Pentacyclic Triterpenoids from Sabia discolor Dunn and Their α-Glycosidase Inhibitory Activities
Abstract
:1. Introduction
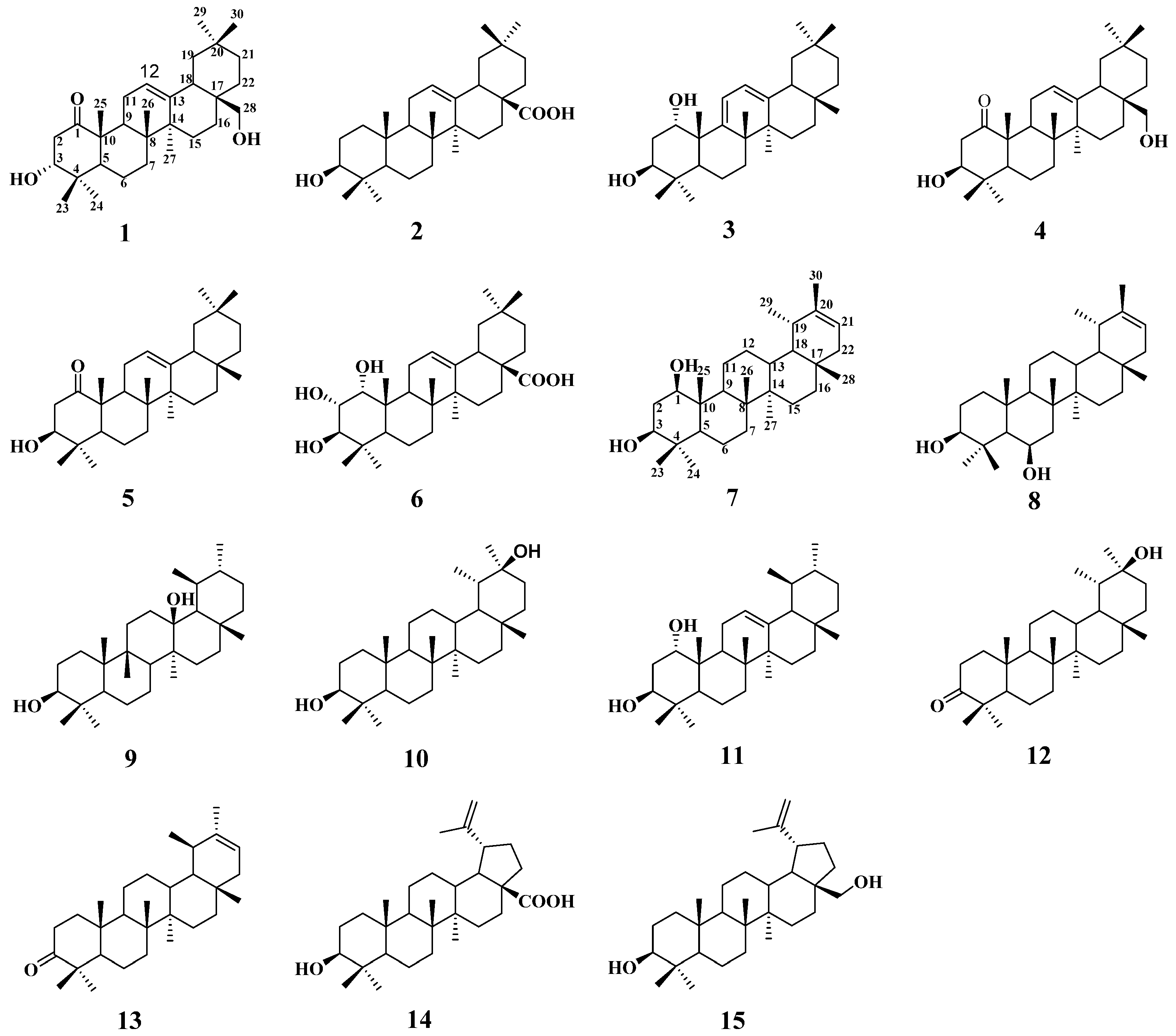
2. Results and Discussion
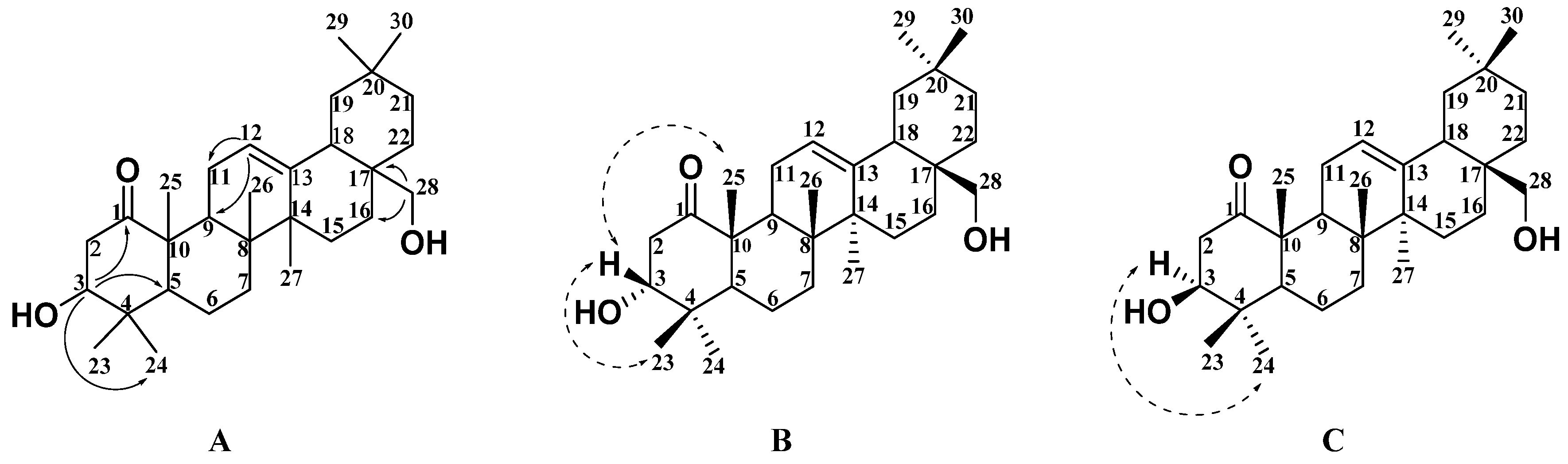
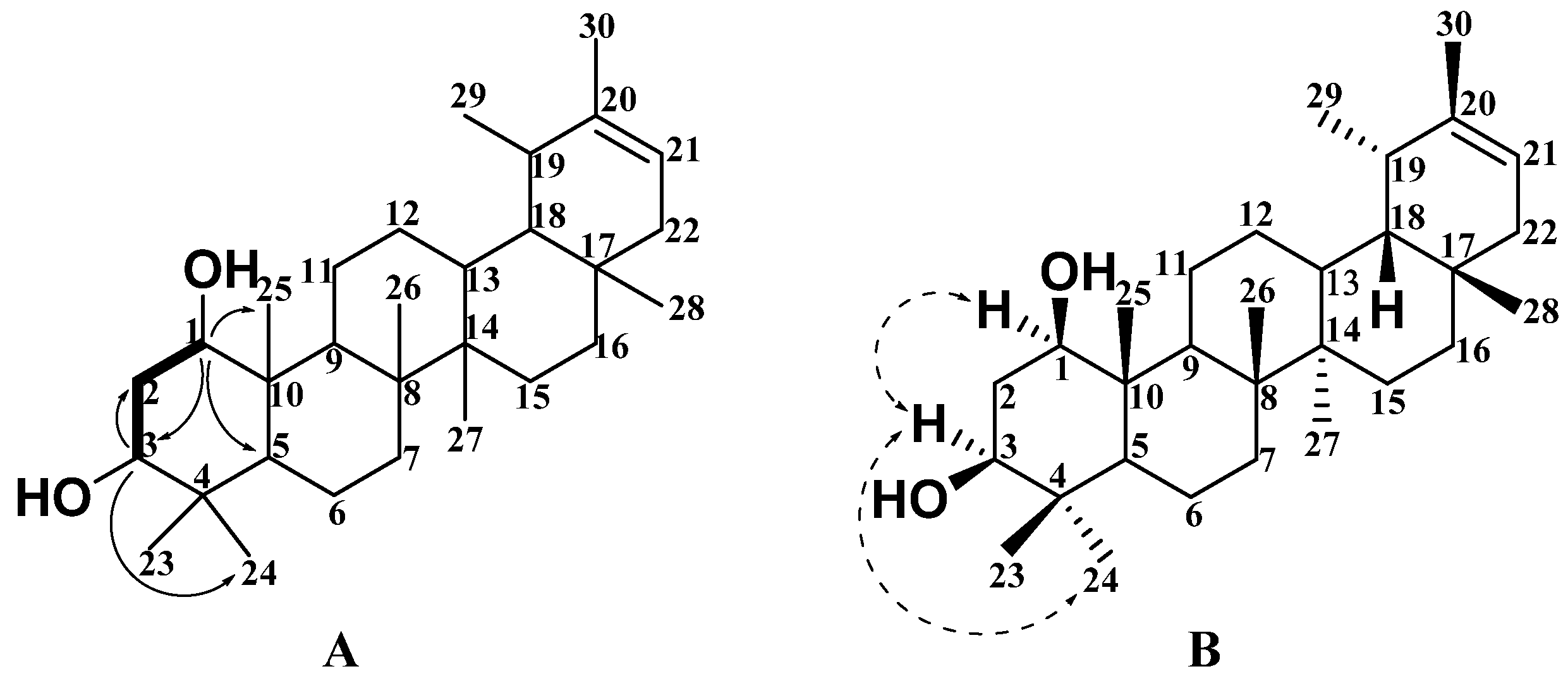
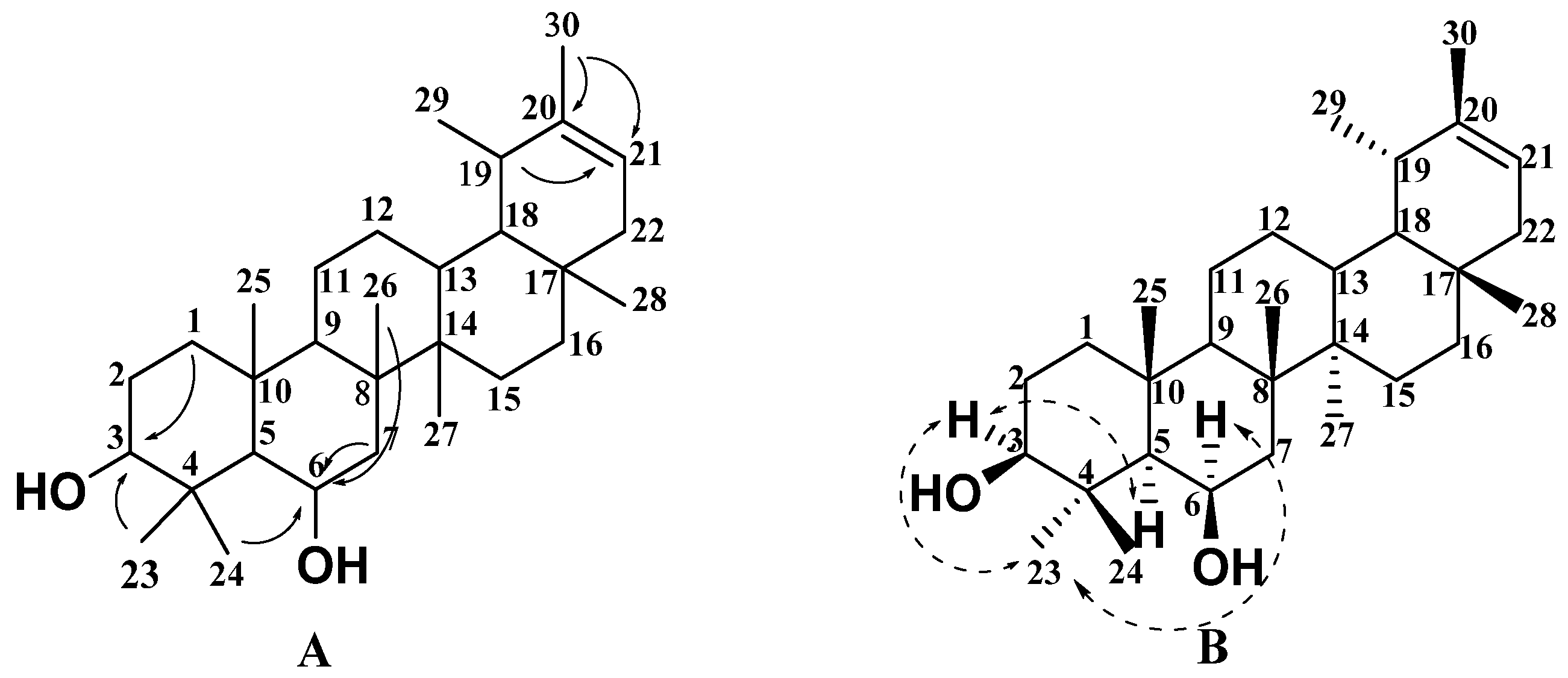
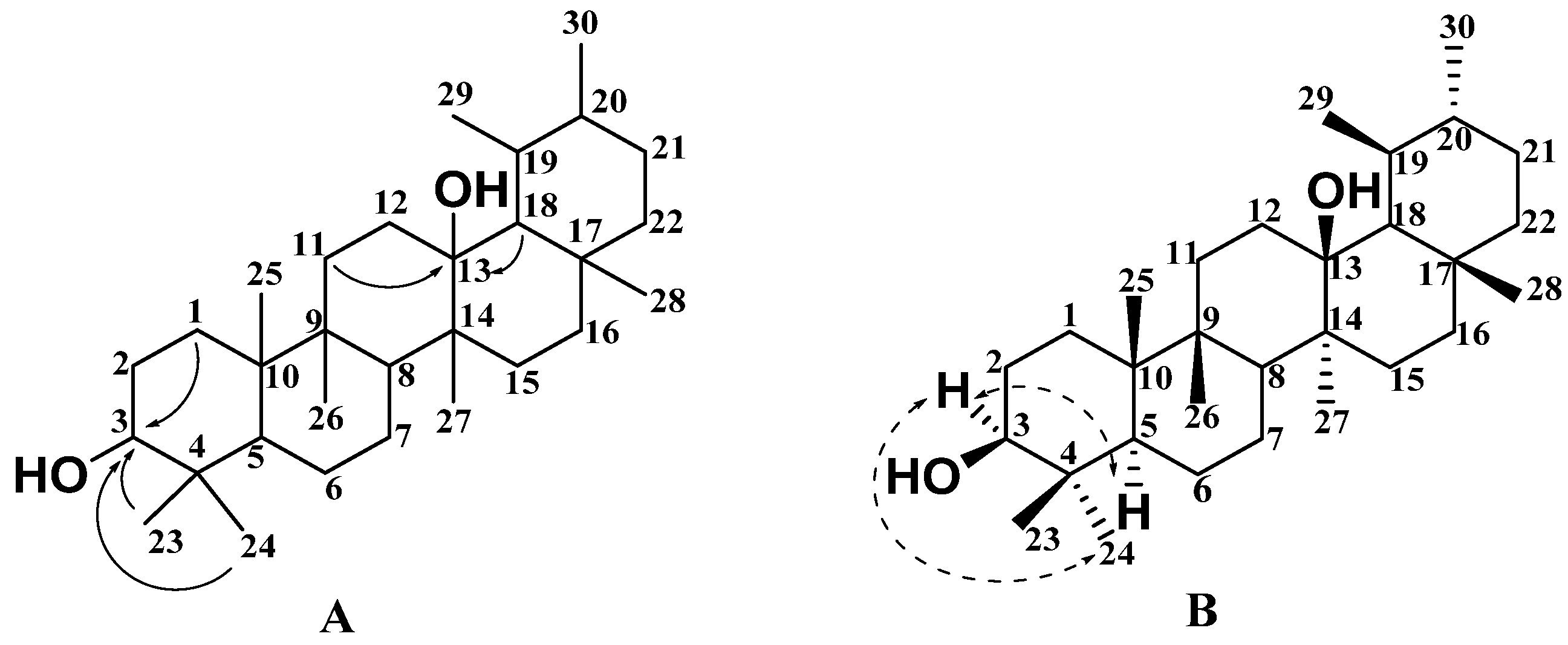
2.1. Structural Analysis of New Compounds
2.2. α-Glycosidase Inhibitory Activities
2.3. Discussion
3. Materials and Methods
3.1. General Experimental Materials
3.2. Plant Materials
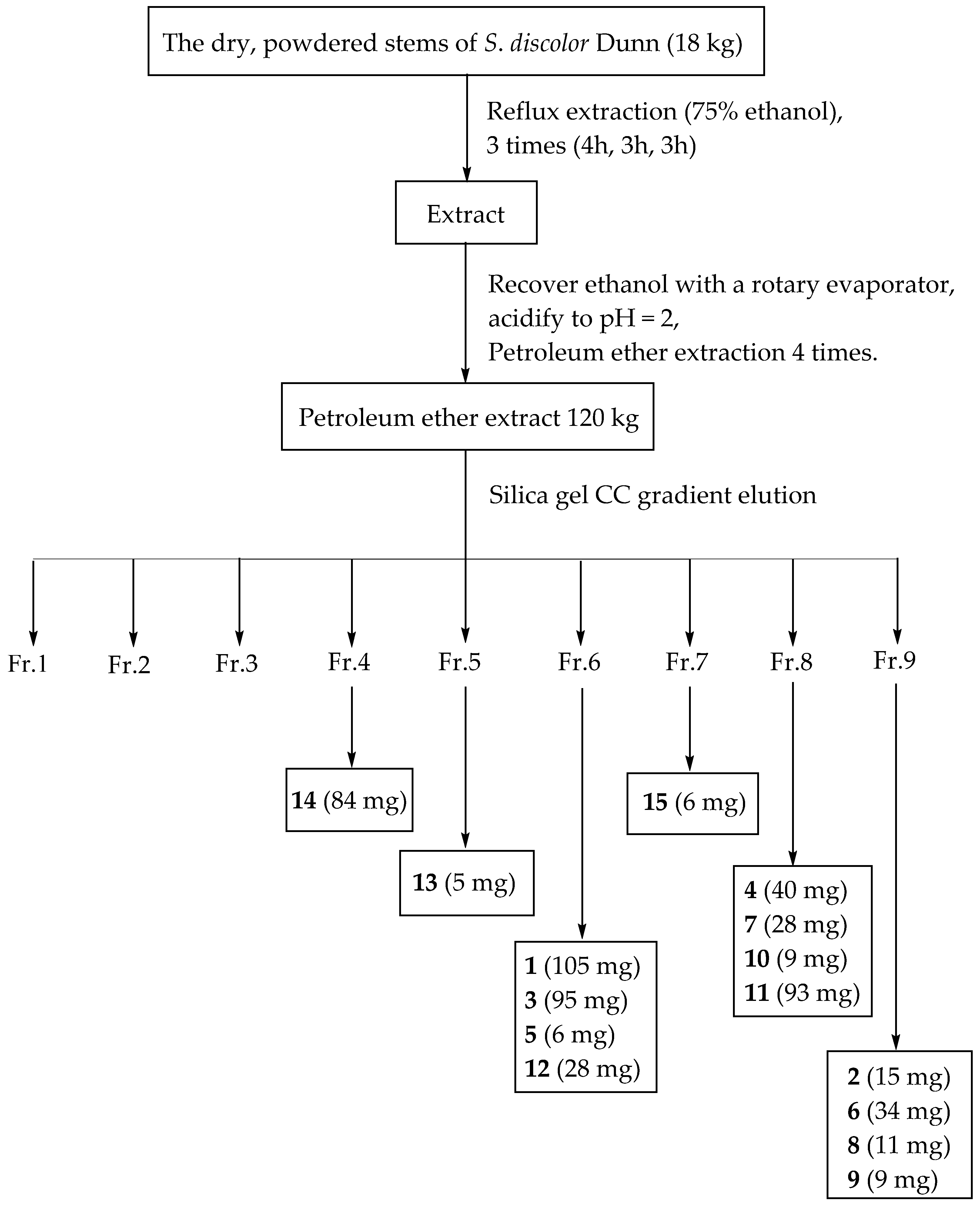
3.3. Extraction and Isolation
3.4. Assay of α-glycosidase Inhibition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mahomoodally, M.F.; Lobine, D.; Picot-Allain, M.C.N.; Sadeer, N.; Jugreet, S.; Zengin, G. Conventional and non-conventional targets of natural products in the management of diabetes mellitus and associated complications. Curr. Med. Chem. 2021, 28, 4638–4669. [Google Scholar] [CrossRef] [PubMed]
- Alomari, A.S.; Al-Harithy, R.N. Intergenic lnc-LEP-2:6 and lnc-LEP-2:7 as novel biomarkers associated with type 2 diabetes mellitus. Arch. Phy. Biochem. 2021, 6, 1–6. [Google Scholar] [CrossRef]
- Simos, Y.V.; Spyrou, K.; Patila, M.; Karouta, N.; Stamatis, H.; Gournis, D. Trends of nanotechnology in type 2 diabetes mellitus treatment. Asian. J. Phar. Sci. 2021, 16, 15–30. [Google Scholar] [CrossRef]
