pereira, C.; Dias, M.I.; Calhelha, R.C.; Alves, M.J.; Rhourri-Frih, B.; Charrouf, Z.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Phytochemical Characterization and Bioactive Properties of Cinnamon Basil (Ocimum basilicum cv. ‘Cinnamon’) and Lemon Basil (Ocimum × citriodorum). Antioxidants 2020, 9, 369. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.d.C.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K.; et al. A systematic review on the neuroprotective perspectives of beta-caryophyllene. Phyther. Res. 2018, 32, 2376–2388. [Google Scholar] [CrossRef] [PubMed]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; Torre, C.L.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene A Sesquiterpene with Countless.pdf. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef] [Green Version]
- Marchioni, I.; Najar, B.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Bioactive Compounds and Aroma Profile of Some Lamiaceae Edible Flowers. Plants 2020, 9, 691. [Google Scholar] [CrossRef] [PubMed]
- Casiglia, S.; Bruno, M.; Bramucci, M.; Quassinti, L.; Lupidi, G.; Fiorini, D.; Maggi, F. Kundmannia sicula (L.) DC: A rich source of germacrene D. J. Essent. Oil Res. 2017, 29, 437–442. [Google Scholar] [CrossRef]
- Malik, S.; Mesquita, L.S.S.d.; Silva, C.R.; Mesquita, J.W.C.d.; Rocha, E.d.S.; Bose, J.; Abiri, R.; Figueiredo, P.D.M.S.; Costa, L.M. Chemical profile and biological activities of essential oil from Artemisia vulgaris L. Cultivated in Brazil. Pharmaceuticals 2019, 12, 49. [Google Scholar] [CrossRef] [Green Version]
