Research Progress of Natural Small-Molecule Compounds Related to Tumor Differentiation
Abstract
1. Introduction
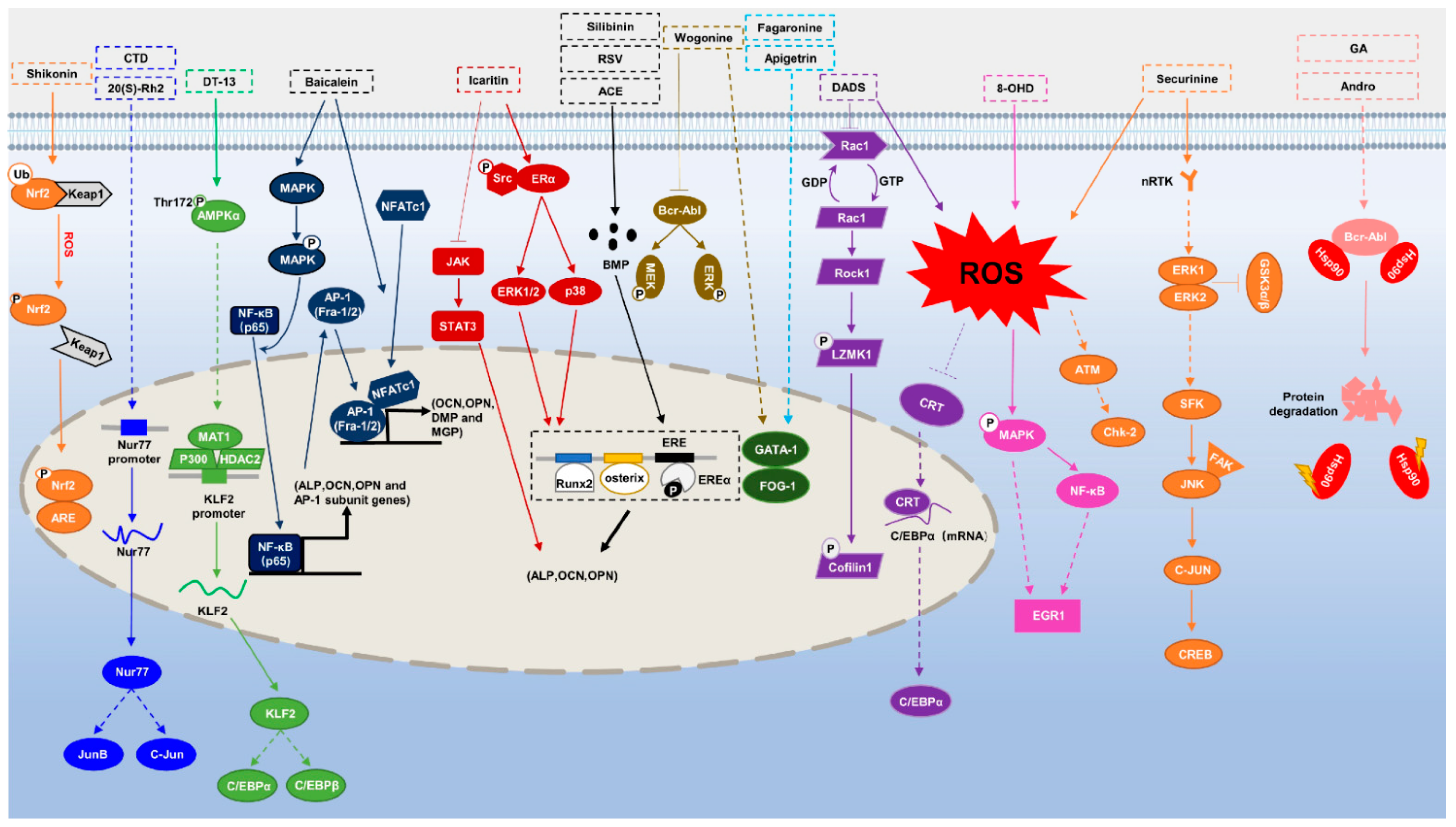
2. Hematologic Tumors
2.1. Myeloid Leukemia
2.1.1. Acute Myeloid Leukemia (AML)
2.1.2. Chronic Myeloid Leukemia (CML)
| Tumor | Compound | Source | Target | Structure | IC50/GI50/Concentration of Induction of Differentiation | Clinical Trial (Phase) | Reference |
|---|---|---|---|---|---|---|---|
| AML | Securinine | Securinega suffruticosal | ATM/ATK, chk1 |  | 15 μM (HL-60 cells) | / | [17,18,19,20] |
| DADS | Garlic | ROS, rac1-rock1-limk1-cofilin 1 | 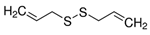 | 1.25 mg/L (HL-60 cells) | / | [21,22,23,24] | |
| Shikonin | Arnebia | Nrf2/ARE | 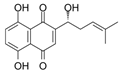 | 100 ng/mL (HL-60 cells) | Hepatitis C (Phase 2), HCV Recurrence After Liver Transplantation (Phase 2) | [25,26,27] | |
| CAE | Caesalpinia sappan L. | ROS | / | 0.19 mg/mL (HL-60 cells), 0.15 mg/mL (Kasumi-1 cells) | / | [28] | |
| 20 (s)-Rh2 | Ginseng | Nur77 | 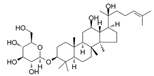 | 25.59 μM (HL-60 cells) and 60.06 μM (Kasumi-1 cells) | / | [32,33] | |
| CTD | Cantharides | Nur77 | 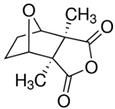 | 6.21 μM (HL-60 cells) and 8.00 μM (Kasumi-1 cells) | Molluscum Contagiosum Skin Infection (Phase 4) | [34] | |
| Notopterol | N. incisum | c-Jun, JunB | 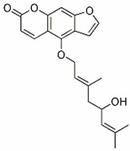 | 40.32 μM (HL-60 cells), 56.68 μM (Kasumi-1 cells) and 50.69 μM (U937 cells) | / | [40] | |
| DT-13 | Liriope muscari (Decne.) Baily | AMPKα | 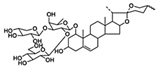 | 17.04 μM (HL-60 cells) and 19.34 μM (Kasumi-1 cells) | / | [32,44] | |
