Characterization and Valorization of the Agricultural Waste Obtained from Lavandula Steam Distillation for Its Reuse in the Food and Pharmaceutical Fields
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolic and Total Flavonoid Content
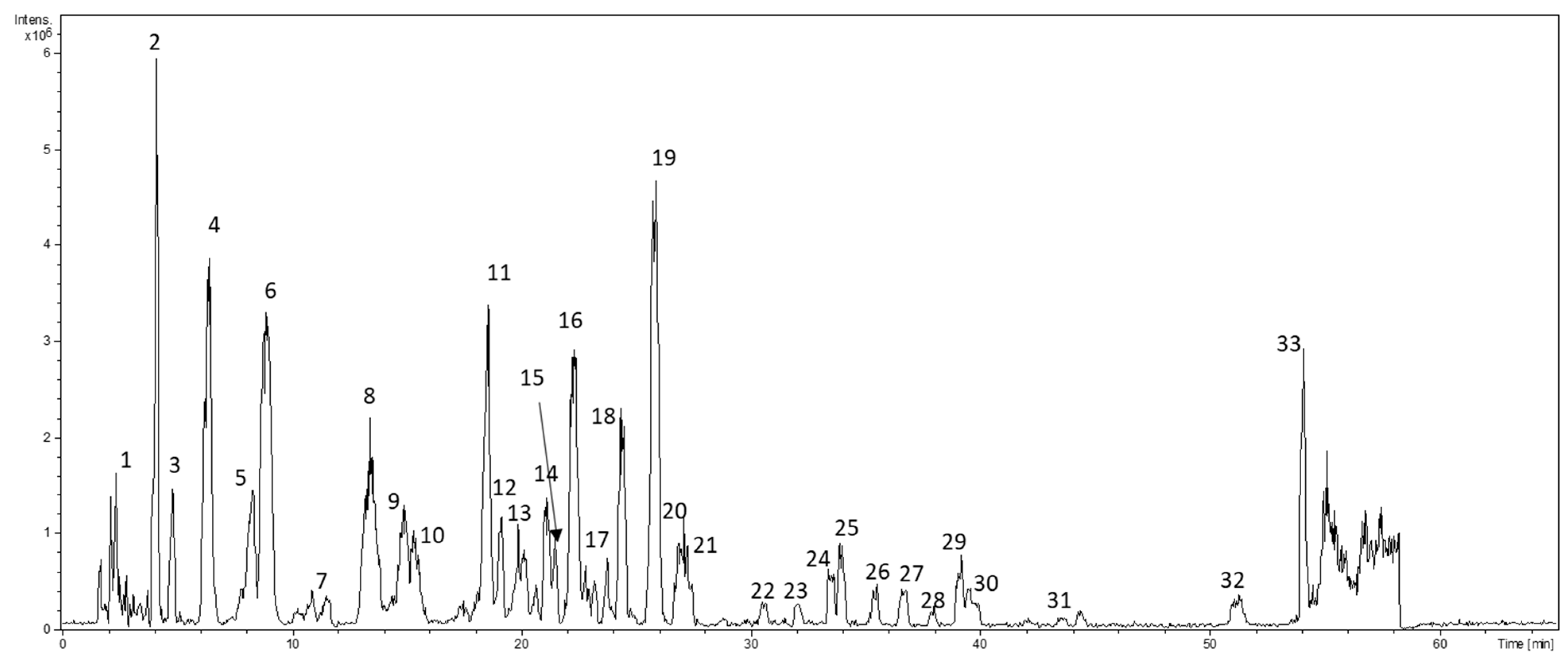
2.2. LC−ESI−MS and MS/MS Analysis
2.3. Antioxidant Activity
2.4. In Vitro Acetylcholinesterase (AChE) and Tyrosinase Inhibition Assay
3. Materials and Methods
3.1. Sample Materials and Chemicals
3.2. Lavender Steam Distillation
3.3. Plant Material and Extraction Procedure
3.4. Total Polyphenolic and Flavonoid Content
3.5. Identification of Polyphenols by LC−ESI−MS and MS2
3.6. Evaluation of Antioxidant Activity
3.6.1. Determination of DPPH Free Radical-Scavenging and Fe2+ Chelating Activities
3.6.2. Reducing Power Activity
3.7. Acetylcholinesterase and Tyrosinase Inhibitory Assays
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- European Commission European Commission Website. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1583933814386&uri=COM:2020:98:FIN (accessed on 24 January 2022).
