Synthesis and Anti-Trypanosoma cruzi Biological Evaluation of Novel 2-Nitropyrrole Derivatives
Abstract
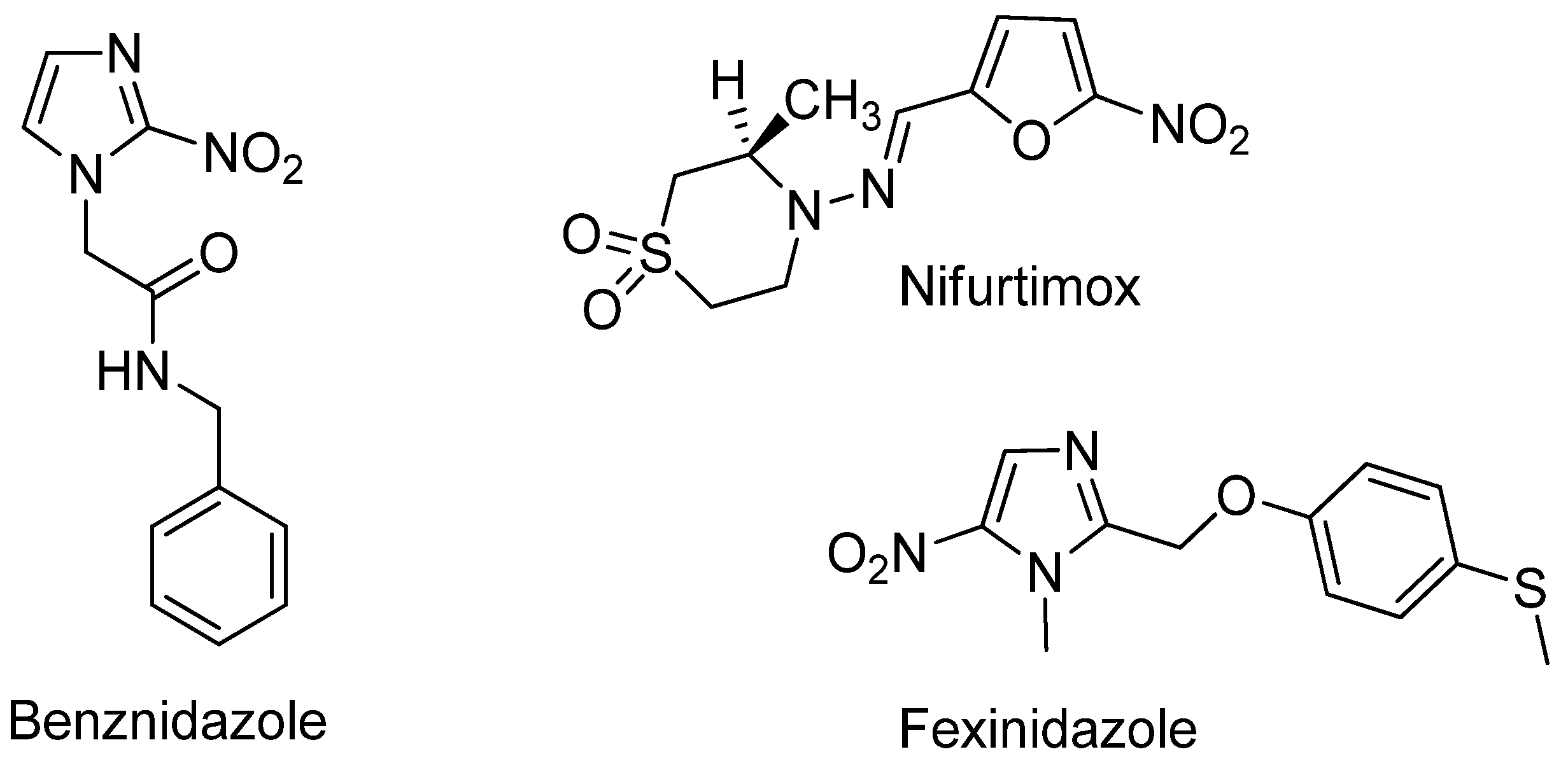
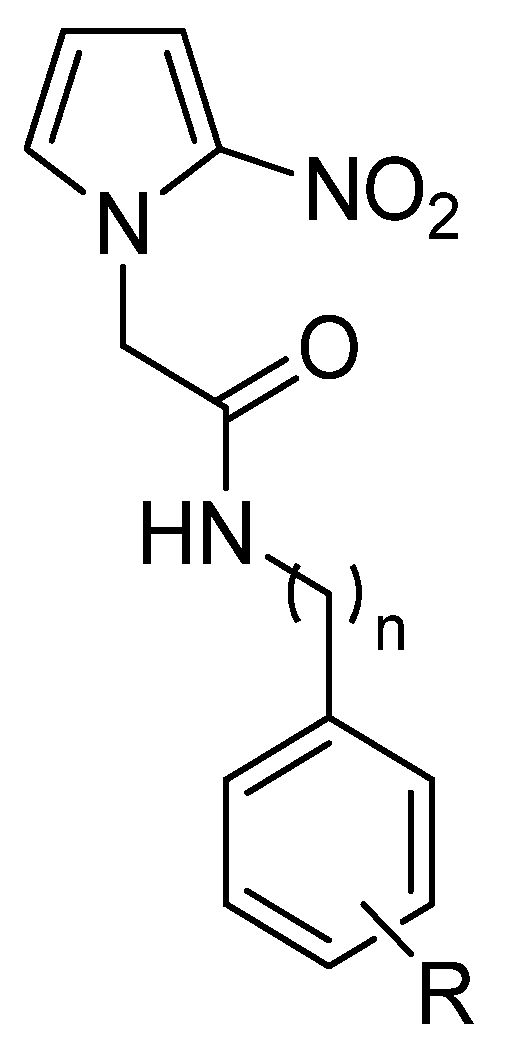
1. Introduction
2. Results and Discussion
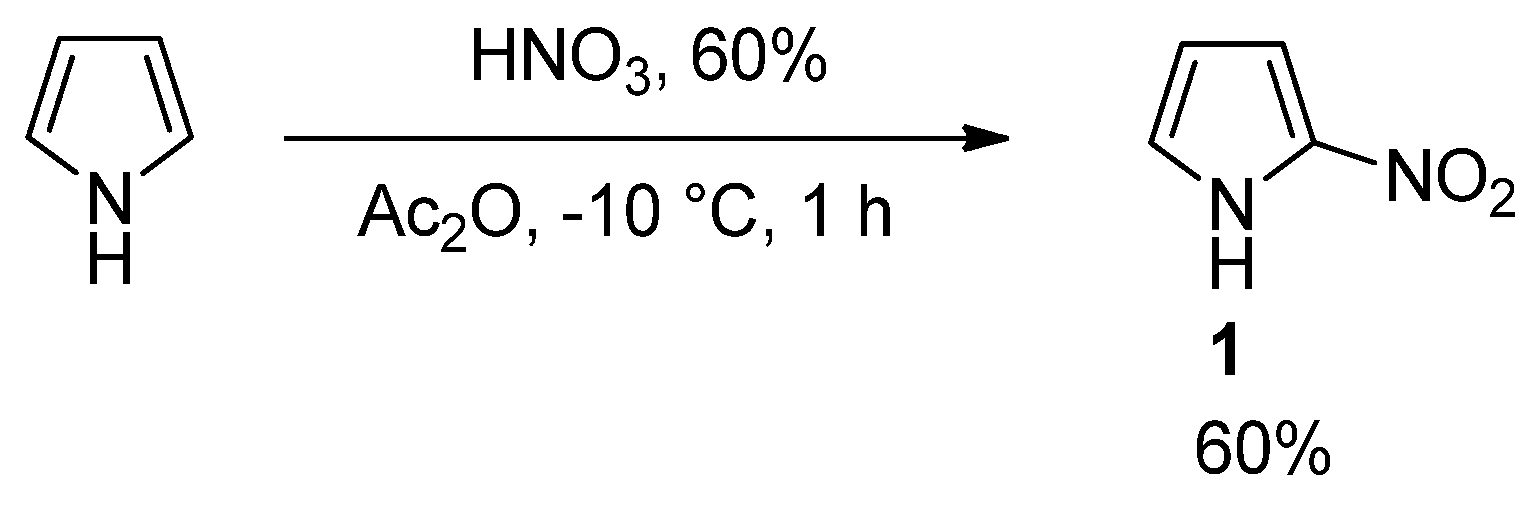
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Cytotoxicity and anti-T. cruzi Biological Evaluation
2.2.2. In Silico ADME Properties
3. Materials and Methods
3.1. Chemistry
3.1.1. Generality
3.1.2. Synthesis of Ethyl 2-(2-nitro-1H-pyrrol-1-yl)acetate (2)
3.1.3. General Procedure for the Synthesis of Bromoacetamide Intermediate Derivatives
- N-Benzyl-2-bromoacetamide (5) [24]
- 2-Bromo-N-[3-(trifluoromethyl)benzyl]acetamide (7) [27]
- 2-Bromo-N-(4-bromophenyl)acetamide (8) [29]
- 2-Bromo-N-(2-chlorophenyl)acetamide (9)
- 2-Bromo-N-[3-(trifluoromethyl)phenyl]acetamide (10) [28]
- 2-Bromo-N-(3-methoxyphenyl)acetamide (11) [30]
- 2-Bromo-N-isopropylacetamide (12) [31]
- 2-Bromo-N-cyclopentylacetamide (13) [31]
- 2-Bromo-N-(2-bromo-5-fluorophenyl)acetamide (14)
3.1.4. General Procedure for the Synthesis of 2-Nitropyrrole Derivatives
- N-Benzyl-2-(2-nitro-1H-pyrrol-1-yl)acetamide (3)
- 2-(2-Nitro-1H-pyrrol-1-yl)-N-phenylacetamide (4)
- 2-(2-Nitro-1H-pyrrol-1-yl)-N-[3-(trifluoromethyl)benzyl]acetamide (15)
- N-(4-Bromophenyl)-2-(2-nitro-1H-pyrrol-1-yl)acetamide (16)
- N-(2-Chlorophenyl)-2-(2-nitro-1H-pyrrol-1-yl)acetamide (17)
- 2-(2-Nitro-1H-pyrrol-1-yl)-N-[3-(trifluoromethyl)phenyl]acetamide (18)
- N-(3-Methoxyphenyl)-2-(2-nitro-1H-pyrrol-1-yl)acetamide (19)
- N-Isopropyl-2-(2-nitro-1H-pyrrol-1-yl)acetamide (20)
- N-Cyclopentyl-2-(2-nitro-1H-pyrrol-1-yl)acetamide (21)
- N-(2-Bromo-5-fluorophenyl)-2-(2-nitro-1H-pyrrol-1-yl)acetamide (22)