| CML | Apigetrin | Celery | GATA-1 |  | 50 μM (K562 cells) | / | [47,48] |
| Fagaronine | Fagara zanthoxyloides Lam | GATA-1 |  | >10 μM (K562 cells) | / | [49] | |
| Wogonine | Scutellaria baicalensis | GATA-1, FOG-1 | 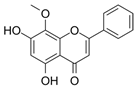 | 80 μM (K562 cells) | / | [53] | |
| GA | gamboges | Bcr-Abl | 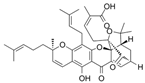 | 0.24 µmol/L (KBM5 cells), 0.34 µmol/L(KBM5-T315Icells) and 0.62 µmol/L (K562clls) | / | [55,56,57] | |
| Andro | Andrographis paniculata | Bcr-Abl | 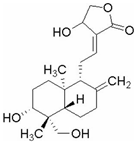 | 23.66 μM (K562 cells), 8.50 μM (KBM5 cells) and 8.50 μM (KBM5R cells) | Acute Tonsillitis (Phase 4), Acute Bronchitis (Phase 4) | [59,60,61] | |
| 8-OHD | Soybean | MAPK, NF-κB |  | 91.8 μM (24 h) and 49.4 μM (48 h) (K562 cells) | / | [62,63] | |
| ATL-I | Atractylodes macrocephula | CD14, CD68 | 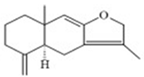 | 44.67 μg/mL (24 h), 37.63 μg/mL (48 h), 24.44 μg/mL(72 h) Jurkat cell and 4.23 μg/mL (24 h, 48 h, 72 h) (U937) cells | / | [64] |
3. Multiple Myeloma (MM) and Osteosarcoma
| Tumor | Compound | Source | Target | Structure | IC50/GI50/Concentration of Induction of Differentiation | Clinical Trial (Phase) | Reference |
|---|---|---|---|---|---|---|---|
| MM | Silibinin | silymarin | BMP, Runx2 |  | 20 μmol/L (hBMSCs) | Transplantation (Phase 2), Hepatitis C (Phase 2) | [67,68] |
| ACE | Acer nikoense Maxim | BMP | 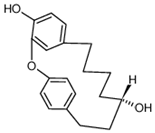 | 30 μM (MC3T3-E1 cells) | / | [69,70] | |
| Baicalein | Scutellaria baicalensis | NF-κB | 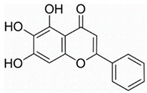 | 30 μM (U266 cells) | Influenz (Phase 2) | [71,72,73,74,75] | |