- Diacono, M.; Persiani, A.; Testani, E.; Montemurro, F.; Ciaccia, C. Recycling agricultural wastes and by-products in organic farming: Biofertilizer production, yield performance and carbon footprint analysis. Sustainability 2019, 11, 3824. [Google Scholar] [CrossRef]
- The NSW Environment Protection Authority Website. Available online: https://www.epa.nsw.gov.au/your-environment/%0Arecycling-and-reuse/warr-strategy/the-waste-hierarchy (accessed on 5 October 2021).
- Demain, A.L.; Fang, A. The natural functions of secondary metabolites. In History of Modern Biotechnology, I.; Fiechter, A., Ed.; Springer: Berlin, Germany, 2000; Volume 69, pp. 1–39. [Google Scholar]
- Hussein, R.A.; El-Anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. In Herbal Medicine; Builders, P., Ed.; IntechOpen: London, UK, 2018; ISBN 978-1-78984-783-3. [Google Scholar]
- Singh, A.; Yau, Y.F.; Leung, K.S.; El-Nezami, H.; Lee, J.C.Y. Interaction of Polyphenols as Antioxidant and Anti-Inflammatory Compounds in Brain–Liver–Gut Axis. Antioxidants 2020, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Cozzolino, A.; de Feo, V.; Coppola, R.; Ombra, M.N.; Nazzaro, F. Polyphenols, Antioxidant, Antibacterial, and Biofilm Inhibitory Activities of Peel and Pulp of Citrus medica L., Citrus bergamia, and Citrus medica cv. Salò Cultivated in Southern Italy. Molecules 2019, 24, 4577. [Google Scholar] [CrossRef] [PubMed]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Khomsi, M.E.; Imtara, H.; Kara, M.; Hmamou, A.; Assouguem, A.; Bourkhiss, B.; Tarayrah, M.; Alzain, M.N.; Alzamel, N.M.; Noman, O.; et al. Antimicrobial and Antioxidant Properties of Total Polyphenols of Anchusa italica Retz. Molecules 2022, 27, 416. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Ahuja, K.; Singh, S. Essential Oils Market Outlook Report 2026|Growth Statistics. Available online: https://www.gminsights.com/industry-analysis/essential-oil-market (accessed on 23 December 2021).
- European Parliament European Council Regulation (EU) 2018/848 of the European parl of 30 May 2018 on organic production and labelling of organic products and repealing Council Regulation (EC) No 834/2007. Off. J. Eur. Communities 2018, 150, 1–92.
- Giray, F.H. An Analysis of World Lavender Oil Markets and Lessons for Turkey. J. Essent. Oil Bear. Plants 2019, 21, 1612–1623. [Google Scholar] [CrossRef]
- Truzzi, E.; Marchetti, L.; Bertelli, D.; Benvenuti, S. Attenuated total reflectance–Fourier transform infrared (ATR–FTIR) spectroscopy coupled with chemometric analysis for detection and quantification of adulteration in lavender and citronella essential oils. Phytochem. Anal. 2021, 1–14. [Google Scholar] [CrossRef]
- Truzzi, E.; Marchetti, L.; Benvenuti, S.; Ferroni, A.; Rossi, M.C.; Bertelli, D. Novel Strategy for the Recognition of Adulterant Vegetable Oils in Essential Oils Commonly Used in Food Industries by Applying 13C NMR Spectroscopy. J. Agric. Food Chem. 2021, 69, 8276–8286. [Google Scholar] [CrossRef]
- Truzzi, E.; Marchetti, L.; Benvenuti, S.; Righi, V.; Rossi, M.C.; Gallo, V.; Bertelli, D. A Novel qNMR Application for the Quantification of Vegetable Oils Used as Adulterants in Essential Oils. Molecules 2021, 26, 5439. [Google Scholar] [CrossRef] [PubMed]