3.1.5. Crystal Data for 2-Nitropyrrole Starting Product 1
3.2. Toxicology
3.3. T. cruzi Intracellular Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO|World Health Organization. Available online: https://www.who.int/news/item/15-11-2021-who-and-bayer-renew-longstanding-collaboration-to-accelerate-control-and-elimination-of-neglected-tropical-diseases (accessed on 22 January 2022).
- Bonney, K.M.; Luthringer, D.J.; Kim, S.A.; Garg, N.J.; Engman, D.M. Pathology and Pathogenesis of Chagas Heart Disease. Annu. Rev. Pathol. 2019, 14, 421–447. [Google Scholar] [CrossRef] [PubMed]
- Chagas Disease-PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/topics/chagas-disease (accessed on 22 January 2022).
- WHO Model Lists of Essential Medicines. Available online: https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists (accessed on 22 January 2022).
- Bern, C.; Montgomery, S.P.; Herwaldt, B.L.; Rassi, A.; Marin-Neto, J.A.; Dantas, R.O.; Maguire, J.H.; Acquatella, H.; Morillo, C.; Kirchhoff, L.V.; et al. Evaluation and Treatment of Chagas Disease in the United States: A Systematic Review. JAMA 2007, 298, 2171–2181. [Google Scholar] [CrossRef]
- Kande Betu Ku Mesu, V.; Mutombo Kalonji, W.; Bardonneau, C.; Valverde Mordt, O.; Ngolo Tete, D.; Blesson, S.; Simon, F.; Delhomme, S.; Bernhard, S.; Mahenzi Mbembo, H.; et al. Oral Fexinidazole for Stage 1 or Early Stage 2 African Trypanosoma Brucei Gambiense Trypanosomiasis: A Prospective, Multicentre, Open-Label, Cohort Study. Lancet Glob. Health 2021, 9, 999–1008. [Google Scholar] [CrossRef]
- Oral Fexinidazole Dosing Regimens for the Treatment of Adults With Chronic Indeterminate Chagas Disease-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03587766 (accessed on 22 January 2022).