| RSV | Peanuts, grapes (red wine), tiger nuts, mulberries | RANKL | 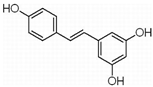 | 100 μmol/L(RPMI 8226, OPM-2 cells) | Multiple Myeloma (Phase 2), Liver Cancer (Phase 2) | [76,77,78,79] | |
| Icaritin | Herba epimedii | ERK1/2, p38 |  | 10 μM (MC3T3-E1 subclone14 cell) | Hepatocellular Carcinoma (Phase 2), Metastatic Breast Cancer (Phase 1) | [80,81,82,83,84] | |
| Osteosarcoma | Quercetin | Rutin (rutin), quercetin, hypericin and other plants | ERα, Runx2, Osterix | 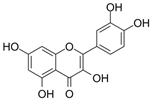 | 2.5 μM (BMSCs) | Diabetes (Phase 2), Alzheimer’s Disease (Phase 2) | [90,91,92,93] |
| Icaritin | Herba epimedii | ERα, ALP, BMP2, Runx2, RANK-RANKL | 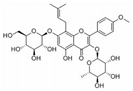 | 1 μM (primary osteoblasts) | Hepatocellular Carcinoma (Phase 2),- Metastatic Breast Cancer (Phase 1) | [94,95,96,97,98] | |
| Genistein | Legumes and bean products | BMP2/ SMAD5/Runx2 | 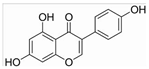 | 10−6 M (hBMSCs) | Sepsis (Phase 4), Metabolic Syndrome (Phase 3) | [99,100,101,102] | |
| KFL | Kaempferia galanga L. | ALP, ERα Runx2, Osterix | 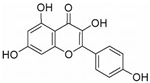 | 10 μM (Osteoblasts) | / | [103,104,105] | |
| Ugonin K | Helminthostachys zeylanica (L.) | SRC, ERα Runx2, Osterix | 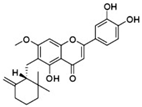 | 10 μM (MC3T3-E1) | / | [106,107] | |
| Galangin | Alpinia officinarum | TGF-β1/Smads | 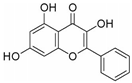 | 67.32 μM (MG-63 cells), 57.09 μM (U-2 OS cells) | / | [108,109,110] | |
| Hyperoside | hypericumperforatum | TGF-β, BMP2, OPN, Runx2 | 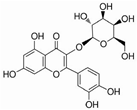 | 223.5 μM (U2OS cells), 239.0 μM (MG63 cells) | / | [111,112,113] | |
| Coleusin | Root of Coleus forskohlii | BMP-2 | 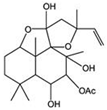 | 100 μmol/L (U2OS cells), MG63 cells) | / | [114] | |