- Capetti, F.; Marengo, A.; Cagliero, C.; Liberto, E.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Adulteration of Essential Oils: A Multitask Issue for Quality Control. Three Case Studies: Lavandula angustifolia Mill., Citrus limon (L.) Osbeck and Melaleuca alternifolia (Maiden & Betche) Cheel. Molecules 2021, 26, 5610. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, J.; Ali, Z.; Avonto, C.; Khan, I.A. A novel approach for lavender essential oil authentication and quality assessment. J. Pharm. Biomed. Anal. 2021, 199, 114050. [Google Scholar] [CrossRef]
- Dubnicka, M.; Cromwell, B.; Levine, M. Investigation of the Adulteration of Essential Oils by GC-MS. Curr. Anal. Chem. 2019, 16, 965–969. [Google Scholar] [CrossRef]
- Beale, D.J.; Morrison, P.D.; Karpe, A.V.; Dunn, M.S. Chemometric analysis of lavender essential oils using targeted and untargeted GC-MS acquired data for the rapid identification and characterization of oil quality. Molecules 2017, 22, 1339. [Google Scholar] [CrossRef] [PubMed]
- Avato, P.; Degli, U.; Di Bari, S.; Moro, A.; Roser Vila, I.; López, V.; Nielsen, B.; Solas, M.; Ramírez, M.J.; Jäger, A.K. Exploring Pharmacological Mechanisms of Lavender (Lavandula angustifolia) Essential Oil on Central Nervous System Targets. Front. Pharmacol. 2017, 8, 280. [Google Scholar] [CrossRef]
- Caputo, L.; Piccialli, I.; Ciccone, R.; De Caprariis, P.; Massa, A.; De Feo, V.; Pannaccione, A. Lavender and coriander essential oils and their main component linalool exert a protective effect against amyloid-β neurotoxicity. Phyther. Res. 2021, 35, 486–493. [Google Scholar] [CrossRef]
- Cardia, G.F.E.; Silva-Filho, S.E.; Silva, E.L.; Uchida, N.S.; Cavalcante, H.A.O.; Cassarotti, L.L.; Salvadego, V.E.C.; Spironello, R.A.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of Lavender (Lavandula angustifolia) Essential Oil on Acute Inflammatory Response. Evid. Based. Complement. Alternat. Med. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Xu, P.; Wang, K.; Lu, C.; Dong, L.; Gao, L.; Yan, M.; Aibai, S.; Yang, Y.; Liu, X. The Protective Effect of Lavender Essential Oil and Its Main Component Linalool against the Cognitive Deficits Induced by D-Galactose and Aluminum Trichloride in Mice. Evid.-Based Complement. Altern. Med. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Nikolova, G.; Karamalakova, Y.; Kovacheva, N.; Stanev, S.; Zheleva, A.; Gadjeva, V. Protective effect of two essential oils isolated from Rosa damascena Mill. and Lavandula angustifolia Mill, and two classic antioxidants against L-dopa oxidative toxicity induced in healthy mice. Regul. Toxicol. Pharmacol. 2016, 81, 1–7. [Google Scholar] [CrossRef]
- Sánchez-Vidaña, D.I.; Po, K.K.T.; Fung, T.K.H.; Chow, J.K.W.; Lau, W.K.W.; So, P.K.; Lau, B.W.M.; Tsang, H.W.H. Lavender essential oil ameliorates depression-like behavior and increases neurogenesis and dendritic complexity in rats. Neurosci. Lett. 2019, 701, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Said, L.A.; Zahlane, K.; Ghalbane, I.; Messoussi, S.E.; Romane, A.; Cavaleiro, C.; Salgueiro, L. Chemical composition and antibacterial activity of Lavandula coronopifolia essential oil against antibiotic-resistant bacteria. Nat. Prod. Res. 2015, 29, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Varona, S.; Rodríguez Rojo, S.; Martín, Á.; Cocero, M.J.; Serra, A.T.; Crespo, T.; Duarte, C.M.M. Antimicrobial activity of lavandin essential oil formulations against three pathogenic food-borne bacteria. Ind. Crops Prod. 2013, 42, 243–250. [Google Scholar] [CrossRef]
- Germinara, G.S.; Giovanna, M.; Stefano, D.I.; De Acutis, L.; Pati, S.; Delfine, S.; De Cristofaro, A.; Rotundo, G. Bioactivities of Lavandula angustifolia essential oil against the stored grain pest Sitophilus granarius. Bull. Insectol. 2017, 70, 129–138. [Google Scholar]
