Ferroptosis: A New Road towards Cancer Management
Abstract
1. Introduction
2. Mechanism of Ferroptosis
2.1. Ferroptosis Due to Suppression of System Xc−
2.2. Ferroptosis Involving Gpx4
2.3. Ferroptosis Involving Mitochondrial Voltage-Dependent Anions Channels
2.4. Some Other Pathways Involving Ferroptosis
2.5. Role of Mitochondria in Ferroptosis
2.6. Role of Lysosome in Ferroptosis
2.7. Role of Fe Metabolism in Ferroptosis
3. Connections between Ferroptosis and Other Forms of Cell Death
3.1. Necrosis and Ferroptosis
3.2. Ferroptosis and Oxytosis
3.3. Autophagy and Ferroptosis
4. Ferroptosis and Cancer
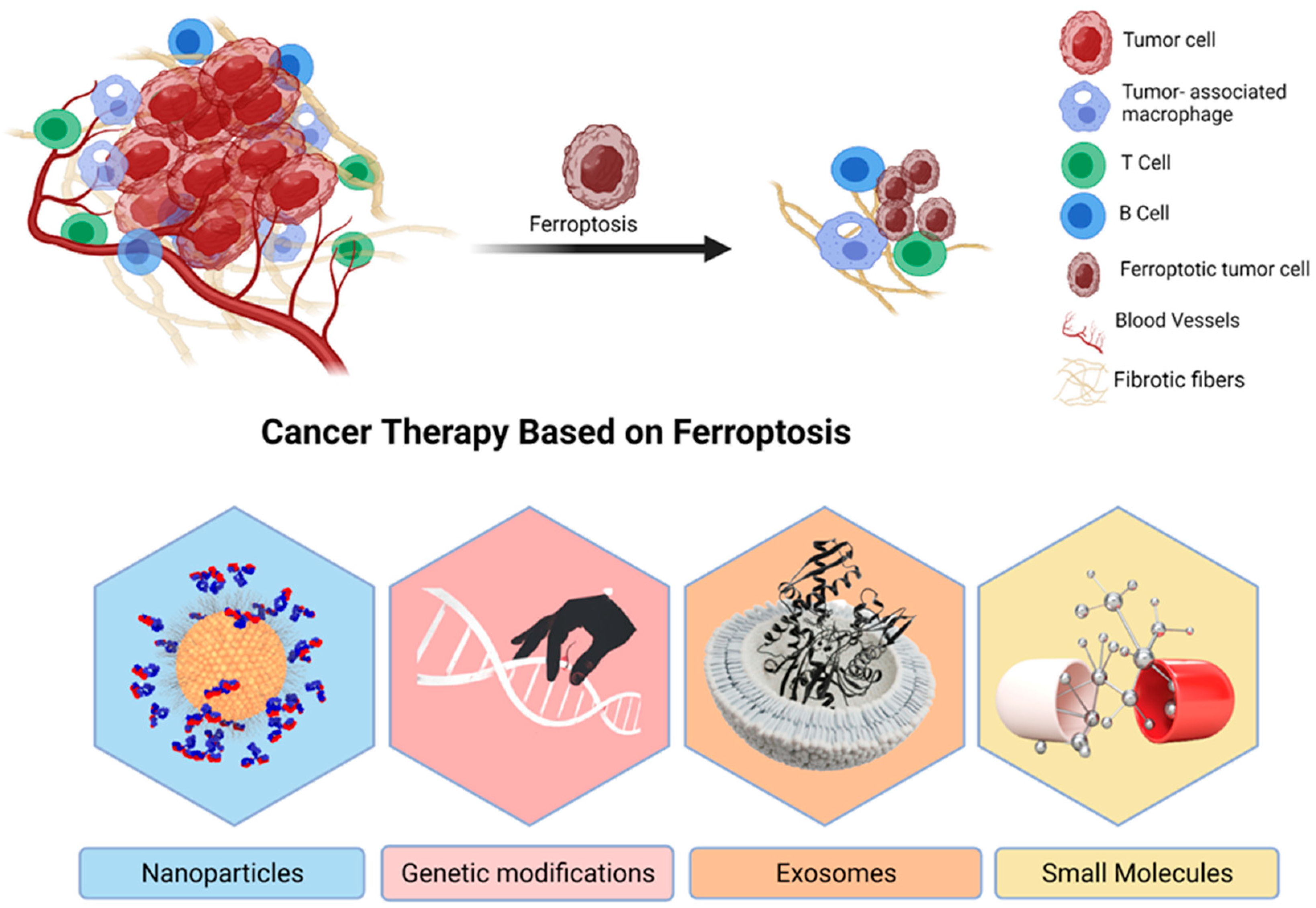
4.1. Ferroptosis and Tumor Microenvironment
4.2. Ferroptosis and Tumor Suppressor p53
5. Ferroptosis Inhibition in Different Forms of Cancer
5.1. Breast Carcinoma
5.2. Pancreatic Carcinoma
5.3. Lung Carcinoma
5.4. Ovarian Cancer
5.5. Brain Tumors
5.6. Hepatocellular Carcinoma
5.7. Fibrosarcoma
6. Ferroptosis in Cancer Treatment
6.1. Nanoparticles
6.2. Genetic Modifications
6.3. Exosomes
6.4. Small Molecules
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Full Form | Abbreviation |
| Acyl-CoA synthase family member 2 | ACSF2 |
| Acyl-CoA synthetase long-chain family member 4 | ACSL4 |
| Apoptosis-inducing factor | AIF |
| Apoptosis-inducing factor mitochondria associated 2 | AIFM2 |
| Apoptosis-inducing factor-1 | AIF-1 |
| Arachidonate 15-lipoxygenase | 15-LOXs |
| Arachidonoyl peroxidation | AA |
| Artesunate | ART |
| Autophagy related-5, autophagy related-7, and nuclear receptor coactivator autophagy related-4 pathways | ATG5-ATG7-NCOA4 |
| Autophagy-dependent ferroptosis | ADF |
| Autophagy-related protein-13 | ATG-13 |
| Autophagy-related-3 protein | ATG-3 |
| BH3 interacting domain death agonist | BID |
| BRCA1-associated protein-1 | BAP-1 |
| Carbonic anhydrase-9 | CA9 |
| CDGSH iron sulphur domain 2 | CISD2 |
| CDGSH iron sulphur domain 1 | CISD1 |
| Clear-cell carcinoma | CCC |
| Colorectal cancer | CRC |
| Competitor endogenous RNAs | ceRNAs |
| Cysteinyl-tRNA synthetase | CARS |
| Diffuse large B-cell lym-phoma | DLBCL |
| Dipeptidyl peptidase-4 | DPP4 |
| Divalent metal transporter-1 | DMT1 |
| Electron transport chain | ETC |
| Ferritin heavy chain-1 | FTH-1 |
| Ferritin light chain | FTL |
| Ferroportin | Fpn |
| Ferroptosis suppressor protein-1 | FSP-1 |
| Gamma-glutamyl-cysteine | g-GCS |
| Glioblastoma steam-like cells | GSCs |
| Glutaminase-2 | GLS2 |
| Glutathione | GSH |
| Glutathione peroxidase 4 | Gpx4 |
| Head and neck squamous cell carcinomas | HNSCC |
| Heat shock protein beta-1 | HSPB1 |
| Heat shock protein-5 | HSPA5 |
| Hepatocellular carci-noma | HCC |
| Hepatocyte growth factor-2 | HGF-2 |
| Hypoxia-inducible factor | HIF |
| Hypoxia-inducible factor-1 | HIF-1 |
| Hypoxia-inducible protein-2 | HIG-2 |
| Iron | Fe |
| Iron regulatory proteins | IRP1 |
| Iron response element-binding protein-2 | IREB-2 |
| Kaplan–Meier | KM |
| Labile iron pool | LIP |
| Lung cancer | LC |
| Lysophosphatidylcholine acyltransferase 3 | LPCAT3 |
| Metallothionein 1G | MT-IG |
| Mitochondrial ferritin | FTMT |
| Mucin 1 cell surface associated | MUC1-C |
| Multiple myeloma | MM |
| NADPH oxidase 1 | NOX1 |
| Nanoparticles | NPs |
| Nicotinamide adenine dinucleotide diphosphate oxidase-1 | NADPH Oxidase/NOX-1 |
| Noncoding RNA | ncRNA |
| Nuclear receptor coactivator-4 | NCOA-4 |
| Ovarian cancer | OVCA |
| Ovarian cancer stem cells | OCSCs |
| Oxidative stress | OS |
| P62-Kelch-like ECH associated protein 1- nuclear factor erythroid 2-related factor 2 | p62-Keap1-Nrf2 |
| Pancreatic cancer | PC |
| Pancreatic ductal adenocarcinoma | PDACs |
| Pentaspanin protein | Prominin-2 |
| Photo-thermal treatment | PTT |
| Polyunsaturated fatty acids | PUFAs |
| Prostaglandin-endoperoxide synthase-2 | PTGS-2 |
| Radiation-induced bystander effect | RIBE |
| RAS selective lethal | RSL3 |
| Receptor-interacting protein kinase-1 | RIPK-1 |
| Regulated cell death | RCD |
| Renal cell carcinoma | RCC |
| Retinoblastoma | Rb |
| Serine/threonine/tyrosine kinase-1 | STYK-1 |
| Signal transducer and activator of transcription 3 | STAT3 |
| Six transmembrane epithelial antigens of the prostate-3 | STEAP-3 |
| Solute carrier family 11 member-A2 | SLC11A2 |
| Solute carrier family 7 member 11 | SLC7A11 |
| Spermidine/spermine N1-acetyltransferase 1 | SAT1 |
| Squamous cell carcinomas | SCCs |
| Temozolomide | TMZ |