| Vitamin D3 | Animal offal, beef, lamb | p73 | 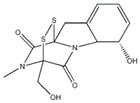 | 200 nmol/L (Osteoblasts) | Vitamin D Deficiency (Phase 4), Pancreatitis, Chronic (Phase 4) | [115,116,117,118,119] |
4. Other Tumors
| Tumor | Compound | Source | Target | Structure | IC50/GI50/Concentration of Induction of Differentiation | Clinical Trial (Phase) | Reference |
|---|---|---|---|---|---|---|---|
| Melanoma | Theo | Camellia sinensis (L.) | MEK1/2, Wnt/β- Catenin | 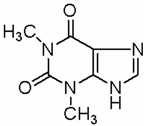 | 2 mM (B16 cells) | Asthma (Phase 3), Leukemia (Phase 2) | [121,122,123] |
| ISL | Licorice | MAPK | 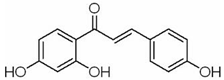 | 10 μM (A375, A2058 cells) | / | [3,124,125,126] | |
| KFL | Kaempferia galanga L. | m-TOR/PI3K/AKT, ERs, ROS | 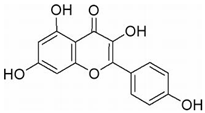 | 20 μM (A375 cells) | / | [127,128,129,130,131] | |
| Genistein | Soybean | FAK/paxillin, MAPK |  | 12.5 μM (B16F10) | Sepsis (Phase 4), Metabolic Syndrome (Phase 3) | [132,133,134] | |
| DIM | Cabbage, Brussels sprouts and cabbage | PTEN/Akt | 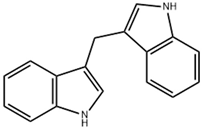 | 150 μM (A375 cells) | Breast Cancer (Phase 3) | [135,136] | |
| Neuroblastoma | KFL | Kaempferia galanga L. | IRE1 α | 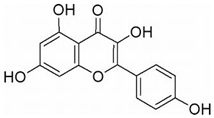 | 50 μM (IMR32 and Neuro2a cell) | / | [139,140] |
| Melatonin | Pinecone | hyaluronic acid synthase 3 | 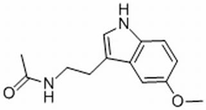 | 0.1 nmol/L (N2a cels) | Coronary Artery Calcification (Phase 4), cancer Malignancies (Phase 3) | [141,142,143,144,145] | |
| TCE | Tinospora cordifolia | NF200, MAP-2, NeuN | / | 200 μg/mL(IMR-32 cells) | / | [146,147] | |