- Wu, X.F.; Xu, Y.; Li, Y.P. Research progress of screening models for α-glucosidase inhibitors. Int. J. Phar. 2008, 35, 9–12. [Google Scholar]
- Cai, Y.H.; Guo, Y.Q.; Li, Z.; Wu, D.Y.; Li, X.L.; Zhang, H.; Yang, J.J.; Lu, H.; Sun, Z.W.; Luo, H.B.; et al. Discovery and modelling studies of natural ingredients from Gaultheria yunnanensis (FRANCH) against phosphodiesterase-4. Eur. J. Med. Chem. 2016, 114, 134–140. [Google Scholar] [CrossRef]
- Li, W.; Lou, L.L.; Zhu, J.Y.; Zhang, J.S.; Liang, A.A.; Bao, J.M.; Tang, G.H.; Yin, S. New lanostane-type triterpenoids from the fruiting body of Ganoderma hainanense. Fitoterapia 2016, 115, 24–30. [Google Scholar] [CrossRef]
- Luo, S.Y.; Pu, R.; Tang, Y.Q.; Fan, R.Z.; Yin, S.; Tang, G.H. Euphane- and 19 (10→9) abeo-euphane-type triterpenoids from Jatropha gossypiifolia. Fitoterapia 2020, 143, 104582–104586. [Google Scholar] [CrossRef]
- Liu, J.C.; Yang, Q.M.; Zhu, Z.F. Studies on the chemical constttuents of Coriaria sinica maxim. Nat. Sci. 1996, 30, 196–198. [Google Scholar]
- Deng, Y.; Li, X.; Wu, F.E. Study on the chemical constituents of Sabia yunnanensis Franch. Chin. Tradit. Herb. Med. 2006, 37, 183–185. [Google Scholar]