- Mazzeti, A.L.; Capelari-Oliveira, P.; Bahia, M.T.; Mosqueira, V.C.F. Review on Experimental Treatment Strategies Against Trypanos. Cruzi. JEP 2021, 13, 409–432. [Google Scholar] [CrossRef] [PubMed]
- Primas, N.; Ducros, C.; Vanelle, P.; Verhaeghe, P. The Renewal of Interest in Nitroaromatic Drugs Towards New Anti-Kinetoplastid Agents. In Medicinal Chemistry of Neglected and Tropical Diseases; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Russell, S.; Rahmani, R.; Jones, A.J.; Newson, H.L.; Neilde, K.; Cotillo, I.; Rahmani Khajouei, M.; Ferrins, L.; Qureishi, S.; Nguyen, N.; et al. Hit-to-Lead Optimization of a Novel Class of Potent, Broad-Spectrum Trypanosomacides. J. Med. Chem. 2016, 59, 9686–9720. [Google Scholar] [CrossRef]
- Brand, S.; Ko, E.J.; Viayna, E.; Thompson, S.; Spinks, D.; Thomas, M.; Sandberg, L.; Francisco, A.F.; Jayawardhana, S.; Smith, V.C.; et al. Discovery and Optimization of 5-Amino-1,2,3-Triazole-4-Carboxamide Series against Trypanos. Cruzi. J. Med. Chem. 2017, 60, 7284–7299. [Google Scholar] [CrossRef]
- Fersing, C.; Boudot, C.; Castera-Ducros, C.; Pinault, E.; Hutter, S.; Paoli-Lombardo, R.; Primas, N.; Pedron, J.; Seguy, L.; Bourgeade-Delmas, S.; et al. 8-Alkynyl-3-Nitroimidazopyridines Display Potent Antitrypanosomal Activity against Both Trypanosoma brucei and Cruzi. Eur. J. Med. Chem. 2020, 202, 112558. [Google Scholar] [CrossRef]
- Saccoliti, F.; Madia, V.N.; Tudino, V.; De Leo, A.; Pescatori, L.; Messore, A.; De Vita, D.; Scipione, L.; Brun, R.; Kaiser, M.; et al. Design, Synthesis, and Biological Evaluation of New 1-(Aryl-1H-Pyrrolyl)(Phenyl)Methyl-1H-Imidazole Derivatives as Antiprotozoal Agents. J. Med. Chem. 2019, 62, 1330–1347. [Google Scholar] [CrossRef]