| TF receptor-1 | TFR-1 |
| The activating transcription factor-4 | ATF4 |
| Transferrin | TF |
| Transferrin receptor | TFRC |
| Transferrin receptor-1 | TFR1 |
| Tricarboxylic acid | TCA |
| Tumor microenvironment | TME |
| Tumor-associated macrophages | TAMs |
| Tumor-cell-released microparticles | RT-MPs |
| Ubiquinone | CoQ10 |
| Voltage-dependent anions channels | VDACs |
| Zinc–Iron regulatory protein family 8/14 | ZIP8/14 |
References
- Bedoui, S.; Herold, M.J.; Strasser, A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. Cell Biol. 2020, 21, 678–695. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, L.; Yuan, H.; Wu, S. Interconnections among major forms of regulated cell death. Apoptosis 2020, 25, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Santagostino, S.F.; Assenmacher, C.A.; Tarrant, J.C.; Adedeji, A.O.; Radaelli, E. Mechanisms of Regulated Cell Death: Current Perspectives. Vet. Pathol. 2021, 58, 596–623. [Google Scholar] [CrossRef]
- Liu, Z.; Chan, F.K.M. Regulatory mechanisms of RIPK1 in cell death and inflammation. Semin. Cell Dev. Biol. 2021, 109, 70–75. [Google Scholar] [CrossRef]
- Tang, D.; Kroemer, G. Ferroptosis. Curr. Biol. 2020, 30, R1292–R1297. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef]
- Bayır, H.; Anthonymuthu, T.S.; Tyurina, Y.Y.; Patel, S.J.; Amoscato, A.A.; Lamade, A.M.; Yang, Q.; Vladimirov, G.K.; Philpott, C.C.; Kagan, V.E. Achieving Life through Death: Redox Biology of Lipid Peroxidation in Ferroptosis. Cell Chem. Biol. 2020, 27, 387–408. [Google Scholar] [CrossRef]
- Li, L.; Qiu, C.; Hou, M.; Wang, X.; Huang, C.; Zou, J.; Liu, T.; Qu, J. Ferroptosis in Ovarian Cancer: A Novel Therapeutic Strategy. Front. Oncol. 2021, 11, 1364. [Google Scholar] [CrossRef]
- Xu, G.; Wang, H.; Li, X.; Huang, R.; Luo, L. Recent progress on targeting ferroptosis for cancer therapy. Biochem. Pharmacol. 2021, 190, 114584. [Google Scholar] [CrossRef]
- Cosialls, E.; El Hage, R.; Dos Santos, L.; Gong, C.; Mehrpour, M.; Hamaï, A. Ferroptosis: Cancer Stem Cells Rely on Iron until “to Die for” It. Cells 2021, 10, 2981. [Google Scholar] [CrossRef]
- Tang, D. Ferroptosis in Health and Disease; Springer Nature: Berlin/Heidelberg, Germany, 2019; ISBN 9783030267803. [Google Scholar]
- Conrad, M.; Sato, H. The oxidative stress-inducible cystine/glutamate antiporter, system x c-: Cystine supplier and beyond. Amino Acids 2012, 42, 231–246. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, W.; Pei, D. System Xc−: A key regulatory target of ferroptosis in cancer. Investig. New Drugs 2021, 39, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, M. Cysteine. In Food Science and Technology; Academic Press: London, UK, 2003; pp. 348–356. ISBN 978-0-12-417762-8. [Google Scholar]
- Zuo, L.; Zhou, T.; Pannell, B.K.; Ziegler, A.C.; Best, T.M. Biological and physiological role of reactive oxygen species—the good, the bad and the ugly. Acta Physiol. 2015, 214, 329–348. [Google Scholar] [CrossRef]
- Shimizu, S. Apoptosis and Non-Apoptotic Cell Death; Springer: Berlin/Heidelberg, Germany, 2011; Volume 52, ISBN 9783319239125. [Google Scholar]
- Dixon, S.J.; Patel, D.; Welsch, M.; Skouta, R.; Lee, E.; Hayano, M.; Thomas, A.G.; Gleason, C.; Tatonetti, N.; Slusher, B.S.; et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 2014, 2014, e02523. [Google Scholar] [CrossRef] [PubMed]
- Seibt, T.M.; Proneth, B.; Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic. Biol. Med. 2019, 133, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, Y.; Li, H.; Han, L. FIN56, a novel ferroptosis inducer, triggers lysosomal membrane permeabilization in a TFEB-dependent manner in glioblastoma. J. Cancer 2021, 12, 6610–6619. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, W.K.; Bae, K.H.; Lee, S.C.; Lee, E.W. Lipid metabolism and ferroptosis. Biology 2021, 10, 184. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Kang, R.; Tang, D. Iron Metabolism in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 1089. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Zhang, R.; Wang, F.; Wang, T.; Jiao, Y. The role of Erastin in ferroptosis and its prospects in cancer therapy. Onco-Targets Ther. 2020, 13, 5429–5441. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Heslop, K.A.; Milesi, V.; Maldonado, E.N. VDAC Modulation of Cancer Metabolism: Advances and Therapeutic Challenges. Front. Physiol. 2021, 12, 742839. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ni, S.; Zhuge, A.; Li, B.; Li, L. Iron Regulates the Warburg Effect and Ferroptosis in Colorectal Cancer. Front. Oncol. 2021, 11, 1491. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Geng, L.; He, J.; Chen, L.; Sun, Q.; Zhao, J.; Wang, X. Inhibition of Acyl-CoA Synthetase Long-Chain Family Member 4 Facilitates Neurological Recovery After Stroke by Regulation Ferroptosis. Front. Cell. Neurosci. 2021, 15, 632354. [Google Scholar] [CrossRef] [PubMed]