- Kagawa, M.; Minami, H.; Mai, N.; Takahashi, H.; Takaoka, S.; Fukuyama, Y. Oleanane-type triterpenes from Viburnum awabuki. Phytochemistry 1998, 47, 1337–1341. [Google Scholar] [CrossRef]
- Chatterjee, P.; Pezzuto, J.M.; Kouzi, S.A. Glucosidation of betulinic acid by Cunninghamella species. J. Nat. Prod. 1999, 62, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.M.; Kuo, Y.H. Taraxastane-type triterpenes from the aerial roots of Ficus macrocarpa. J. Nat. Prod. 2000, 63, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Mashitah, Y.; Hazrulrizawati, H.; Peter, H. Anticholinesterase inhibitory activity of quaternary alkaloids from Tinospora crispa. Molecules 2014, 19, 1201–1211. [Google Scholar]
- Li, L.L.; Sun, Z.; Shang, X.Y.; Li, J.J.; Wang, R.; Zhu, J. Studies on triterpenes from Cirsium setosum. J. Chin. Mater. Med. 2012, 37, 951–955. [Google Scholar]
- Wang, P.; Song, Q.S.; Xu, W.; Li, S.Z. Study on chemical Constituents of Leaves and branches of Beaumontia grandi flova. Chin. Tradit. Herb. Med. 2009, 40, 1549–1551. [Google Scholar]
- Zheng, H.T.; Zhang, G.G.; Zheng, Y.F.; Jin, Y.P.; Du, S.S.; Deng, Z.W. Study on lipid soluble Chemical Constituents of Tibetan medicine Dracocephalum tanguticum Maxim. Chin. J. Med. Chem. 2007, 17, 314–315. [Google Scholar]