- Hall, B.S.; Wilkinson, S.R. Activation of Benznidazole by Trypanosomal Type I Nitroreductases Results in Glyoxal Formation. Antimicrob. Agents Chemother. 2012, 56, 115–123. [Google Scholar] [CrossRef]
- Hall, B.S.; Bot, C.; Wilkinson, S.R. Nifurtimox Activation by Trypanosomal Type I Nitroreductases Generates Cytotoxic Nitrile Metabolites. J. Biol. Chem. 2011, 286, 13088–13095. [Google Scholar] [CrossRef]
- Kneeteman, M.; Baena, A.; Rosa, C.; Mancini, P. Polar Diels-Alder Reactions Using Heterocycles as Electrophiles. Influence of Microwave Irradiation. IRJPAC 2015, 8, 229–235. [Google Scholar] [CrossRef][Green Version]
- Cooksey, A.R.; Morgan, K.J.; Morrey, D.P. Nitropyrroles—II. Tetrahedron 1970, 26, 5101–5111. [Google Scholar] [CrossRef]
- WO2017205622 Method of Making Benznidazole (wipo.int). Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2017205622 (accessed on 25 January 2022).
- Allais, C.; Baslé, O.; Grassot, J.-M.; Fontaine, M.; Anguille, S.; Rodriguez, J.; Constantieux, T. Cooperative Heterogeneous Organocatalysis and Homogeneous Metal Catalysis for the One-Pot Regioselective Synthesis of 2-Pyridones. Adv. Synth. Catal. 2012, 354, 2084–2088. [Google Scholar] [CrossRef]
- Tang, Z.; Li, X.; Yao, Y.; Qi, Y.; Wang, M.; Dai, N.; Wen, Y.; Wan, Y.; Peng, L. Design, Synthesis, Fungicidal Activity and Molecular Docking Studies of Novel 2-((2-Hydroxyphenyl)Methylamino)Acetamide Derivatives. Bioorg. Med. Chem. 2019, 27, 2572–2578. [Google Scholar] [CrossRef]