- Nozohour, Y.; Jalilzadeh-amin, G. Comparison of Antibacterial Activity of Trans-cinnamaldehyde, 1, 8 Cineole, and Pulegone Against Streptococcus equi subsp equi Isolated from Horse. Iran. J. Med. Microbiol. 2021, 15, 606–611. [Google Scholar] [CrossRef]
- Roy, A.; Park, H.J.; Abdul, Q.A.; Jung, H.A.; Choi, J.S. Pulegone exhibits anti-inflammatory activities through the regulation of NF-κb and Nrf-2 signaling pathways in LPS-stimulated RAW 264.7 cells. Nat. Prod. Sci. 2018, 24, 28–35. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Hǎncianu, M.; Costache, I.I.; Miron, A. Linalool: A review on a key odorant molecule with valuable biological properties. Flavour Fragr. J. 2014, 29, 193–219. [Google Scholar] [CrossRef]
- Dias, I.J.; Trajano, E.R.I.S.; Castro, R.D.; Ferreira, G.L.S.; Medeiros, H.C.M.; Gomes, D.Q.C. Antifungal activity of linalool in cases of Candida spp. isolated from individuals with oral candidiasis. Brazilian J. Biol. 2017, 78, 368–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Habib, S.; Sahu, D.; Gupta, J. Chemical Properties and Therapeutic Potential of Citral, a Monoterpene Isolated from Lemongrass. Med. Chem. 2020, 17, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Nejad, S.; Özgüneş, H.; Başaran, N. Öjenolün Farmakolojik Ve Toksikolojik Özellikleri. Turkish J. Pharm. Sci. 2017, 14, 201–206. [Google Scholar] [CrossRef]
- Singh, H.; Pathak, A. Review on Estimation of Eugenol in Herbal Plants. World Wide J. Multidiscip. Res. Dev. 2018, 4, 344–347. [Google Scholar]