- Chang, Y.; Harmon, P.F.; Treadwell, D.D.; Carrillo, D.; Sarkhosh, A.; Brecht, J.K. Biocontrol Potential of Essential Oils in Organic Horticulture Systems: From Farm to Fork. Front. Nutr. 2022, 8, 1275. [Google Scholar] [CrossRef] [PubMed]
- Khater, H.F.; Ali, A.M.; Abouelella, G.A.; Marawan, M.A.; Govindarajan, M.; Murugan, K.; Abbas, R.Z.; Vaz, N.P.; Benelli, G. Toxicity and growth inhibition potential of vetiver, cinnamon, and lavender essential oils and their blends against larvae of the sheep blowfly, Lucilia sericata. Int. J. Dermatol. 2018, 57, 449–457. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Phytotoxic Effects of Commercial Eucalyptus citriodora, Lavandula angustifolia, and Pinus sylvestris Essential Oils on Weeds, Crops, and Invasive Species. Molecules 2019, 24, 2847. [Google Scholar] [CrossRef]
- Spiridon, I.; Colceru, S.; Anghel, N.; Teaca, C.A.; Bodirlau, R.; Armatu, A. Antioxidant capacity and total phenolic contents of oregano (Origanum vulgare), lavender (Lavandula angustifolia) and lemon balm (Melissa officinalis) from Romania. Nat. Prod. Res. 2011, 25, 1657–1661. [Google Scholar] [CrossRef]
- Duda, S.C.; Mărghitaş, L.A.; Dezmirean, D.; Duda, M.; Mărgăoan, R.; Bobiş, O. Changes in major bioactive compounds with antioxidant activity of Agastache foeniculum, Lavandula angustifolia, Melissa officinalis and Nepeta cataria: Effect of harvest time and plant species. Ind. Crops Prod. 2015, 77, 499–507. [Google Scholar] [CrossRef]
- Turrini, F.; Beruto, M.; Mela, L.; Curir, P.; Triglia, G.; Boggia, R.; Zunin, P.; Monroy, F. Ultrasound-assisted extraction of lavender (Lavandula angustifolia miller, cultivar rosa) solid by-products remaining after the distillation of the essential oil. Appl. Sci. 2021, 11, 5495. [Google Scholar] [CrossRef]
- Slavov, A.; Karneva, K.; Vasileva, I.; Denev, P.; Denkova, R.; Shikov, V.; Monolova, M.; Lazarova, Y.; Ivanova, V. Valorization of lavender waste–obtaining and characteristics of polyphenol rich extracts. Food Sci. Appl. Biotechnol. 2018, 1, 11–18. [Google Scholar]
- Méndez-Tovar, I.; Herrero, B.; Pérez-Magariño, S.; Pereira, J.A.; Asensio-S-Manzanera, M.C. By-product of Lavandula latifolia essential oil distillation as source of antioxidants. J. Food Drug Anal. 2015, 23, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.d.M.; Algieri, F.; Rodriguez-Nogales, A.; Gálvez, J.; Segura-Carretero, A. Phytochemical profiling of anti-inflammatory Lavandula extracts via RP–HPLC–DAD–QTOF–MS and –MS/MS: Assessment of their qualitative and quantitative differences. Electrophoresis 2018, 39, 1284–1293. [Google Scholar] [CrossRef]
- Algieri, F.; Rodriguez-Nogales, A.; Vezza, T.; Garrido-Mesa, J.; Garrido-Mesa, N.; Utrilla, M.P.; González-Tejero, M.R.; Casares-Porcel, M.; Molero-Mesa, J.; Del Mar Contreras, M.; et al. Anti-inflammatory activity of hydroalcoholic extracts of Lavandula dentata L. and Lavandula stoechas L. J. Ethnopharmacol. 2016, 190, 142–158. [Google Scholar] [CrossRef]