- Molinspiration Cheminformatics. Available online: https://www.Molinspiration.com/ (accessed on 25 January 2022).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- McKerrow, J.H.; Lipinski, C.A. The Rule of Five Should Not Impede Anti-Parasitic Drug Development. Int. J. Parasitol. Drugs Drug. Resist. 2017, 7, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Ng, D.; Savinov, S.N.; Dey, B.; Kwong, P.D.; Wyatt, R.; Smith, A.B.; Hendrickson, W.A. Structure−Activity Relationships in the Binding of Chemically Derivatized CD4 to Gp120 from Human Immunodeficiency Virus. J. Med. Chem. 2007, 50, 4898–4908. [Google Scholar] [CrossRef] [PubMed]
- Kushner, S.; Cassell, R.I.; Morton, J.; Williams, J.H. Anticonvulsants. N-Benzylamides. J. Org. Chem. 1951, 16, 1283–1288. [Google Scholar] [CrossRef]
- Ferraccioli, R.; Forni, A. Selective Synthesis of Isoquinolin-3-One Derivatives Combining Pd-Catalysed Aromatic Alkylation/Vinylation with Addition Reactions: The Beneficial Effect of Water. Eur. J. Org. Chem. 2009, 2009, 3161–3166. [Google Scholar] [CrossRef]
- Kadjane, P.; Platas-Iglesias, C.; Boehm-Sturm, P.; Truffault, V.; Hagberg, G.E.; Hoehn, M.; Logothetis, N.K.; Angelovski, G. Dual-Frequency Calcium-Responsive MRI Agents. Chem. Eur. J. 2014, 20, 7351–7362. [Google Scholar] [CrossRef]
- Jogula, S.; Krishna, V.S.; Meda, N.; Balraju, V.; Sriram, D. Design, Synthesis and Biological Evaluation of Novel Pseudomonas Aeruginosa DNA Gyrase B Inhibitors. Bioorg. Chem. 2020, 100, 103905. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Shi, J.; Liao, L.; Zhang, L.; Liu, J.; Wang, Y.; Lao, Y.; Zhang, J. 5-[2-(N-(Substituted Phenyl)Acetamide)]Amino-1,3,4-thiadiazole-2-sulfonamides as Selective Carbonic Anhydrase II Inhibitors with Neuroprotective Effects. ChemMedChem 2020, 15, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Lim, T.L.; Lam, Y. Synthesis