- Huang, Y.; Zhang, C.L.; Lang, W.W.; Chen, M.S.; Liu, Y.; Liu, B.M. A new oxoaporphine from Sabia limoniacea var. ardisioides. Acta Pharm. Sin. 2018, 5, 778–781. [Google Scholar]
- Chen, J.; Chen, B. Two New Pentacyclic Triterpenes from Sabia parviflora. Chin. Chem. Lett. 2002, 13, 345–348. [Google Scholar]
- Zhang, Y.W.; Lin, H.; Bao, Y.L.; Wu, Y.; Yu, C.L.; Huang, Y.X.; Li, Y.X. A new triterpenoid and other constituents from the stem bark of Juglans mandshurica. Biochem. Syst. Ecol. 2012, 44, 136–140. [Google Scholar] [CrossRef]
- Mahato, S.B.; Kundu, A.P. 13 C NMR Spectra of pentacyclic triterpenoids—a compilation and some salient features. Phytochemistry 1994, 37, 1517–1575. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wu, D.G. Two new triterpenoids from Tripterygium wilfordii. Acta Botanica. Yunnanica 1993, 15, 309–310. [Google Scholar]
- Zhang, X.Q.; Li, M.S.; Li, Y.L.; Zhang, X.X.; Lu, S.G.; Wen, X.F.; Hu, J.Z.; Zou, K. An overview of the research on medicinal plants of the genus Sabia in China. Biol. Resources. 2018, 40, 4–17. [Google Scholar]
- Wen, D.; Sun, Q.W.; Pan, G.J.; Chen, J.Z.; Lu, X. Research progress of medicinal plants of Pterygophyllum. Guizhou Sci. 2016, 34, 25–31. [Google Scholar]
- Chen, Z.B.; Hao, J.J.; Wang, L.P.; Wang, Y.; Kong, F.D.; Zhu, W.M. New α-glucosidase inhibitors from marine algae-derived Streptomycessp.OUCMDZ-3434. Sci. Rep. 2016, 6, 20004–20012. [Google Scholar] [CrossRef]