- González, A.M.; Tracanna, M.I.; Amani, S.M.; Schuff, C.; Poch, M.J.; Bach, H.; Catalán, C.A.N. Chemical composition, antimicrobial and antioxidant properties of the volatile oil and methanol extract of Xenophyllum poposum. Nat. Prod. Commun. 2012, 7, 1663–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najar, B.; Marchioni, I.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Volatilomic Analysis of Four Edible Flowers from Agastache Genus. Molecules 2019, 24, 4480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
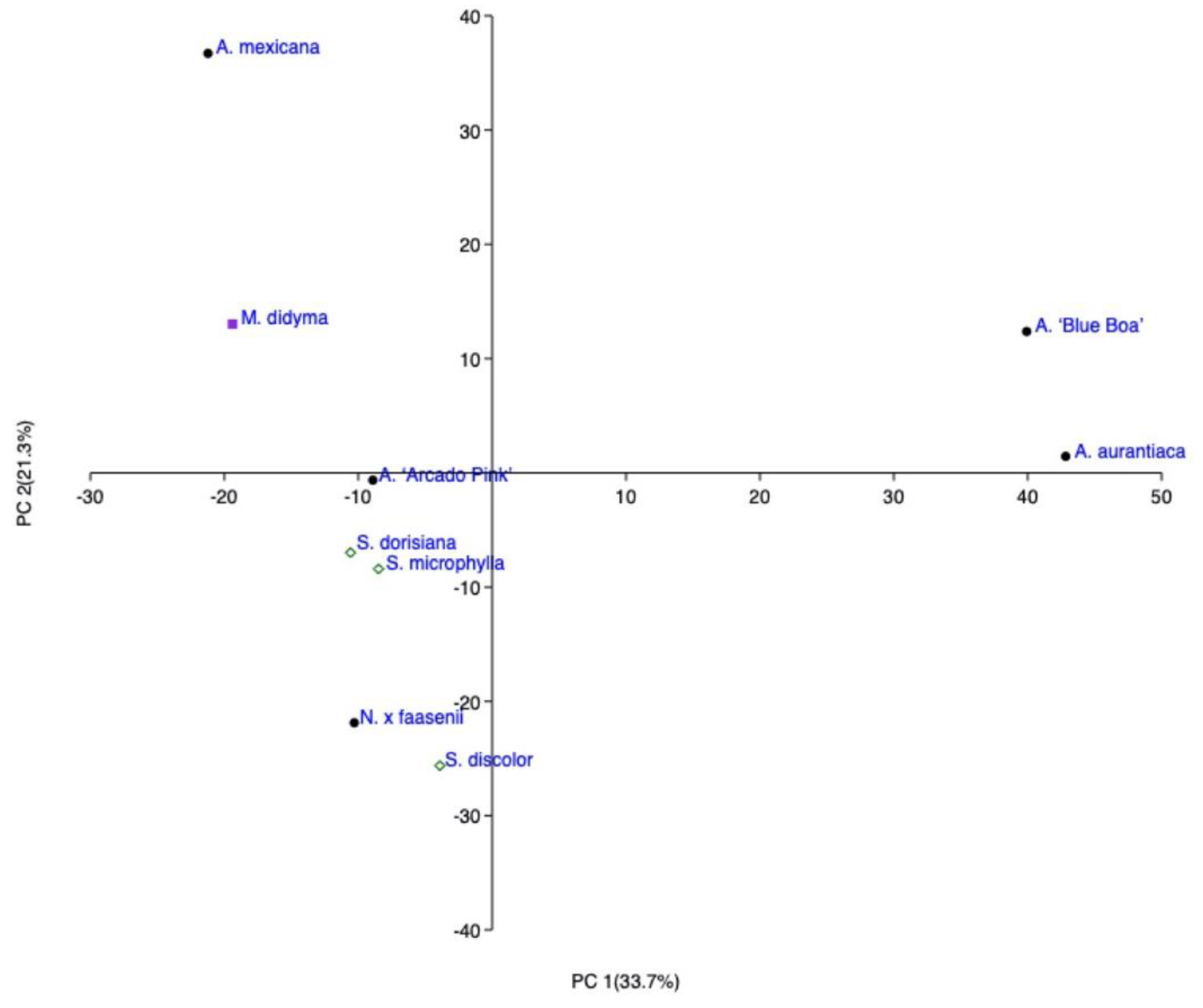
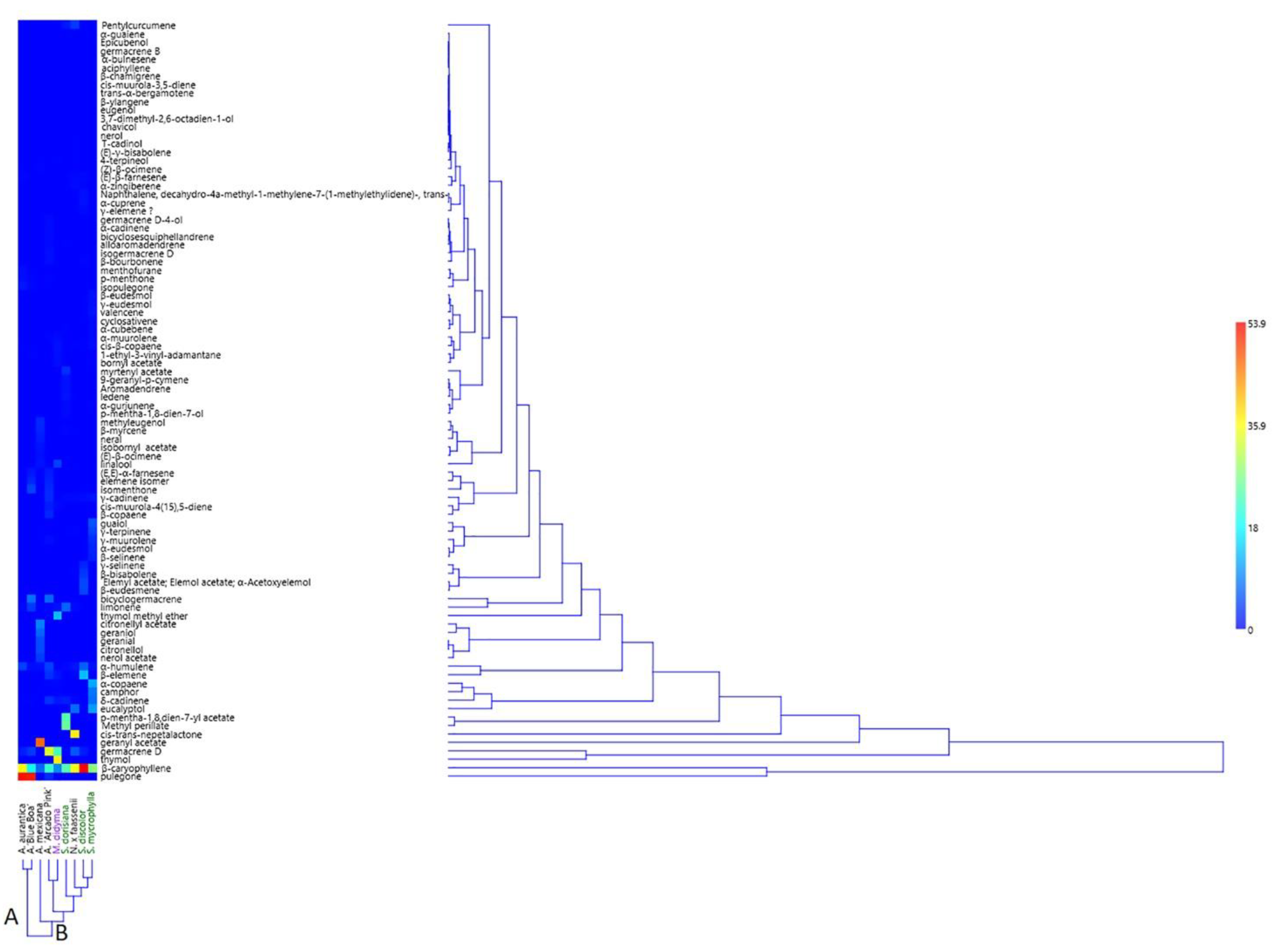
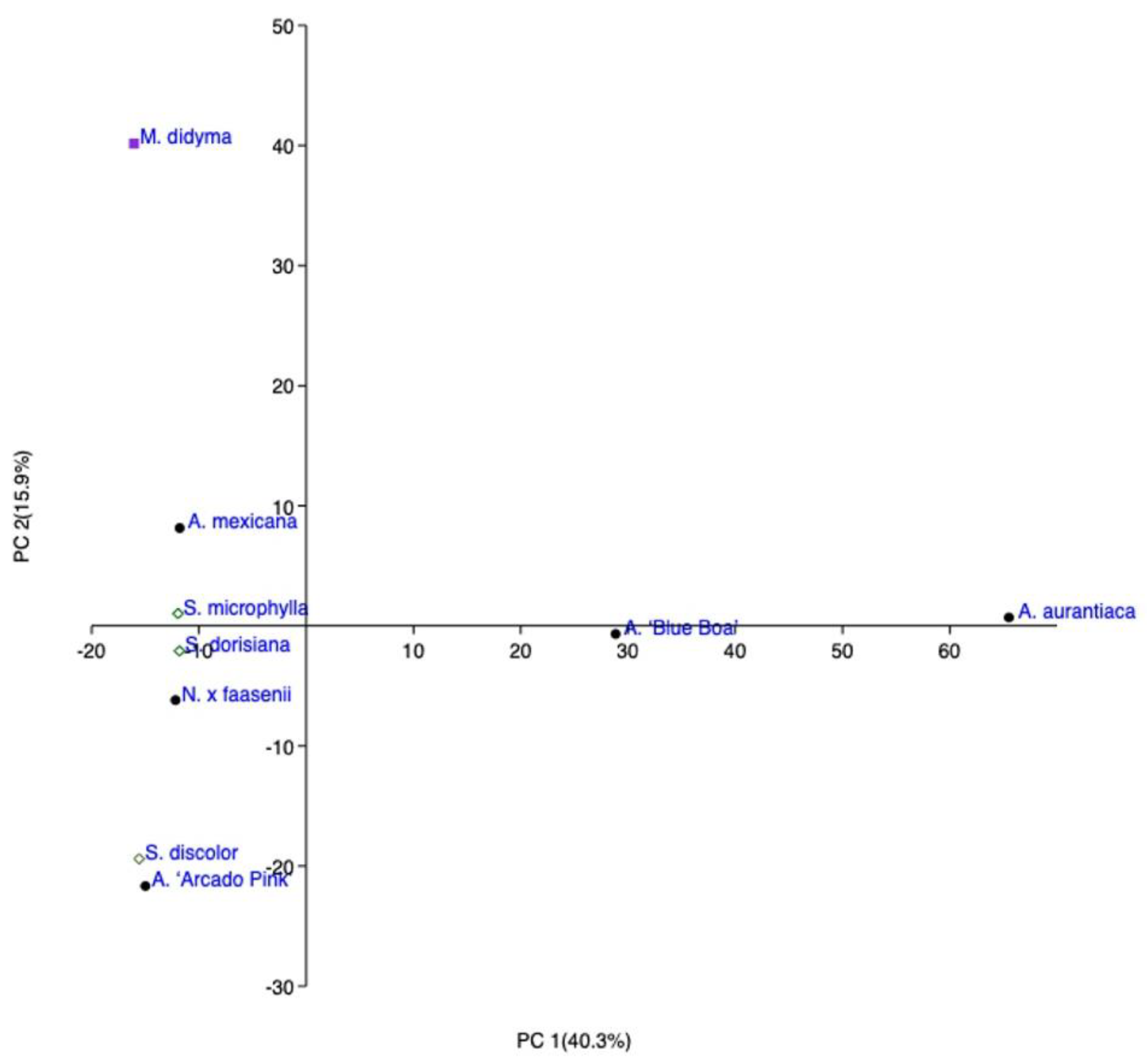


| A | B | ||
|---|---|---|---|
| Permutation N: | 9999 | Permutation N: | 9999 |
| Total sum of squares: | 4.629 | Total sum of squares: | 6.007 |
| Within-group sum of squares: | 3.282 | Within-group sum of squares: | 4.727 |
| F: | 3.079 | F: | 4.332 |
| p (same): | 0.0018 | p (same): | 0.0002 |
| A1 | B1 | |||||
|---|---|---|---|---|---|---|
| Subtribe Nepetinae | Subtribe Menthinae | Subtribe Salviinae | Mentheae Tribe | Ocimeae Tribe | ||
| Subtribe Nepetinae | 0.0975 | 0.0327 | Mentheae Tribe | 0.0001 | ||
| Subtribe Menthinae | 0.0975 | 0.1119 | Ocimeae Tribe | 0.0001 | ||
| Subtribe Salviinae | 0.0327 | 0.1119 | ||||
| A2 | B2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | Av. dissim | Contrib. % | Cumulative % | Mean Nep.Str | Mean Salv.Str | Compounds | Av. dissim | Contrib. % | Cumulative % | Mean Men.Tr | Mean Oci.Tr | ||
| 1 | Pulegone | 10.3 | 14.1 | 14.1 | 20.3 | 0.0 | 1 | β-Caryophyllene | 11.9 | 13.2 | 13.2 | 26.7 | 3.4 |
| 2 | β-Caryophyllene | 8.2 | 11.2 | 25.3 | 25.1 | 35.2 | 2 | Eugenol | 11.2 | 12.4 | 25.6 | 0.0 | 22.1 |