and in Vitro Evaluation of West Nile Virus Protease Inhibitors Based on the 2-{6-[2-(5-Phenyl-4 H -{1,2,4]Triazol-3-Ylsulfanyl)Acetylamino]Benzothiazol-2-Ylsulfanyl}acetamide Scaffold. ChemMedChem 2013, 8, 994–1001. [Google Scholar] [CrossRef]
- Lucas, R.L.; Zart, M.K.; Murkerjee, J.; Sorrell, T.N.; Powell, D.R.; Borovik, A.S. A Modular Approach toward Regulating the Secondary Coordination Sphere of Metal Ions: Differential Dioxygen Activation Assisted by Intramolecular Hydrogen Bonds. J. Am. Chem. Soc. 2006, 128, 15476–15489. [Google Scholar] [CrossRef]
- De Rycker, M.; Thomas, J.; Riley, J.; Brough, S.J.; Miles, T.J.; Gray, D.W. Identification of Trypanocidal Activity for Known Clinical Compounds Using a New Trypanosoma Cruzi Hit-Discovery Screening Cascade. PLoS Negl. Trop. Dis. 2016, 10, e0004584. [Google Scholar] [CrossRef] [PubMed]
- De Rycker, M.; Hallyburton, I.; Thomas, J.; Campbell, L.; Wyllie, S.; Joshi, D.; Cameron, S.; Gilbert, I.H.; Wyatt, P.G.; Frearson, J.A.; et al. Comparison of a High-Throughput High-Content Intracellular Leishmania Donovani Assay with an Axenic Amastigote Assay. Antimicrob. Agents Chemother. 2013, 57, 2913–2922. [Google Scholar] [CrossRef] [PubMed]
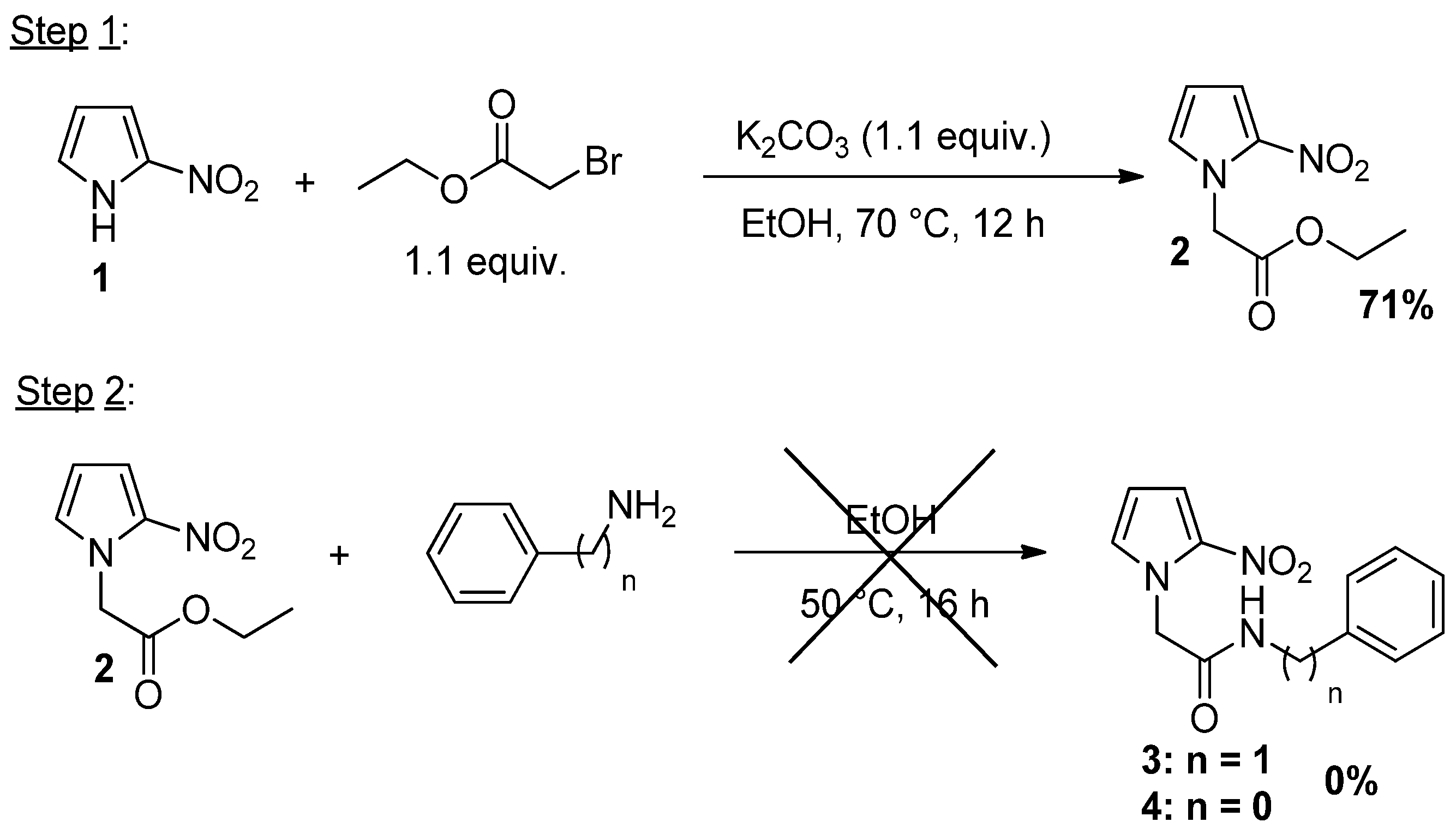
| Entry | Amine | Base | Temp (°C) | Time (h) | Solvent | 2 (%) | 3 (%) | 4 (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Benzylamine | K2CO3 | 50 | 24 | EtOH | 0 | 0 | - |
| 2 | Benzylamine | - | 50 | 48 | EtOH | 100 | 0 | - |
| 3 | Phenylamine | - | 80 | 48 | EtOH | 100 | - | 0 |
| 4 a | Phenylamine | NaH | rt | 24 | DMSO | 0 | - | 0 |
| 5 b | Phenylamine | DMAP | 150 | 1 | Toluene | 0 | - | 0 |
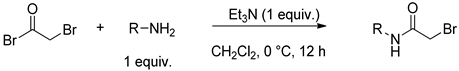 | ||