| CA | Eucommia almoides oliver or Lonicera confuse | Tuj1, GFAP |  | 50 µM (Huh7 cells) | Type 2 Diabetes Nonalcoholic Fatty Liver (Phase 3), Advanced Lung Cancer (Phase 2) | [148,149,150] | |
| Glioblastoma | RSV | Peanuts, grapes (red wine), tiger nuts, mulberries | p53/p21 | 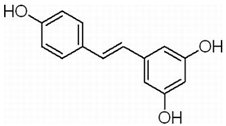 | 50 μM (GSCs) | Multiple Myeloma (Phase 2), Liver Cancer (Phase 2) | [152,153,154,155] |
| Norswertianin | Gentiana dinarica transformed roots | Akt/mTOR, ROS | 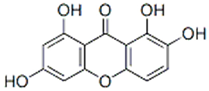 | 86.44 µM (U251 cells) | / | [156,157] | |
| Curcumin | Curcuma longa | autophagy | 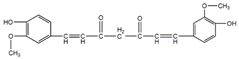 | 2 μM (SU-2, SU-3) | Type 2 Diabetes (Phase 4), Multiple Myeloma (Phase 2) | [158,159,160] | |
| FAD | North Sea Cucumber | Notch | 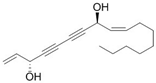 | 40 μM (U373 cells) | / | [161] |
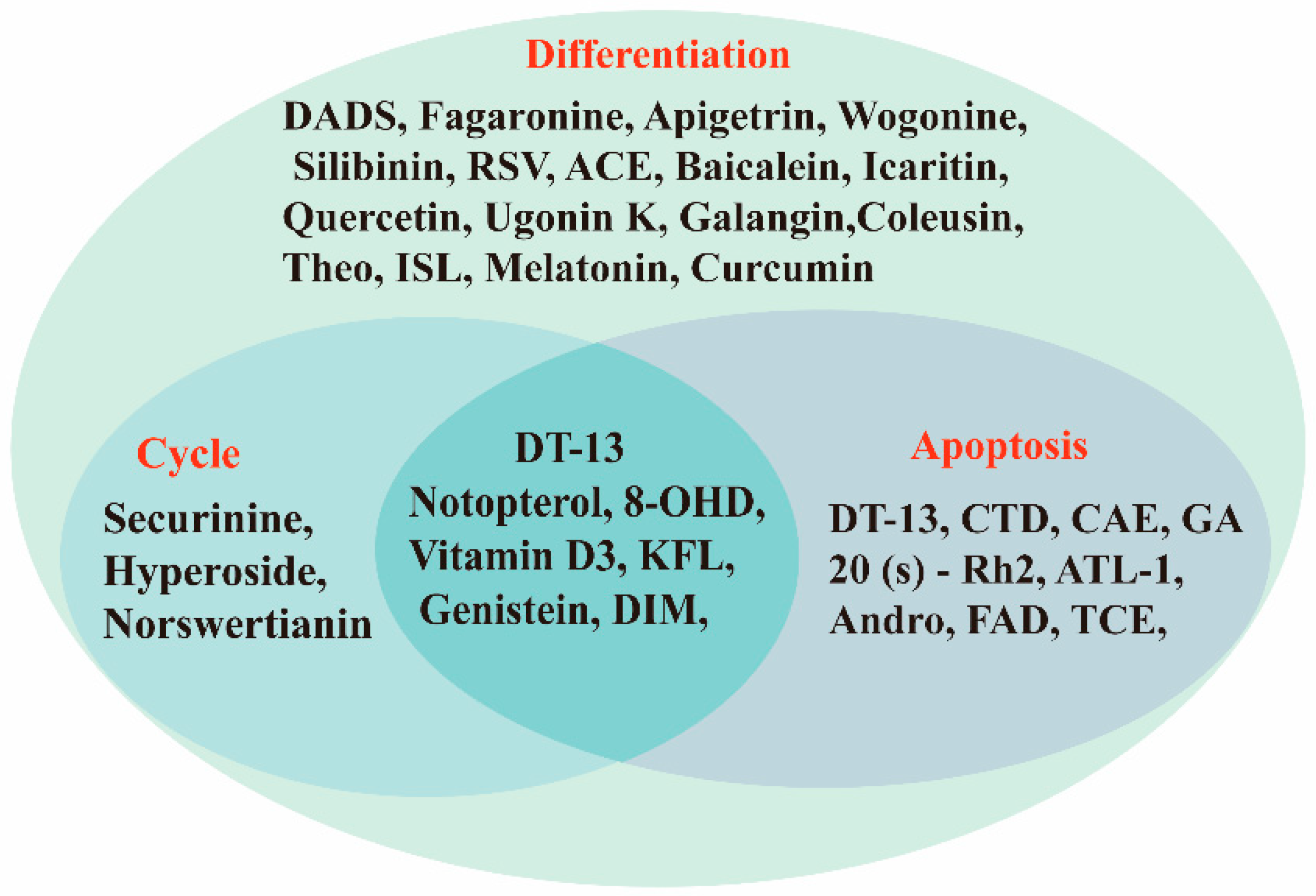
5. Discussion
- Using medicinal chemistry, natural small-molecule compounds can be structurally modified and optimized to obtain new chemical entities with high drug resistance and low toxic side effects.
- Using computer simulations of the drug screening process to predict the likely activity of compounds allows multiple biologically relevant pathways to be probed in a target-agnostic manner, which allows for targeted screening and greatly reduces the number of compounds to be experimentally screened, thereby shortening development cycles and saving money.
- The development of innovative target-prediction tools to help identify biomolecular targets or potential off-target effects of drugs may help to determine the biological activity of natural products and guide the biochemical screening of natural products to reduce the number of experiments and save valuable resources.