| 3 | Geranyl acetate | 4.7 | 6.5 | 31.8 | 9.3 | 0.0 | 3 | Pulegone | 5.7 | 6.3 | 31.9 | 11.3 | 0.0 |
| 4 | Germacrene D | 4.3 | 5.8 | 37.6 | 8.9 | 0.8 | 5 | β-Bisabolene | 4.5 | 5.0 | 42.5 | 0.5 | 8.8 |
| 5 | Methyl perillate | 4.2 | 5.8 | 43.4 | 0.0 | 8.4 | 6 | Germacrene B | 4.2 | 4.7 | 47.2 | 0.0 | 8.3 |
| 7 | cis-trans-Nepetalactone | 3.8 | 5.2 | 54.1 | 7.4 | 0.0 | 7 | Geranial | 4.1 | 4.5 | 51.7 | 0.6 | 7.9 |
| 8 | β-Elemene | 2.4 | 3.3 | 57.4 | 0.7 | 4.5 | 8 | Germacrene D | 4.1 | 4.5 | 56.2 | 7.8 | 5.3 |
| 9 | Eucalyptol | 2.1 | 2.9 | 60.3 | 1.5 | 3.7 | 9 | Geranyl acetate | 2.6 | 2.9 | 59.1 | 5.2 | 0.0 |
| 10 | α-Copaene | 2.1 | 2.8 | 63.1 | 0.1 | 4.0 | 10 | β-Elemene | 2.4 | 2.7 | 61.8 | 2.1 | 4.1 |
| 11 | δ-Cadinene | 1.6 | 2.2 | 65.3 | 0.8 | 3.3 | 11 | γ-Cadinene | 2.3 | 2.5 | 64.3 | 0.7 | 4.3 |
| 12 | Bicyclogermacrene | 1.5 | 2.1 | 67.4 | 3.0 | 0.0 | 12 | Thymol | 2.1 | 2.3 | 66.6 | 4.1 | 0.0 |
| 13 | Camphor | 1.4 | 1.9 | 69.3 | 0.0 | 2.8 | 13 | cis-trans-Nepetalactone | 2.1 | 2.3 | 68.9 | 4.1 | 0.0 |
| 14 | Limonene | 1.4 | 1.9 | 71.2 | 1.3 | 2.3 | 14 | Neral | 1.5 | 1.6 | 70.5 | 0.2 | 2.9 |
| 15 | α-Humulene | 1.3 | 1.8 | 73.0 | 2.6 | 2.9 | 15 | Methyl perillate | 1.4 | 1.6 | 72.1 | 2.8 | 0.0 |
| 16 | Guaiol | 1.0 | 1.3 | 74.3 | 0.0 | 1.9 | 16 | T-Cadinol | 1.4 | 1.5 | 73.6 | 0.0 | 2.7 |
| 17 | Citronellyl acetate | 0.9 | 1.3 | 75.6 | 1.9 | 0.0 | 17 | p-Mentha-1,8,dien-7-yl acetate | 1.3 | 1.5 | 75.1 | 2.6 | 0.0 |
| 18 | δ-Guaiene | 1.3 | 1.4 | 76.5 | 0.0 | 2.6 | |||||||
| 19 | trans-α-Bergamotene | 1.2 | 1.4 | 77.9 | 0.0 | 2.5 | |||||||
| 20 | eucalyptol | 1.1 | 1.2 | 79.1 | 2.1 | 0.1 | |||||||