- Forcina, G.C.; Dixon, S.J. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics 2019, 19, 1800311. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Jiang, X. The Chemistry and Biology of Ferroptosis. Cell Chem. Biol. 2020, 27, 365–375. [Google Scholar] [CrossRef]
- Kurz, T.; Gustafsson, B.; Brunk, U.T. Cell sensitivity to oxidative stress is influenced by ferritin autophagy. Free Radic. Biol. Med. 2011, 50, 1647–1658. [Google Scholar] [CrossRef]
- Dai, E.; Zhang, W.; Cong, D.; Kang, R.; Wang, J.; Tang, D. AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochem. Biophys. Res. Commun. 2020, 523, 966–971. [Google Scholar] [CrossRef]
- Capelletti, M.M.; Manceau, H.; Puy, H.; Peoc’h, K. Ferroptosis in liver diseases: An overview. Int. J. Mol. Sci. 2020, 21, 4908. [Google Scholar] [CrossRef]
- Baschiera, E.; Sorrentino, U.; Calderan, C.; Desbats, M.A.; Salviati, L. The multiple roles of coenzyme Q in cellular homeostasis and their relevance for the pathogenesis of coenzyme Q deficiency. Free Radic. Biol. Med. 2021, 166, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Bao, J. FerrDb: A manually curated resource for regulators and markers of ferroptosis and ferroptosis-disease associations. Database 2020, 2020, baaa021. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Qi, W.; Liu, J.; Zhang, Z.; Wang, Z.; Bao, J.; Wu, C.; Liang, F. Research Progress of Ferroptosis: A Bibliometrics and Visual Analysis Study. J. Healthc. Eng. 2021, 2021, 2178281. [Google Scholar] [CrossRef] [PubMed]
- Bano, I.; Malhi, M.; Khatri, P.; Soomro, S.A.; Sajjad, H.; Leghari, A.; Awais, M.; Kandhro, S.; Lakho, S.A.; Soomro, M. Effect of dietary selenium yeast supplementation on morphology and antioxidant status in testes of young goat. Pak. J. Zool. 2019, 51, 979–988. [Google Scholar] [CrossRef]
- Latunde-Dada, G.O. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1893–1900. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Yang, M.; Dong, X. Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv. Mater. 2019, 31, 1904197. [Google Scholar] [CrossRef]
- Yu, H.; Guo, P.; Xie, X.; Wang, Y.; Chen, G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J. Cell. Mol. Med. 2017, 21, 648–657. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Chen, S.; Hou, M.; Yang, Y.; Xie, M. Construction and Validation of a Ferroptosis-Related Prognostic Model for Endometrial Cancer. Front. Genet. 2021, 12, 1680. [Google Scholar] [CrossRef]
- Mao, L.; Zhao, T.; Song, Y.; Lin, L.; Fan, X.; Cui, B.; Feng, H.; Wang, X.; Yu, Q.; Zhang, J.; et al. The emerging role of ferroptosis in non-cancer liver diseases: Hype or increasing hope? Cell Death Dis. 2020, 11, 518. [Google Scholar] [CrossRef]
- Miess, H.; Dankworth, B.; Gouw, A.M.; Rosenfeldt, M.; Schmitz, W.; Jiang, M.; Saunders, B.; Howell, M.; Downward, J.; Felsher, D.W.; et al. The glutathione redox system is essential to prevent ferroptosis caused by impaired lipid metabolism in clear cell renal cell carcinoma. Oncogene 2018, 37, 5435–5450. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The hallmarks of ferroptosis. Annu. Rev. Cancer Biol. 2019, 3, 35–54. [Google Scholar] [CrossRef]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363.e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, C.; Zhao, Y.; Gao, G. Mitochondria regulation in ferroptosis. Eur. J. Cell Biol. 2020, 99, 151058. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, S.; Gong, X.; Tam, S.; Xiao, D.; Liu, S.; Tao, Y. The epigenetic regulators and metabolic changes in ferroptosis-Associated cancer progression. Mol. Cancer 2020, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, A.M.; Chirillo, R.; Aversa, I.; Sacco, A.; Costanzo, F.; Biamonte, F. Ferroptosis and Cancer: Mitochondria Meet the “Iron Maiden” Cell Death. Cells 2020, 9, 1505. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef]