|---|---|---|
| Compound | Structure | Yield (%) |
| 5 | 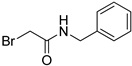 | 82 |
| 6 | 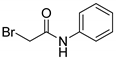 | 99 |
| 7 | 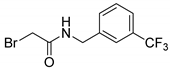 | 18 |
| 8 | 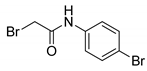 | 91 |
| 9 | 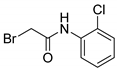 | 44 |
| 10 | 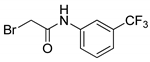 | 48 |
| 11 | 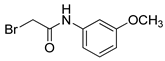 | 46 |
| 12 | 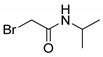 | 25 |
| 13 | 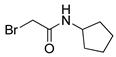 | 87 |
| 14 | 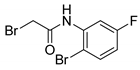 | 25 |
 | ||
|---|---|---|
| Compound | Structure | Yield (%) |
| 3 | 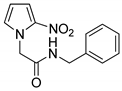 | 73 |
| 4 | 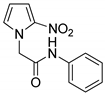 | 78 |
| 15 |  | 60 |
| 16 | 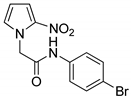 | 86 |
| 17 | 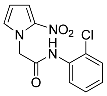 | 96 |
| 18 | 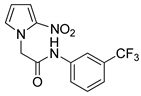 | 81 |
| 19 | 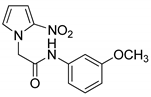 | 88 |
| 20 | 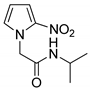 | 30 |
| 21 | 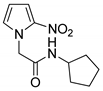 | 59 |
| 22 | 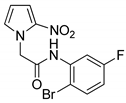 | 77 |
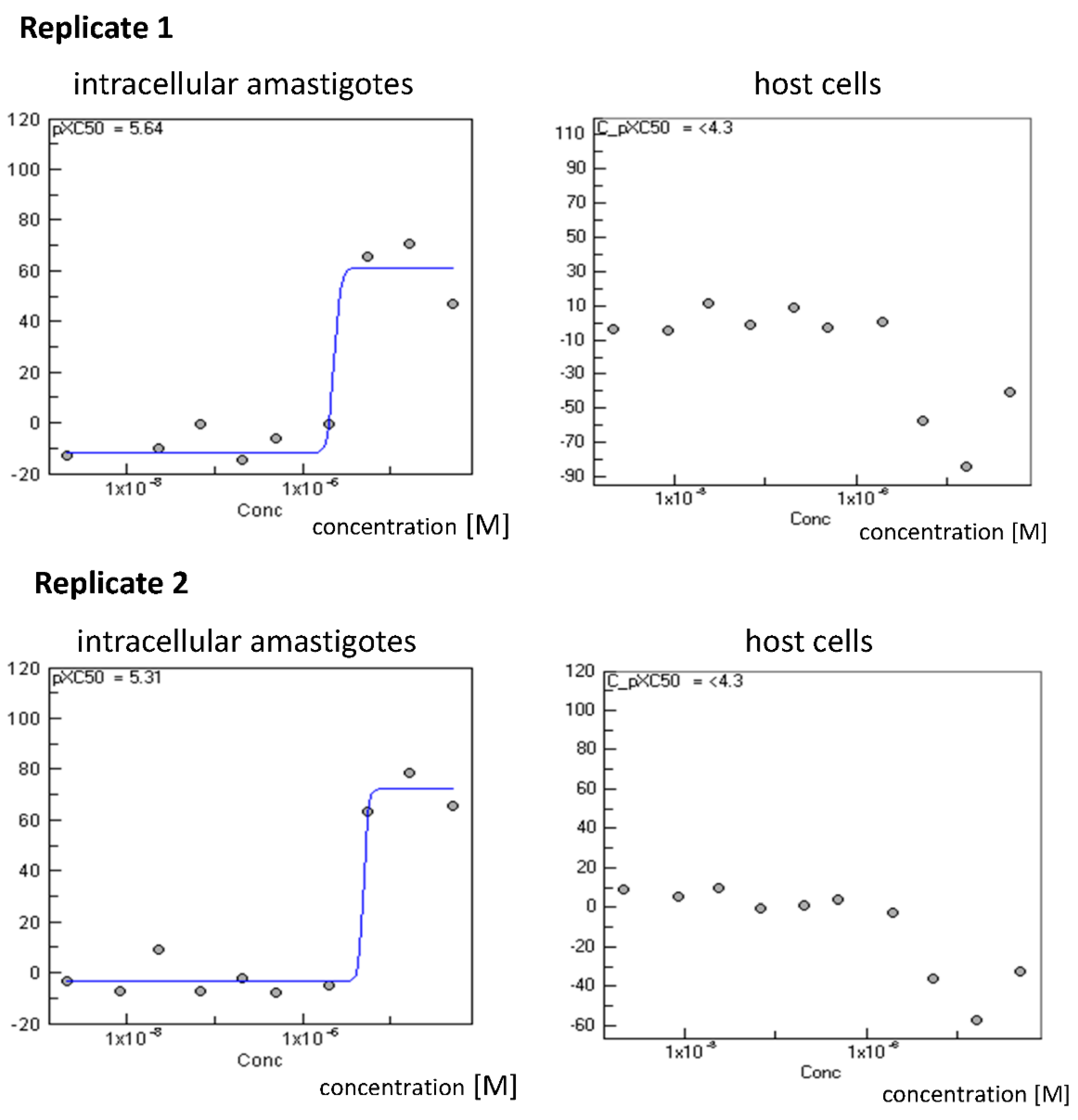
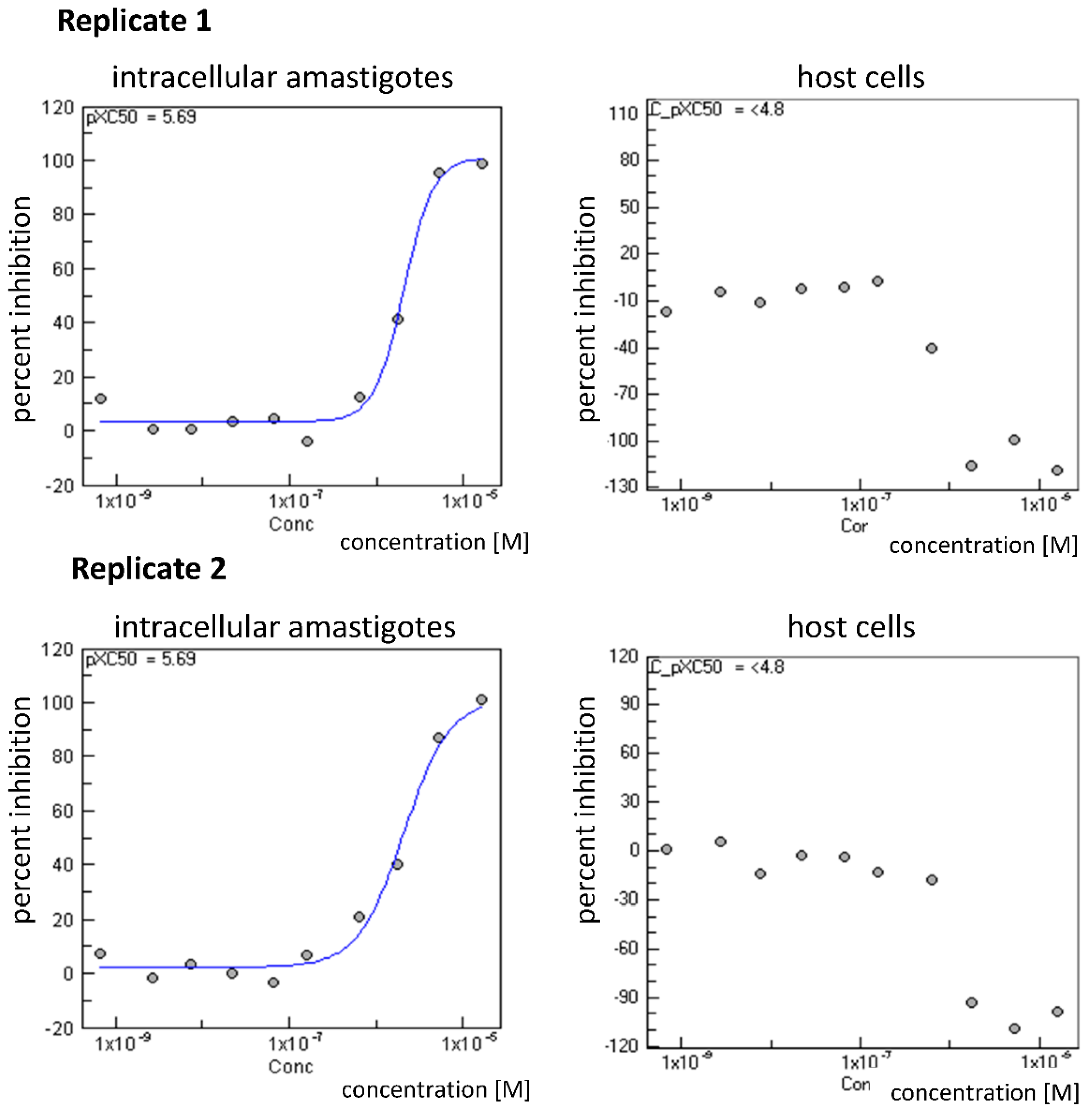
| Compound | Activity | Toxicity | |
|---|---|---|---|
| EC50 T. cruzi (µM) a | Vero cells EC50 (µM) | CC50 CHO (µM) b | |
| 3 | 34.5 ± 21.9 | 42 ± 11.3 | 24.2 ± 1.2 |
| 4 | 6.0 | 14.0 | 262.1 ± 3.9 |
| 15 | 41.0 ± 12.7 | 43 ± 9.9 | 184.9 ± 5.1 |
| 16 | 6.5 ± 0.6 | 14.5 ± 0.7 | 173.0 ± 4.6 |
| 17 | 50.0 | >50 | 198.3 ± 5.7 |
| 18 | 3.6 ± 1.8 | >50 | 155.6 ± 2.9 |
| 19 | 50.0 | >50 | 260.1 ± 6.3 |
| 20 | - | - | 321.1 ± 5.3 |
| 21 | - | - | 303.3 ± 6.1 |