- Lavorgna, M.; Pacifico, S.; Nugnes, R.; Russo, C.; Orlo, E.; Piccolella, S.; Isidori, M. Theobroma cacao criollo var. Beans: Biological properties and chemical profile. Foods 2021, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhao, X.; Li, X.; Wu, S.; Yu, S.; Lou, Y.; Fan, G. High-Throughput Determination of Sodium Danshensu in Beagle Dogs by the LCMS/MS Method, Employing Liquid-Liquid Extraction Based on 96-Well Format Plates. Molecules 2017, 22, 667. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Ezzat, S.M.; Salama, M.M.; Tadros, M.G. Anti-acetylcholinesterase potential and metabolome classification of 4 Ocimum species as determined via UPLC/qTOF/MS and chemometric tools. J. Pharm. Biomed. Anal. 2016, 125, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Møller, J.K.S.; Catharino, R.R.; Eberlin, M.N. Electrospray ionization mass spectrometry fingerprinting of whisky: Immediate proof of origin and authenticity. Analyst 2005, 130, 890–897. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oliveira-Alves, S.C.; Vendramini-Costa, D.B.; Betim Cazarin, C.B.; Maróstica Júnior, M.R.; Borges Ferreira, J.P.; Silva, A.B.; Prado, M.A.; Bronze, M.R. Characterization of phenolic compounds in chia (Salvia hispanica L.) seeds, fiber flour and oil. Food Chem. 2017, 232, 295–305. [Google Scholar] [CrossRef]
- Zeng, G.; Xiao, H.; Liu, J.; Liang, X. Identification of phenolic constituents in Radix Salvia miltiorrhizae by liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, L.L.; Vilegas, W.; Dokkedal, A.L. Characterization of flavonoids and phenolic acids in Myrcia bella cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS combined with NMR. Molecules 2013, 18, 8402–8416. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; You, L.; Zeng, S. Studies on the flavonoid substrates of human UDP-glucuronosyl transferase (UGT) 2B7. Pharmazie 2007, 62, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Han, L.; Li, J.; Liu, C.; Sun, C. Role of Flavonoids in the Treatment of Iron Overload. Front. Cell Dev. Biol. 2021, 9, 685364. [Google Scholar] [CrossRef] [PubMed]
- Miliauskas, G.; Venskutonis, P.R.; Van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Sánchez-Vioque, R.; Polissiou, M.; Astraka, K.; De los Mozos-Pascual, M.; Tarantilis, P.; Herraiz-Peñalver, D.; Santana-Méridas, O. Polyphenol composition and antioxidant and metal chelating activities of the solid residues from the essential oil industry. Ind. Crops Prod. 2013, 49, 150–159. [Google Scholar] [CrossRef]
- Baptista, R.; Madureira, A.M.; Jorge, R.; Adão, R.; Duarte, A.; Duarte, N.; Lopes, M.M.; Teixeira, G. Antioxidant and antimycotic activities of two native Lavandula species from Portugal. Evid.-Based Complement. Altern. Med. 2015, 2015, 570521. [Google Scholar] [CrossRef]
- Blažeković, B.; Vladimir-Knežević, S.; Brantner, A.; Štefan, M.B. Evaluation of Antioxidant Potential of Lavandula x intermedia Emeric ex Loisel. ‘Budrovka’: A Comparative Study with L. angustifolia Mill. Molecules 2010, 15, 5971–5987. [Google Scholar] [CrossRef]
- Robu, S.; Aprotosoaie, A.C.; Miron, A.; Cioancă, O.; Stănescu, U.; Hăncianu, M. In vitro antioxidant activity of ethanolic extracts from some lavandula species cultivated in romania. Farmacia 2012, 60, 3. [Google Scholar]
- Lakey-Beitia, J.; Burillo, A.M.; Penna, G.L.; Hegde, M.L.; Rao, K.S. Polyphenols as Potential Metal Chelation Compounds Against Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 82, S335. [Google Scholar] [CrossRef]