| 21 | α-humulene | 1.0 | 1.1 | 80.2 | 2.4 | 1.9 | |||||||
| A | B | ||
|---|---|---|---|
| Permutation N: | 9999 | Permutation N: | 9999 |
| Total sum of squares: | 6.559 | Total sum of squares: | 6.912 |
| Within-group sum of squares: | 4.75 | Within-group sum of squares: | 5.994 |
| F: | 2.857 | F: | 2.451 |
| p (same): | 0.0012 | p (same): | 0.0014 |
| A1 | B1 | |||||
|---|---|---|---|---|---|---|
| Subtribe Nepetinae | Subtribe Menthinae | Subtribe Salviinae | Mentheae Tribe | Ocimeae Tribe | Mentheae Tribe | |
| Subtribe Nepetinae | 0.0885 | 0.0942 | Mentheae | 0.0024 | ||
| Subtribe Menthinae | 0.0885 | 0.1011 | Ocimeae | 0.0024 | ||
| Subtribe Salviinae | 0.0942 | 0.1011 | ||||
| Compounds | Av. dissim | Contrib. % | Cumulative % | Mean Mentheae Tribe | Mean Ocimeae Tribe | |
|---|---|---|---|---|---|---|
| 1 | Geranial | 7.5 | 8.1 | 8.1 | 0.0 | 14.7 |
| 2 | Eugenol | 6.8 | 7.3 | 15.4 | 0.0 | 13.2 |
| 3 | Neral | 6.5 | 7.0 | 22.4 | 0.0 | 12.8 |
| 4 | β-Caryophyllene | 4.9 | 5.2 | 27.6 | 10.3 | 1.5 |
| 5 | Linalool | 4.3 | 4.6 | 32.2 | 7.3 | 2.8 |
| 6 | T-Cadinol | 3.9 | 4.2 | 36.4 | 0.2 | 7.5 |
| 7 | Germacrene B | 3.8 | 4.1 | 40.5 | 0.0 | 7.4 |
| 8 | Germacrene D | 3.7 | 4.0 | 44.5 | 5.4 | 6.4 |
| 9 | β-Bisabolene | 3.4 | 3.6 | 48.1 | 0.8 | 6.2 |
| 10 | Guaiol | 2.4 | 2.6 | 50.7 | 4.8 | 0.0 |
| 11 | p-Mentha-1,8-dien-7-yl acetate | 2.3 | 2.5 | 53.2 | 4.2 | 0.0 |
| 12 | Geranyl acetate | 2.1 | 2.3 | 55.5 | 4.2 | 0.0 |
| 13 | Thymol | 2.1 | 2.2 | 57.7 | 4.1 | 0.0 |
| 14 | Elemol acetate | 2.1 | 2.2 | 59.9 | 4.1 | 0.0 |
| 15 | Tetracosane | 2.0 | 2.2 | 62.1 | 0.4 | 3.9 |