- Torii, S.; Shintoku, R.; Kubota, C.; Yaegashi, M.; Torii, R.; Sasaki, M.; Suzuki, T.; Mori, M.; Yoshimoto, Y.; Takeuchi, T.; et al. An essential role for functional lysosomes in ferroptosis of cancer cells. Biochem. J. 2016, 473, 769–777. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, J.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Tang, D. Ferroptosis is a type of autophagy-dependent cell death. Semin. Cancer Biol. 2020, 66, 89–100. [Google Scholar] [CrossRef]
- Kuang, F.; Liu, J.; Li, C.; Kang, R.; Tang, D. Cathepsin B is a mediator of organelle-specific initiation of ferroptosis. Biochem. Biophys. Res. Commun. 2020, 533, 1464–1469. [Google Scholar] [CrossRef]
- Li, D.; Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target. Ther. 2020, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Coates, T.D. Physiology and Pathophysiology of Iron in Hemoglobin- Associated Diseases. Free Radic. Biol. Med. 2014, 72, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.F. Regulation of Folate Homeostasis. In 8 Montreal, Canada, June 15–20, 1986; De Gruyter: Berlin, Germany; Boston, MA, USA, 2019; Volume 37, pp. 925–928. [Google Scholar] [CrossRef]
- De Domenico, I.; Ward, D.M.V.; Di Patti, M.C.B.; Jeong, S.Y.; David, S.; Musci, G.; Kaplan, J. Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. EMBO J. 2007, 26, 2823–2831. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Parker Siburt, C.J.; Mistry, S.; Noto, J.M.; Dearmond, P.; Fitzgerald, M.C.; Lambert, L.A.; Cornelissen, C.N.; Crumbliss, A.L. Evidence of Fe3+ interaction with the plug domain of the outer membrane transferrin receptor protein of Neisseria gonorrhoeae: Implications for Fe transport. Metallomics 2012, 4, 361–372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Atanasiu, V.; Manolescu, B.; Stoian, I. Hepcidin—Central regulator of iron metabolism. Eur. J. Haematol. 2007, 78, 1–10. [Google Scholar] [CrossRef]
- Yao, Q.; Sun, R.; Bao, S.; Chen, R.; Kou, L. Bilirubin Protects Transplanted Islets by Targeting Ferroptosis. Front. Pharmacol. 2020, 11, 907. [Google Scholar] [CrossRef]
- Fuhrmann, D.C.; Mondorf, A.; Beifuß, J.; Jung, M.; Brüne, B. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 2020, 36, 101670. [Google Scholar] [CrossRef]
- Yao, F.; Cui, X.; Zhang, Y.; Bei, Z.; Wang, H.; Zhao, D.; Wang, H.; Yang, Y. Iron regulatory protein 1 promotes ferroptosis by sustaining cellular iron homeostasis in melanoma. Oncol. Lett. 2021, 22, 657. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, Y.; Xu, T.; Wu, Q. Ferroptosis: Opportunities and Challenges in Myocardial Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2021, 2021, 9929687. [Google Scholar] [CrossRef]
- Dixon, S.J. Ferroptosis: Bug or feature? Immunol. Rev. 2017, 277, 150–157. [Google Scholar] [CrossRef]
- Tang, R.; Xu, J.; Zhang, B.; Liu, J.; Liang, C.; Hua, J.; Meng, Q.; Yu, X.; Shi, S. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J. Hematol. Oncol. 2020, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, J.; Ates, G.; Methner, A.; Conrad, M.; Maher, P. Oxytosis/ferroptosis-(Re-) emerging roles for oxidative stress-dependent non-apoptotic cell death in diseases of the central nervous system. Front. Neurosci. 2018, 12, 214. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Andreyev, A.; Murphy, A.N.; Perkins, G.A.; Ellisman, M.H.; Newmeyer, D.D. Mitochondria frozen with trehalose retain a number of biological functions and preserve outer membrane integrity. Cell Death Differ. 2007, 14, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Lorenz, S.M.; Proneth, B. Targeting Ferroptosis: New Hope for As-Yet-Incurable Diseases. Trends Mol. Med. 2021, 27, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kuang, F.; Kroemer, G.; Klionsky, D.J.; Kang, R.; Tang, D. Autophagy-Dependent Ferroptosis: Machinery and Regulation. Cell Chem. Biol. 2020, 27, 420–435. [Google Scholar] [CrossRef]
- Hou, W.; Xie, Y.; Song, X.; Sun, X.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 2016, 12, 1425–1428. [Google Scholar] [CrossRef]
- Lei, P.; Bai, T.; Sun, Y. Mechanisms of ferroptosis and relations with regulated cell death: A review. Front. Physiol. 2019, 10, 139. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, C.; Luo, M.; Cen, C.; Qiu, J.; Zhang, S.; Hu, K. Ferroptosis in Cancer Treatment: Another Way to Rome. Front. Oncol. 2020, 10, 1924. [Google Scholar] [CrossRef]