| Position | 1 a | 4 a | 7 b | 8 a | 9 b |
|---|---|---|---|---|---|
| 1 | 3.80 (dd, 11.1, 4.2) | 1.85 (m) 1.63 (t, 1.7) | 1.22 (s) 1.42 (s) | ||
| 2 | 3.23 (d, 10.9) 3.30 (d, 10.9) | 3.57 (d, 10.9) 3.23 (d, 10.9) | 2.34 (q, 12.0) 2.42 (dt, 12.6) | 2.04 (m) 1.74 (m) | 1.01 (s) 1.75 (d, 4.6) |
| 3 | 3.86 (dd, 12.0, 4.6) | 3.87 (dd, 12.0, 4.6) | 3.63 (dd, 12.0, 4.4) | 3.48 (dd, 12.4, 4.3) | 3.49 (dd, 10.6, 5.6) |
| 5 | 1.59 (d, 2.8) | 2.0 (d, 4.3) | 1.80 (d, 3.2) | 0.59 (dd, 11.8, 2.2) | 0.84 (m) |
| 6 | 1.51 (d, 4.0) 1.59 (d, 2.9) | 2.25 (d, 5.6) 2.0 (d, 4.3) | 1.76 (d, 3.0) 1.68 (d, 1.8) | 3.26 (dd, 12.4, 4.3) | 1.60 (dt, 10.4, 2.6) 1.19 (s) |
| 7 | 1.35 (d, 3.2) 1.53 (d, 4.2) | 1.35 (d, 3.2) 1.53 (d, 4.2) | 1.76 (d, 3.0) 1.68 (d, 1.8) | 1.52 (d, 3.2) 1.57 (s) | 1.30 (d, 3.8) 1.42 (s) |
| 8 | 1.23 (s) | ||||
| 9 | 2.30 (dd, 11.3, 5.6) | 2.25 (dd, 11.3, 5.6) | 2.42 (dd, 12.6, 4.5) | 1.52 (d, 3.2) | |
| 11 | 2.40 (dd, 12.0, 4.8) 2.25 (dd, 11.3, 5.6) | 2.40 (dd, 12.0, 4.8) 2.25 (dd, 11.3, 5.6) | 1.68 (t, 1.8) 1.80 (d, 3.2) | 1.43 (d, 2.9) 2.35 (m) | |
| 12 | 5.20 (dd, 4.5, 2.9) | 5.22 (dd, 4.4, 2.7) | 1.80 (d, 3.2) 1.75 (d, 3.0) | 1.34 (s) 1.43 (d, 2.9) | 1.71 (m) 1.90 (m) |