- Arya, A.; Chahal, R.; Rao, R.; Rahman, M.H.; Kaushik, D.; Akhtar, M.F.; Saleem, A.; Khalifa, S.M.A.; El-Seedi, H.R.; Kamel, M.; et al. Acetylcholinesterase inhibitory potential of various sesquiterpene analogues for alzheimer’s disease therapy. Biomolecules 2021, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Proença, C.; Serralheiro, M.L.M.; Araújo, M.E.M. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Świątek, Ł.; Sieniawska, E.; Mahomoodally, M.F.; Sadeer, N.B.; Wojtanowski, K.K.; Rajtar, B.; Polz-Dacewicz, M.; Paksoy, M.Y.; Zengin, G. Phytochemical Profile and Biological Activities of the Extracts from Two Oenanthe Species (O. aquatica and O. silaifolia). Pharmaceuticals 2021, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Jabir, N.R.; Khan, F.R.; Tabrez, S. Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of Alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 753. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Guo, Y.; Zhang, Y.; Zhuang, Y. Antioxidant and Anti-tyrosinase Activities of Phenolic Extracts from Rape Bee Pollen and Inhibitory Melanogenesis by cAMP/MITF/TYR Pathway in B16 Mouse Melanoma Cells. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Fan, L.; Duan, Z. Five individual polyphenols as tyrosinase inhibitors: Inhibitory activity, synergistic effect, action mechanism, and molecular docking. Food Chem. 2019, 297, 124910. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Farag, M.A.; Ezzat, S.M.; Salama, M.M.; Tadros, M.G.; Serya, R.A.T. Anti-acetylcholinesterase activity of essential oils and their major constituents from four Ocimum species. Z. Naturforsch–Sect. C J. Biosci. 2016, 71, 393–402. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and anti-aging potentials of essential oils from aromatic and medicinal plants. Front. Aging Neurosci. 2017, 9, 1–16. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Kasprzak, K.; Oniszczuk, T.; Oniszczuk, A. Natural Monoterpenes: Much More than Only a Scent. Chem. Biodivers. 2019, 16, e1900434. [Google Scholar] [CrossRef]
- Vladimir-Knezevic, S.; Blazekovic, B.; Kindl, M.; Vladic, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Grosso, C.; Gonçalves, S.; Andrade, P.B.; Valentão, P.; Gabriela Bernardo-Gil, M.; Romano, A. Supercritical fluid extraction and hydrodistillation for the recovery of bioactive compounds from Lavandula viridis L’Hér. Food Chem. 2012, 135, 112–121. [Google Scholar] [CrossRef]
- Hsu, C.K.; Chang, C.T.; Lu, H.Y.; Chung, Y.C. Inhibitory effects of the water extracts of Lavendula sp. on mushroom tyrosinase activity. Food Chem. 2007, 105, 1099–1105. [Google Scholar] [CrossRef]
- Truzzi, E.; Benvenuti, S.; Bertelli, D.; Francia, E.; Ronga, D. Effects of Biostimulants on the Chemical Composition of Essential Oil and Hydrosol of Lavandin (Lavandula x intermedia Emeric ex Loisel.) Cultivated in Tuscan-Emilian Apennines. Molecules 2021, 26, 6157. [Google Scholar] [CrossRef] [PubMed]
- Papotti, G.; Bertelli, D.; Bortolotti, L.; Plessi, M. Chemical and Functional Characterization of Italian Propolis Obtained by Different Harvesting Methods. J. Agric. Food Chem. 2012, 60, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Fiocco, D.; Fiorentino, D.; Frabboni, L.; Benvenuti, S.; Orlandini, G.; Pellati, F.; Gallone, A. Lavender and peppermint essential oils as effective mushroom tyrosinase inhibitors: A basic study. Flavour Fragr. J. 2011, 26, 441–446. [Google Scholar] [CrossRef]
- Renaud, E.N.C.; Charles, D.J.; Simon, J.E. Composition from 10 Cultivars of Organically Grown Lavender and Lavandin. J. Essent. Oil Res. 2001, 13, 269–273. [Google Scholar] [CrossRef]
- Lafhal, S.; Vanloot, P.; Bombarda, I.; Kister, J.; Dupuy, N. Identification of metabolomic markers of lavender and lavandin essential oils using mid-infrared spectroscopy. Vib. Spectrosc. 2016, 85, 79–90. [Google Scholar] [CrossRef]