- Xia, X.; Fan, X.; Zhao, M.; Zhu, P. The Relationship between Ferroptosis and Tumors: A Novel Landscape for Therapeutic Approach. Curr. Gene Ther. 2019, 19, 117–124. [Google Scholar] [CrossRef]
- Bartolacci, C.; Andreani, C.; El-Gammal, Y.; Scaglioni, P.P. Lipid Metabolism Regulates Oxidative Stress and Ferroptosis in RAS-Driven Cancers: A Perspective on Cancer Progression and Therapy. Front. Mol. Biosci. 2021, 8, 383. [Google Scholar] [CrossRef]
- Li, H.; Fan, X.; Houghton, J.M. Tumor microenvironment: The role of the tumor stroma in cancer. J. Cell. Biochem. 2007, 101, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Ossowski, L.; Aguirre-Ghiso, J.A. Dormancy of metastatic melanoma. Pigment Cell Melanoma Res. 2010, 23, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ye, D.; Ren, M.; Zhang, H.; Bi, F. Ferroptosis in the tumor microenvironment: Perspectives for immunotherapy. Trends Mol. Med. 2021, 27, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Aprelikova, O.; Wood, M.; Tackett, S.; Chandramouli, G.V.R.; Barrett, J.C. Role of ETS transcription factors in the hypoxia-inducible factor-2 target gene selection. Cancer Res. 2006, 66, 5641–5647. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Björndahl, M.A.; Gallego, M.I.; Chen, S.; Religa, P.; Hansen, A.J.; Cao, Y. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood 2006, 107, 3531–3536. [Google Scholar] [CrossRef]
- Ventura, A. NORAD: Defender of the Genome. Trends Genet. 2016, 32, 390–392. [Google Scholar] [CrossRef]
- Bieging, K.T.; Mello, S.S.; Attardi, L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer 2014, 14, 359–370. [Google Scholar] [CrossRef]
- Gnanapradeepan, K.; Basu, S.; Barnoud, T.; Budina-Kolomets, A.; Kung, C.P.; Murphy, M.E. The p53 tumor suppressor in the control of metabolism and ferroptosis. Front. Endocrinol. 2018, 9, 124. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Krysko, D.V.; Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 2019, 19, 405–414. [Google Scholar] [CrossRef]
- Milković, L.; Tomljanović, M.; Čipak Gašparović, A.; Novak Kujundžić, R.; Šimunić, D.; Konjevoda, P.; Mojzeš, A.; Đaković, N.; Žarković, N.; Gall Trošelj, K. Nutritional Stress in Head and Neck Cancer Originating Cell Lines: The Sensitivity of the NRF2-NQO1 Axis. Cells 2019, 8, 1001. [Google Scholar] [CrossRef]
- Pu, F.; Chen, F.; Zhang, Z.; Shi, D.; Zhong, B.; Lv, X.; Tucker, A.B.; Fan, J.; Li, A.J.; Qin, K.; et al. Ferroptosis as a novel form of regulated cell death: Implications in the pathogenesis, oncometabolism and treatment of human cancer. Genes Dis. 2020, 9, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, B. TP53 mutation in colorectal cancer. Hum. Mutat. 2003, 21, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhu, S.; Song, X.; Sun, X.; Fan, Y.; Liu, J.; Zhong, M.; Yuan, H.; Zhang, L.; Billiar, T.R.; et al. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017, 20, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xie, Y.; Chen, Y.; Liu, X. Epigenetic Regulation and Nonepigenetic Mechanisms of Ferroptosis Drive Emerging Nanotherapeutics in Tumor. Oxid. Med. Cell. Longev. 2021, 2021, 8854790. [Google Scholar] [CrossRef]
- Anjum, K.; Shagufta, B.I.; Abbas, S.Q.; Patel, S.; Khan, I.; Shah, S.A.A.; Akhter, N.; Hassan, S.S.U. Current status and future therapeutic perspectives of glioblastoma multiforme (GBM) therapy: A review. Biomed. Pharmacother. 2017, 92, 681–689. [Google Scholar] [CrossRef]
- Bruno, A.; Bassani, B.; Pelosi, G.; Boni, L.; Dominioni, L.; Mortara, L.; Noonan, D.M.; Redfern, A. Proffered abstracts from the 17th Biennial Congress of the Metastasis Research Society. Clin. Exp. Metastasis 2019, 36, 139–170. [Google Scholar] [CrossRef]
- Liu, J.; Xia, X.; Huang, P. xCT: A Critical Molecule That Links Cancer Metabolism to Redox Signaling. Mol. Ther. 2020, 28, 2358–2366. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Jiang, R.; Xue, R.; Yin, X.; Wu, M.; Meng, Q. Ferroptosis in liver disease: New insights into disease mechanisms. Cell Death Discov. 2021, 7, 276. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, Y.; Mao, C.; Liu, S.; Xiao, D.; Huang, J.; Tao, Y. Emerging mechanisms and targeted therapy of ferroptosis in cancer. Mol. Ther. 2021, 29, 2185–2208. [Google Scholar] [CrossRef]