| 13 | 1.62 (s) | 1.57 (s) | |||
| 15 | 0.98 (s) 1.70 (d, 4.6) | 1.01 (s) 1.32 (d, 4.6) | 1.68 (t, 1.8) 1.28 (s) | 1.34 (s) 1.63 (d, 1.7) | 1.23 (s) 1.90 (m) |
| 16 | 1.19 (s) 1.90 (d, 4.5) | 1.21 (s) 2.0 (d, 4.5) | 1.68 (t, 1.8) 1.28 (s) | 1.26 (s) | 1.81 (t, 3.3) 2.15 (d, 3.8) |
| 18 | 1.97 (dd, 13.5, 4.2) | 2.10 (dd, 13.6, 4.3) | 1.28 (s) | 1.06 (s) | 1.33 (s) |
| 19 | 1.14 (s) 1.74 (s) | 0.91 (s) 1.32 (s) | 1.96 (s) | 1.65 (s) | 1.41(s) |
| 20 | 2.44 (td, 7.3, 3.9) | ||||
| 21 | 1.17 (d, 2.2) 1.31 (s) | 1.17 (d, 2.2) 1.02 (s) | 5.33 (dd, 6.7, 2.0) | 5.30 (s) | 1.79 (d, 3.2) 2.11 (m) |
| 22 | 1.37 (d, 3.7) 1.53 (d, 4.2) | 1.35 (d, 3.7) 1.51 (d, 4.3) | 1.62 (s) 1.28 (s) | 1.57 (s) 1.74 (m) | 1.40 (s) 1.19 (s) |
| 23 | β 1.10 (s) | β 1.08 (s) | β 1.24 (s) | α 0.93 (s) | β 1.27 (s) |
| 24 | α 1.03 (s) | α 1.04 (s) | α 1.09 (s) | β 0.96 (s) | α 0.90 (s) |
| 25 | β 1.32 (s) | β 1.32 (s) | β 1.26 (s) | β 0.96 (s) | β 1.08 (s) |
| 26 | β 1.00 (s) | β 1.02 (s) | β 1.17 (s) | β 1.06 (s) | β 1.33 (s) |
| 27 | α 1.21 (s) | α 1.21 (s) | α 1.01 (s) | α 0.76 (s) | α 1.42 (s) |
| 28 | 3.30 (d, 3.2) 3.21 (d, 10.9) | 3.57 (d, 3.2) 3.23 (d, 10.9) | β 0.80 (s) | β 0.74 (s) | β 1.01 (s) |
| 29 | α 0.90 (s) | α 0.91 (s) | α 1.65 (s) | α 0.99 (d, 6.4) | β 1.03 (d, 2.8) |
| 30 | β 0.88 (s) | β 0.89 (s) | β 0.99 (s) | β 1.57 (s) | α 1.03 (d, 2.8) |