| 16 | α-Bulnesene | 1.7 | 1.9 | 64.0 | 0.0 | 3.3 |
| 17 | Methyl perillate | 1.6 | 1.7 | 65.7 | 2.9 | 0.0 |
| 18 | Thymol methyl ether | 1.5 | 1.6 | 67.3 | 3.0 | 0.0 |
| 19 | β-Eudesmol | 1.4 | 1.5 | 68.8 | 2.6 | 0.3 |
| 20 | Citral | 1.4 | 1.5 | 70.3 | 2.8 | 0.0 |
| 21 | Estragole | 1.3 | 1.4 | 71.8 | 0.0 | 2.6 |
| 22 | γ-Cadinene | 1.3 | 1.4 | 73.2 | 0.4 | 2.4 |
| 23 | (Z)-α-trans-Bergamotol | 1.2 | 1.3 | 74.5 | 2.3 | 0.0 |
| 24 | α-Humulene | 1.1 | 1.2 | 75.7 | 1.2 | 2.3 |
| 25 | Eucalyptol | 1.1 | 1.2 | 76.8 | 2.0 | 0.5 |
| 26 | β-Citral | 1.0 | 1.1 | 77.9 | 2.0 | 0.0 |
| 27 | Citronellol | 1.0 | 1.1 | 79.0 | 2.0 | 0.0 |
| 28 | α-Eudesmol | 1.0 | 1.1 | 80.1 | 2.0 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najar, B.; Pistelli, L.; Marchioni, I.; Pistelli, L. Valorization of a Waste Product of Edible Flowers: Volatile Characterization of Leaves. Molecules 2022, 27, 2172. https://doi.org/10.3390/molecules27072172
Najar B, Pistelli L, Marchioni I, Pistelli L. Valorization of a Waste Product of Edible Flowers: Volatile Characterization of Leaves. Molecules. 2022; 27(7):2172. https://doi.org/10.3390/molecules27072172
Chicago/Turabian StyleNajar, Basma, Laura Pistelli, Ilaria Marchioni, and Luisa Pistelli. 2022. "Valorization of a Waste Product of Edible Flowers: Volatile Characterization of Leaves" Molecules 27, no. 7: 2172. https://doi.org/10.3390/molecules27072172
APA StyleNajar, B., Pistelli, L., Marchioni, I., & Pistelli, L. (2022). Valorization of a Waste Product of Edible Flowers: Volatile Characterization of Leaves. Molecules, 27(7), 2172. https://doi.org/10.3390/molecules27072172