- Truman-Rosentsvit, M.; Berenbaum, D.; Spektor, L.; Cohen, L.A.; Belizowsky-Moshe, S.; Lifshitz, L.; Ma, J.; Li, W.; Kesselman, E.; Abutbul-Ionita, I.; et al. Ferritin is secreted via 2 distinct nonclassical vesicular pathways. Blood 2018, 131, 342–352. [Google Scholar] [CrossRef]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Chen, X.; Li, J.; Comish, P.; Kang, R.; Tang, D. Transcription factors in ferroptotic cell death. Cancer Gene Ther. 2020, 27, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhuang, L.; Gan, B. BAP1 suppresses tumor development by inducing ferroptosis upon SLC7A11 repression. Mol. Cell. Oncol. 2019, 6, 1536845. [Google Scholar] [CrossRef]
- Murphy, M.P. Metabolic control of ferroptosis in cancer. Nature 2018, 20, 1104–1105. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Liu, X.; Feng, L.; Gong, Z.; Koppula, P.; Sirohi, K.; Li, X.; Wei, Y.; Lee, H.; et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol. 2018, 20, 1181–1192. [Google Scholar] [CrossRef]
- Liang, J.Y.; Wang, D.S.; Lin, H.C.; Chen, X.X.; Yang, H.; Zheng, Y.; Li, Y.H. A novel ferroptosis-related gene signature for overall survival prediction in patients with hepatocellular carcinoma. Int. J. Biol. Sci. 2020, 16, 2430–2441. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Zhuang, L.; Olszewski, K.; Gan, B. NADPH debt drives redox bankruptcy: SLC7A11/xCT-mediated cystine uptake as a double-edged sword in cellular redox regulation. Genes Dis. 2021, 8, 731–745. [Google Scholar] [CrossRef]
- Lai, Y.; Lin, F.; Wang, X.; Zhang, J.; Xia, J.; Sun, Y.; Wen, M.; Li, X.; Zhang, Z.; Zhao, J. STYK1/NOK Promotes Metastasis and Epithelial-Mesenchymal Transition in Non-small Cell Lung Cancer by Suppressing FoxO1 Signaling. Front. Cell Dev. Biol. 2021, 9, 621147. [Google Scholar] [CrossRef]
- Pectasides, D.; Fountzilas, G.; Papaxoinis, G.; Pectasides, E.; Xiros, N.; Sykiotis, C.; Koumarianou, A.; Psyrri, A.; Panayiotides, J.; Economopoulos, T. Carboplatin and paclitaxel in metastatic or recurrent cervical cancer. Int. J. Gynecol. Cancer 2009, 19, 777–781. [Google Scholar] [CrossRef]
- Muñoz-Galván, S.; Carnero, A. Targeting Cancer Stem Cells to Overcome Therapy Resistance in Ovarian Cancer. Cells 2020, 9, 1402. [Google Scholar] [CrossRef] [PubMed]
- Al-Alem, L.F.; Pandya, U.M.; Baker, A.T.; Bellio, C.; Zarrella, B.D.; Clark, J.; DiGloria, C.M.; Rueda, B.R. Ovarian cancer stem cells: What progress have we made? Int. J. Biochem. Cell Biol. 2019, 107, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Lei, G.; Chen, X.; Li, H.; Zhang, X.; Wu, N.; Zhao, Y.; Zhang, Y.; Wang, J. PARP inhibition promotes ferroptosis via repressing SLC7A11 and synergizes with ferroptosis inducers in BRCA-proficient ovarian cancer. Redox Biol. 2021, 42, 101928. [Google Scholar] [CrossRef] [PubMed]
- Narang, V.S.; Pauletti, G.M.; Gout, P.W.; Buckley, D.J.; Buckley, A.R. Sulfasalazine-induced reduction of glutathione levels in breast cancer cells: Enhancement of growth-inhibitory activity of doxorubicin. Chemotherapy 2007, 53, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, M.; Saverio, V.; Monzani, R.; Ferrari, E.; Piacentini, M.; Corazzari, M. Ferroptosis: A new unexpected chance to treat metastatic melanoma? Cell Cycle 2020, 19, 2411–2425. [Google Scholar] [CrossRef]
- Shams ul Hassan, S.; Ishaq, M.; Zhang, W.; Jin, H.-Z. An overview of the mechanisms of marine fungi-derived antiinflammatory and anti-tumor agents and their novel role in drug targeting. Curr. Pharm. Des. 2021, 27, 2605–2614. [Google Scholar] [CrossRef]
- Shin, D.; Kim, E.H.; Lee, J.; Roh, J.L. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic. Biol. Med. 2018, 129, 454–462. [Google Scholar] [CrossRef]
- Panieri, E.; Telkoparan-Akillilar, P.; Suzen, S.; Saso, L. The nrf2/keap1 axis in the regulation of tumor metabolism: Mechanisms and therapeutic perspectives. Biomolecules 2020, 10, 791. [Google Scholar] [CrossRef]
- Denysenko, T.; Gennero, L.; Roos, M.A.; Melcarne, A.; Juenemann, C.; Faccani, G.; Morra, I.; Cavallo, G.; Reguzzi, S.; Pescarmona, G.; et al. Glioblastoma cancer stem cells: Heterogeneity, microenvironment and related therapeutic strategies. Cell Biochem. Funct. 2010, 28, 343–351. [Google Scholar] [CrossRef]
- D’Alessio, A.; Proietti, G.; Sica, G.; Scicchitano, B.M. Pathological and molecular features of glioblastoma and its peritumoral tissue. Cancers 2019, 11, 469. [Google Scholar] [CrossRef]