- Jianu, C.; Pop, G.; Gruia, A.T.; Horhat, F.G. (Lavandula angustifolia) and lavandin (Lavandula x intermedia) grown in Western Romania. Int. J. Agric. Biol. 2013, 15, 772–776. [Google Scholar]
- Council of Europe (Ed.) European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, Germany, 2021. [Google Scholar]
- Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Lavandin (Lavandula × intermedia Emeric ex Loiseleur) essential oil from Spain: Determination of aromatic profile by gas chromatography-mass spectrometry, antioxidant and lipoxygenase inhibitory bioactivities. Nat. Prod. Res. 2016, 30, 1123–1130. [Google Scholar] [CrossRef]
- Bombarda, I.; Dupuy, N.; Le Van Da, J.P.; Gaydou, E.M. Comparative chemometric analyses of geographic origins and compositions of lavandin var. Grosso essential oils by mid infrared spectroscopy and gas chromatography. Anal. Chim. Acta 2008, 613, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Barocelli, E.; Calcina, F.; Chiavarini, M.; Impicciatore, M.; Bruni, R.; Bianchi, A.; Ballabeni, V. Antinociceptive and gastroprotective effects of inhaled and orally administered Lavandula hybrida Reverchon “grosso” essential oil. Life Sci. 2004, 76, 213–223. [Google Scholar] [CrossRef]
- Usano-Alemany, J.; Herraiz Peñalver, D.; Cuadrado Ortiz, J.; De López, B.B.; Ruiz, O.S.; Palá-Paúl, J. Ecological production of lavenders in Cuenca province (Spain). A study of yield production and quality of the essential oils. Bot. Complut. 2011, 35, 147–152. [Google Scholar]
- Hassiotis, C.N.; Ntana, F.; Lazari, D.M.; Poulios, S.; Vlachonasios, K.E. Environmental and developmental factors affect essential oil production and quality of Lavandula angustifolia during flowering period. Ind. Crops Prod. 2014, 62, 359–366. [Google Scholar] [CrossRef]
- Fernández-Sestelo, M.; Carrillo, J.M. Environmental effects on yield and composition of essential oil in wild populations of spike lavender (Lavandula latifolia medik.). Agric. 2020, 10, 626. [Google Scholar] [CrossRef]
- Moghaddam, M.; Mehdizadeh, L. Chemistry of Essential Oils and Factors Influencing Their Constituents. In Soft Chemistry and Food Fermentation; Elsevier: Amsterdam, The Netherlands, 2017; pp. 379–419. [Google Scholar]
- Arabaci, O.; Bayram, E.; Baydar, H.; Ferhan Savran, A.; Karadogan, T.; Ozay, N. Chemical Composition, Yield and Contents of Essential Oil of Lavandula hybrida Reverchon Grown under Different Nitrogen Fertilizer, Plant Density and Location. Asian J. Chem. 2007, 19, 2184–2192. [Google Scholar]
| Residual Material | LA | LI |
|---|---|---|
| TPC (mg GAE/g) | 19.22 ± 4.16 | 17.06 ± 3.31 |
| TPC % (GAE dry extract) | 9.09 ± 0.33 | 8.84 ± 0.12 |
| TFC (mg QE/g) | 1.56 ± 0.21 | 1.41 ± 0.10 |
| TFC % (QE/dry extract) | 0.74 ± 0.03 | 0.73 ± 0.01 |
| Peak Number | Rt (min) | Tentative Identification | [M-H]− (m/z) | Fragments (m/z) | Molecular Weight (g/mol) | LA | LI |
|---|---|---|---|---|---|---|---|
| 1 | 2.3 | Caffeoyl aspartic acid | 294.1 | 179.0 | 295.24 | + | − |
| 2 | 4.2 | Danshensu | 395.0 (2M-H), 197.0 | 178.9, 135.0 | 198.17 | + | + |
| 3 | 4.9 | Unknown | 501.0 | 336.9, 295.0 | + | + | |
| 4 | 6.5 | p-coumaric acid hexose | 651.3 (2M-H), 325.0 | 162.9, 119.0 | 326.10 | + | + |
| 5 | 8.2 | Unknown | 387.2 | 369.2, 207.0 | + | + | |
| 6 | 8.9 | Ferulic acid hexose | 711.3 (2M-H), 355.1 | 192.9, 148.9 | 356.32 | + | + |
| 7 | 11.5 | Unknown | 351.0 | 248.9, 231.0, 177.0, 113.0 | + | − | |
| 8 | 13.3 | p-coumaric acid hexose | 651.0 (2M-H), 325.1 | 162.9, 119.0 | 326.10 | + | − |
| 9 | 14.7 | Luteolin 7-O-diglucuronide | 637.2 | 461.1, 284.9 | 638.11 | + | − |