| 22 | - | - | 188.2 ± 4.4 |
| Doxorubicin | - | - | 4.6 ± 0.08 |
| Nifurtimox | 2.1 ± 0.2 | - | - |
| Compound | XlogP (o/w) | Mol. Wt. (g·mol−1) | HBA | HDA | nrotb | TPSA (Å2) |
|---|---|---|---|---|---|---|
| 3 | 1.51 | 259.26 | 6 | 1 | 5 | 79.86 |
| 4 | 1.81 | 245.24 | 6 | 1 | 4 | 79.86 |
| 15 | 2.38 | 327.26 | 6 | 1 | 6 | 79.86 |
| 16 | 2.62 | 324.13 | 6 | 1 | 4 | 79.86 |
| 17 | 2.44 | 279.68 | 6 | 1 | 4 | 79.86 |
| 18 | 2.65 | 313.24 | 6 | 1 | 5 | 79.86 |
| 19 | 1.84 | 275.26 | 7 | 1 | 5 | 89.09 |
| 20 | 0.78 | 211.22 | 6 | 1 | 4 | 79.86 |
| 21 | 1.51 | 237.26 | 6 | 1 | 4 | 79.86 |
| 22 | 2.71 | 342.12 | 6 | 1 | 4 | 79.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathias, F.; Kabri, Y.; Brun, D.; Primas, N.; Di Giorgio, C.; Vanelle, P. Synthesis and Anti-Trypanosoma cruzi Biological Evaluation of Novel 2-Nitropyrrole Derivatives. Molecules 2022, 27, 2163. https://doi.org/10.3390/molecules27072163
Mathias F, Kabri Y, Brun D, Primas N, Di Giorgio C, Vanelle P. Synthesis and Anti-Trypanosoma cruzi Biological Evaluation of Novel 2-Nitropyrrole Derivatives. Molecules. 2022; 27(7):2163. https://doi.org/10.3390/molecules27072163
Chicago/Turabian StyleMathias, Fanny, Youssef Kabri, Damien Brun, Nicolas Primas, Carole Di Giorgio, and Patrice Vanelle. 2022. "Synthesis and Anti-Trypanosoma cruzi Biological Evaluation of Novel 2-Nitropyrrole Derivatives" Molecules 27, no. 7: 2163. https://doi.org/10.3390/molecules27072163
APA StyleMathias, F., Kabri, Y., Brun, D., Primas, N., Di Giorgio, C., & Vanelle, P. (2022). Synthesis and Anti-Trypanosoma cruzi Biological Evaluation of Novel 2-Nitropyrrole Derivatives. Molecules, 27(7), 2163. https://doi.org/10.3390/molecules27072163