- Buccarelli, M.; Marconi, M.; Pacioni, S.; De Pasqualis, I.; D’Alessandris, Q.G.; Martini, M.; Ascione, B.; Malorni, W.; Larocca, L.M.; Pallini, R.; et al. Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Cell Death Dis. 2018, 9, 841. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Lin, B.; Zhou, M.; Wu, L.; Zheng, T. Role of ferroptosis in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2018, 144, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Macías-Rodríguez, R.U.; Inzaugarat, M.E.; Ruiz-margáin, A.; Nelson, L.J.; Trautwein, C.; Cubero, F.J. Reclassifying hepatic cell death during liver damage: Ferroptosis—a novel form of non-apoptotic cell death? Int. J. Mol. Sci. 2020, 21, 1651. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, A.; Sundarraj, K.; Arfuso, F.; Sethi, G.; Perumal, E. Dysregulation of Nrf2 in hepatocellular carcinoma: Role in cancer progression and chemoresistance. Cancers 2018, 10, 481. [Google Scholar] [CrossRef]
- Singh, A.; Misra, V.; Thimmulappa, R.K.; Lee, H.; Ames, S.; Hoque, M.O.; Herman, J.G.; Baylin, S.B.; Sidransky, D.; Gabrielson, E.; et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006, 3, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martın, P.; Komatsu, M. p62/SQSTM1—Steering the cell through health and disease. J. Cell Sci. 2018, 131, jcs222836. [Google Scholar] [CrossRef]
- Lin, W.; Wang, C.; Liu, G.; Bi, C.; Wang, X.; Zhou, Q.; Jin, H. SLC7A11/xCT in cancer: Biological functions and therapeutic implications. Am. J. Cancer Res. 2020, 10, 3106–3126. [Google Scholar]
- Asmat, S.; Shah, A.; Shams, S.; Bungau, S.; Si, Y.; Xu, H.; Rahman, H.; Behl, T.; Gitea, D.; Pavel, F.; et al. Chemically Diverse and Biologically Active Secondary Metabolites from Marine Phylum chlorophyta. Mar. Drugs 2020, 18, 493. [Google Scholar]
- Zuo, S.; Yu, J.; Pan, H.; Lu, L. Novel insights on targeting ferroptosis in cancer therapy. Biomark. Res. 2020, 8, 50. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Foresti, R.; Motterlini, R. Heme Oxygenase-1 and Carbon Monoxide in the Heart: The Balancing Act between Danger Signaling and Pro-Survival. Circ. Res. 2016, 118, 1940–1959. [Google Scholar] [CrossRef]
- Chiang, S.K.; Chen, S.E.; Chang, L.C. A dual role of heme oxygenase-1 in cancer cells. Int. J. Mol. Sci. 2019, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kepp, O.; Kroemer, G. Ferroptosis becomes immunogenic: Implications for anticancer treatments. Oncoimmunology 2021, 10, 1862949. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Ang, B.T.; Pervaiz, S. Cancer stem cell: Target for anti-cancer therapy. FASEB J. 2007, 21, 3777–3785. [Google Scholar] [CrossRef]
- Shan, X.; Li, S.; Sun, B.; Chen, Q.; Sun, J.; He, Z.; Luo, C. Ferroptosis-driven nanotherapeutics for cancer treatment. J. Control. Release 2020, 319, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, T.; Liu, W.; Huang, Y. Ferroptosis and Cancer: Complex Relationship and Potential Application of Exosomes. Front. Cell Dev. Biol. 2021, 9, 733751. [Google Scholar] [CrossRef] [PubMed]
- Mousa, D.S.; El-Far, A.H.; Saddiq, A.A.; Sudha, T.; Mousa, S.A. Nanoformulated bioactive compounds derived from different natural products combat pancreatic cancer cell proliferation. Int. J. Nanomed. 2020, 15, 2259–2268. [Google Scholar] [CrossRef]
- He, S.; Jiang, Y.; Li, J.; Pu, K. Semiconducting Polycomplex Nanoparticles for Photothermal Ferrotherapy of Cancer. Angew. Chem. 2020, 132, 10720–10725. [Google Scholar] [CrossRef]
- Guan, Q.; Guo, R.; Huang, S.; Zhang, F.; Liu, J.; Wang, Z.; Yang, X.; Shuai, X.; Cao, Z. Mesoporous polydopamine carrying sorafenib and SPIO nanoparticles for MRI-guided ferroptosis cancer therapy. J. Control Release 2020, 320, 392–403. [Google Scholar] [CrossRef]
- Li, S.L.; Jiang, P.; Jiang, F.L.; Liu, Y. Recent Advances in Nanomaterial-Based Nanoplatforms for Chemodynamic Cancer Therapy. Adv. Funct. Mater. 2021, 31, 2100243. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Yu, S.; Zhang, M.; Liu, X.; Deng, G.; Liu, Y.; Wu, S. Multifunctional MnO2-based nanoplatform-induced ferroptosis and apoptosis for synergetic chemoradiotherapy. Nanomedicine 2021, 16, 2343–2361. [Google Scholar] [CrossRef]