| 10 | 15.5 | Apigenin 7-O-diglucuronide | 621.0 | 445.1, 268.9 | 622.12 | + | − |
| 11 | 18.4 | Ferulic acid hexose | 711.0 (2M-H), 355.1 | 192.9, 149.0 | 356.32 | + | + |
| 12 | 19.2 | Unknown | 521.2 | 358.9, 229.0, 285.0 | + | − | |
| 13 | 20.7 | Quercetin hexose | 463.0 | 301.0, 178.9 | 464.09 | + | − |
| 14 | 21.0 | Luteolin/kaempferol hexose | 447.2 | 284.9 | 448.10 | + | + |
| 15 | 21.5 | Unknown | 441.2 | 395.3, 262.9 | + | + | |
| 16 | 22.3 | Luteolin/kaempferol glucuronide | 461.1 | 284.9 | 462.40 | + | + |
| 17 | 23.7 | Quercetin 3-O-rhamnoside | 447.1 | 300.9, 151.0 | 448.10 | + | − |
| 18 | 24.3 | Apigenin 7-O-glucoside | 431.2 | 269.0 | 432.40 | + | + |
| 19 | 25.8 | Rosmarinic acid | 359.1 | 222.8, 196.9, 178.9, 160.9, | 360.31 | + | + |
| 20 | 26.7 | Luteolin/kaempferol glucuronide | 461.0 | 285.0 | 462.40 | + | + |
| 21 | 27.2 | Apigenin 7-O-glucurunide | 445.1 | 269.0, 174.9 | 446.40 | + | + |
| 22 | 30.5 | Rosmarinic acid methylester | 373.0 | 178.9, 135.0 | 374.30 | + | − |
| 23 | 31.9 | Kaempferol/Luteolin | 285.1 | 254.8, 226.9 | 286.05 | + | − |
| 24 | 33.4 | Unknown | 493.0 | 295.0, 269.1 | + | + | |
| 25 | 33.9 | Unknown | 618.4 | 582.4, 462.3 | + | − | |
| 26 | 35.3 | Quercetin hexose | 463.2 | 301.0 | 464.09 | + | + |
| 27 | 36.6 | Unknown | 507.3 | 345.2, 299.2 | + | + | |
| 28 | 37.9 | Unknown | 329.2 | 221.0, 193.0, 170.9 | + | + | |
| 29 | 39.1 | Ellagic acid | 301.2 | 283.4 | 302.19 | + | − |
| 30 | 39.6 | Unknown | 287.2 | 269.1 | + | − | |
| 31 | 44.4 | Unknown | 307.2 | 289.0, 235.0, 185.0 | + | − | |
| 32 | 51.0 | Unknown | 309.2 | 291.1, 208.9, 184.9 | + | − | |
| 33 | 53.9 | Unknown | 487.5 | 469.4 | + | − |
| LA | LI | |
|---|---|---|
| Antiradical activity | 94.17 ± 6.29 mg eqT/g | 94.51 ± 2.85 mg eqT/g |
| Chelation activity | 1.49 ± 0.03 mg eqEDTA/g | 2.10 ± 0.13 mg eqEDTA/g |
| Fe3+ reduction capacity | 89.36 ± 3.92 mg eqAA/g | 74.53 ± 2.74 mg eqAA/g |
| AChE | Tyrosinase | |
|---|---|---|
| LA | 5.35 ± 0.47 mg/mL | 5.26 ± 0.02 mg/mL |
| LI | 6.67 ± 0.12 mg/mL | 6.56 ± 0.16 mg/mL |
| Galantamine | 18.83 ± 1.05 μg/mL | - |
| Kojic acid | - | 18.13 ± 0.45 μg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truzzi, E.; Chaouch, M.A.; Rossi, G.; Tagliazucchi, L.; Bertelli, D.; Benvenuti, S. Characterization and Valorization of the Agricultural Waste Obtained from Lavandula Steam Distillation for Its Reuse in the Food and Pharmaceutical Fields. Molecules 2022, 27, 1613. https://doi.org/10.3390/molecules27051613
Truzzi E, Chaouch MA, Rossi G, Tagliazucchi L, Bertelli D, Benvenuti S. Characterization and Valorization of the Agricultural Waste Obtained from Lavandula Steam Distillation for Its Reuse in the Food and Pharmaceutical Fields. Molecules. 2022; 27(5):1613. https://doi.org/10.3390/molecules27051613
Chicago/Turabian StyleTruzzi, Eleonora, Mohamed Aymen Chaouch, Gaia Rossi, Lorenzo Tagliazucchi, Davide Bertelli, and Stefania Benvenuti. 2022. "Characterization and Valorization of the Agricultural Waste Obtained from Lavandula Steam Distillation for Its Reuse in the Food and Pharmaceutical Fields" Molecules 27, no. 5: 1613. https://doi.org/10.3390/molecules27051613
APA StyleTruzzi, E., Chaouch, M. A., Rossi, G., Tagliazucchi, L., Bertelli, D., & Benvenuti, S. (2022). Characterization and Valorization of the Agricultural Waste Obtained from Lavandula Steam Distillation for Its Reuse in the Food and Pharmaceutical Fields. Molecules, 27(5), 1613. https://doi.org/10.3390/molecules27051613