| Position | 1 a | 4 a | 7 b | 8 a | 9 b |
|---|---|---|---|---|---|
| 1 | 214.6 | 212.4 | 79.7 | 38.1 | 38.5 |
| 2 | 42.8 | 44.1 | 39.5 | 27.1 | 26.7 |
| 3 | 79.3 | 78.6 | 75.5 | 79.3 | 77.9 |
| 4 | 38.0 | 39.3 | 39.7 | 38.8 | 39.2 |
| 5 | 51.3 | 54 | 52.1 | 53.0 | 55.5 |
| 6 | 18.4 | 17.8 | 18.5 | 75.0 | 18.6 |
| 7 | 32.3 | 32.5 | 34.5 | 17.9 | 40.5 |
| 8 | 41.9 | 42 | 42.5 | 41.6 | 47.9 |
| 9 | 38.9 | 39.1 | 53.8 | 51.4 | 42.2 |
| 10 | 51.9 | 52.3 | 44.1 | 43.4 | 37.0 |
| 11 | 25.3 | 25.3 | 24.8 | 24.4 | 21.3 |
| 12 | 122.9 | 123 | 28.4 | 34.0 | 38.9 |
| 13 | 143.1 | 143.1 | 39.3 | 36.3 | 73.8 |
| 14 | 39.9 | 39.7 | 42.7 | 42.4 | 38.9 |
| 15 | 25.4 | 25.5 | 27.5 | 27.7 | 28.1 |
| 16 | 21.9 | 22.0 | 37.0 | 36.7 | 38.3 |
| 17 | 36.9 | 37 | 34.8 | 34.3 | 35.5 |
| 18 | 42.5 | 42.5 | 48.9 | 48.6 | 49.6 |
| 19 | 46.1 | 46.1 | 36.5 | 38.3 | 43.0 |
| 20 | 30.9 | 30.9 | 140.0 | 139.9 | 41.3 |
| 21 | 34.1 | 34.1 | 119.3 | 118.8 | 28.6 |
| 22 | 31.0 | 31.0 | 42.0 | 42.2 | 34.5 |
| 23 | 22.3 | 16 | 28.7 | 12.0 | 28.4 |
| 24 | 27 | 28.5 | 16.2 | 27.8 | 16.2 |
| 25 | 15 | 15 | 13.2 | 14.6 | 16.2 |
| 26 | 17.5 | 17.5 | 16.8 | 16.3 | 21.6 |
| 27 | 25.8 | 25.7 | 14.9 | 15.0 | 17.8 |
| 28 | 69.7 | 69.9 | 18.0 | 17.7 | 18.4 |
| 29 | 33.2 | 33.2 | 22.6 | 22.4 | 15.9 |