- Poon, J.F.; Zilka, O.; Pratt, D.A. Potent Ferroptosis Inhibitors Can Catalyze the Cross-Dismutation of Phospholipid-Derived Peroxyl Radicals and Hydroperoxyl Radicals. J. Am. Chem. Soc. 2020, 142, 14331–14342. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liao, H.; Li, F.; Ling, D. A mini-review and perspective on ferroptosis-inducing strategies in cancer therapy. Chin. Chem. Lett. 2019, 30, 847–852. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Zhang, Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res. Lett. 2011, 6, 555. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Le Du, F.; Eckhardt, B.L.; Lim, B.; Litton, J.K.; Moulder, S.; Meric-Bernstam, F.; Gonzalez-Angulo, A.M.; Ueno, N.T. Is the future of personalized therapy in triple-negative breast cancer based on molecular subtype? Oncotarget 2015, 6, 12890–12908. [Google Scholar] [CrossRef]
- Strzyz, P. Iron expulsion by exosomes drives ferroptosis resistance. Nat. Rev. Mol. Cell Biol. 2020, 21, 4–5. [Google Scholar] [CrossRef]
- Brown, C.W.; Mercurio, A.M. Ferroptosis resistance mediated by exosomal release of iron. Mol. Cell. Oncol. 2020, 7, 1730144. [Google Scholar] [CrossRef]
- Xu, D.; Lü, Y.; Li, Y.; Li, S.; Wang, Z.; Wang, J. Ferroptosis Resistance in Cancer: An Emerging Crisis of New Hope. BIO Integr. 2021, 2, 22–28. [Google Scholar] [CrossRef]
- Ye, L.F.; Chaudhary, K.R.; Zandkarimi, F.; Harken, A.D.; Kinslow, C.J.; Upadhyayula, P.S.; Dovas, A.; Higgins, D.M.; Tan, H.; Zhang, Y.; et al. Radiation-Induced Lipid Peroxidation Triggers Ferroptosis and Synergizes with Ferroptosis Inducers. ACS Chem. Biol. 2020, 15, 469–484. [Google Scholar] [CrossRef]
- Kim, E.H.; Shin, D.; Lee, J.; Jung, A.R.; Roh, J.L. CISD2 inhibition overcomes resistance to sulfasalazine-induced ferroptotic cell death in head and neck cancer. Cancer Lett. 2018, 432, 180–190. [Google Scholar] [CrossRef]
- Luo, L.; Wang, H.; Tian, W.; Li, X.; Zhu, Z.; Huang, R.; Luo, H. Targeting ferroptosis-based cancer therapy using nanomaterials: Strategies and applications. Theranostics 2021, 11, 9937–9952. [Google Scholar] [CrossRef] [PubMed]
- Eling, N.; Reuter, L.; Hazin, J.; Hamacher-Brady, A.; Brady, N.R. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience 2015, 2, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Kim, E.H.; Jang, H.; Shin, D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017, 11, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chen, X.; Liu, C.; Ge, W.; Wang, Q.; Hao, X.; Wang, M.; Chen, Y.; Zhang, Q. Identification of a small molecule as inducer of ferroptosis and apoptosis through ubiquitination of GPX4 in triple negative breast cancer cells. J. Hematol. Oncol. 2021, 14, 19. [Google Scholar] [CrossRef]

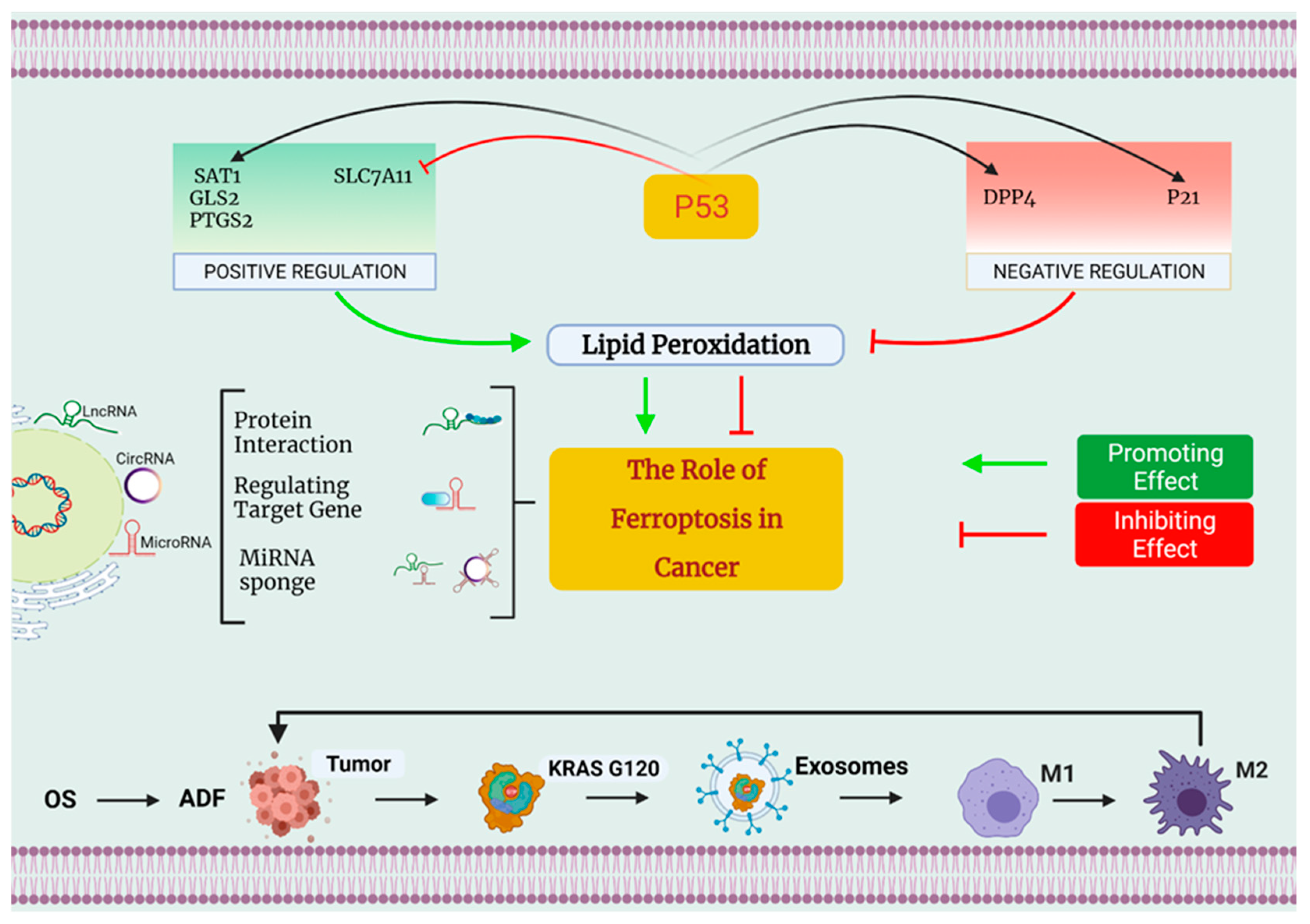
| Factors | Effects on Ferroptosis | Mechanisms Related to Ferroptosis | References |
|---|---|---|---|
| System Xc− | Antiferroptosis | Promotes cystine absorption | [13] |
| HPA5 | Antiferroptosis | Prevents Gpx4 degradation | [18] |
| Gpx4 | Antiferroptosis | Prevents lipid peroxidation reaction | [37] |
| MT-1 | Antiferroptosis | Binds heavy metals | [38] |
| Ferritin | Antiferroptosis | Fe storage inside cell | [5] |
| IRP2 | Antiferroptosis | Manages ferritin transcription | [39] |
| SAT1 | Proferroptosis | Involved in peroxidation of arachidonic acid | [40] |
| ACSL4 | Proferroptosis | Increases concentration of PUFA in plasma membrane | [41] |
| HO-1 | Proferroptosis | Controls degradation of heme and release of Fe | [42] |
| p53 | Proferroptosis | Tumor suppressor and expression of SLC7A11 gene | [43] |
| NOX | Proferroptosis | Promotes ROS production | [39] |
| CARS | Proferroptosis | Protein translation via charging of tRNAs with cystine | [44] |
| TfR1 | Proferroptosis | Promotes the Fe uptake | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bano, I.; Horky, P.; Abbas, S.Q.; Majid, M.; Bilal, A.H.M.; Ali, F.; Behl, T.; Hassan, S.S.u.; Bungau, S. Ferroptosis: A New Road towards Cancer Management. Molecules 2022, 27, 2129. https://doi.org/10.3390/molecules27072129
Bano I, Horky P, Abbas SQ, Majid M, Bilal AHM, Ali F, Behl T, Hassan SSu, Bungau S. Ferroptosis: A New Road towards Cancer Management. Molecules. 2022; 27(7):2129. https://doi.org/10.3390/molecules27072129
Chicago/Turabian StyleBano, Iqra, Pavel Horky, Syed Qamar Abbas, Muhammad Majid, Akram Hafiz Muhammad Bilal, Fawad Ali, Tapan Behl, Syed Shams ul Hassan, and Simona Bungau. 2022. "Ferroptosis: A New Road towards Cancer Management" Molecules 27, no. 7: 2129. https://doi.org/10.3390/molecules27072129
APA StyleBano, I., Horky, P., Abbas, S. Q., Majid, M., Bilal, A. H. M., Ali, F., Behl, T., Hassan, S. S. u., & Bungau, S. (2022). Ferroptosis: A New Road towards Cancer Management. Molecules, 27(7), 2129. https://doi.org/10.3390/molecules27072129