| 30 | 23.5 | 23.5 | 21.8 | 21.6 | 14.7 |
| Compound | IC50 (μM) | Compound | IC50 (μM) |
|---|---|---|---|
| 1 | 0.27 ± 0.0499 | 9 | 0.23 ± 0.0307 |
| 3 | 0.11 ± 0.0222 | 13 | 0.26 ± 0.0383 |
| 7 | 0.56 ± 0.0331 | 15 | 0.09 ± 0.0045 |
| 8 | 0.23 ± 0.0135 | Acarbose b | 0.35 ± 0.0006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.-H.; Hu, D.; Deng, L.-L.; Li, J.; Hao, X.-J.; Mu, S.-Z. Pentacyclic Triterpenoids from Sabia discolor Dunn and Their α-Glycosidase Inhibitory Activities. Molecules 2022, 27, 2161. https://doi.org/10.3390/molecules27072161
Ma J-H, Hu D, Deng L-L, Li J, Hao X-J, Mu S-Z. Pentacyclic Triterpenoids from Sabia discolor Dunn and Their α-Glycosidase Inhibitory Activities. Molecules. 2022; 27(7):2161. https://doi.org/10.3390/molecules27072161
Chicago/Turabian StyleMa, Jin-Hong, Dan Hu, Lu-Lu Deng, Jiang Li, Xiao-Jiang Hao, and Shu-Zhen Mu. 2022. "Pentacyclic Triterpenoids from Sabia discolor Dunn and Their α-Glycosidase Inhibitory Activities" Molecules 27, no. 7: 2161. https://doi.org/10.3390/molecules27072161
APA StyleMa, J.-H., Hu, D., Deng, L.-L., Li, J., Hao, X.-J., & Mu, S.-Z. (2022). Pentacyclic Triterpenoids from Sabia discolor Dunn and Their α-Glycosidase Inhibitory Activities. Molecules, 27(7), 2161. https://doi.org/10.3390/molecules27072161