- Establishing sound testing methods and experimental approaches to promote drug development, improving methods for purification of active ingredients in natural compounds and extracting key components for subsequent experiments.
- The search for or development of lead compounds for novel drugs with improved pharmacokinetic profiles through the study of natural product molecules with specific active backbones, active moieties and excellent biological activity.
- Diverse and well-established animal or neural models are established for screening, development and validation.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACE | Acerogenin A |
| ALP | Alkaline phosphatase |
| AML | Acute myeloid leukemia |
| Andro | Andrographolide |
| APL | Acute promyelocytic leukemia |
| ATL-I | Atractylodes lactone I |
| ATRA | All trans-retinoic acid |
| BC | Blast crisis |
| BMP-2 | Bone morphogenetic protein-2 |
| BMPs | Bone morphogenetic proteins |
| CA | Chlorogenic acid |
| CAE | Ethyl acetate extract |
| CAPE | Caffeic acid phenethyl ester |
| C/EBPα | CCAAT/Enhancer-Binding Proteins -α |
| CCND2 | cell cycle protein D2 |
| CDK6 | Cyclin-dependent kinase 6 |
| CML | Chronic myeloid leukemia |
| c-myc | Cellular-myelocytomatosis viral oncogene |
| CTD | Cantharidin |
| DADS | Diallyl disulfide |
| DIM | 3′3-diindolylmethane |
| DT-13 | Liriodendron chinense saponin C |
| EGR1 | Early growth response 1 |
| ER | Estrogen receptor |
| FAD | Falcarindiol |
| FDA | Food and Drug Administration |
| FOG-1 | friend of GATA-1 |
| GA | Gambogic acid |
| GATA-1 | GATA Binding Protein 1 |
| GBM | Glioblastoma |
| GICs | Glioma-initiating cells |
| HAS3 | Hyaluronate synthase 3 |
| ISL | Isoliquiritigenin |
| KFL | Kaempferol |
| KLF2 | Kruppel-like factor 2 |
| MAPK | Mitogen activated protein kinase |
| MM | Multiple myeloma |
| NB | Neuroblastoma |
| NF-κB | Nuclear factor-κB |
| OCN | Osteocalcin |
| RA | Retinoic acid |
| ROS | Reactive oxygen species |
| RSV | Resveratrol |
| RTK | Receptor tyrosine kinase |
| Runx2 | Transcription factor 2 |
| TBM | Tubeimosides |
| TCE | Tinospora cordifolia |
| TGF-β | transforming growth factor beta |
| Tho | Theophylline |
| TKIs | Tyrosine kinase inhibitors |
| VD3 | 1,25-dihydroxyvitamin D3 |
| 8-OHD | 8-hydroxydaidzein |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Morceau, F.; Chateauvieux, S.; Orsini, M.; Trecul, A.; Dicato, M.; Diederich, M. Natural compounds and pharmaceuticals reprogram leukemia cell differentiation pathways. Biotechnol. Adv. 2015, 33, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, M.; Hao, W.; Han, J.; Ma, J.; Wang, C.; Sun, S.; Zheng, Q. Differentiation-inducing and anti-proliferative activities of isoliquiritigenin and all-trans-retinoic acid on B16F0 melanoma cells: Mechanisms profiling by RNA-seq. Gene 2016, 592, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.B., Jr.; Verney, E.L. An in vitro and in vivo study of differentiation in teratocarcinomas. Cancer 1961, 14, 1017–1029. [Google Scholar] [CrossRef]
- Friend, C.; Scher, W. Stimulation by dimethyl sulfoxide of erythroid differentiation and hemoglobin synthesis in murine virus-induced leukemic cells. Ann. N. Y. Acad. Sci. 1975, 243, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Chen, Z. Acute promyelocytic leukemia: From highly fatal to highly curable. Blood 2008, 111, 2505–2515. [Google Scholar] [CrossRef]
- Kreider, J.W.; Wade, D.R.; Rosenthal, M.; Densley, T. Maturation and differentiation of B16 melanoma cells induced by theophylline treatment. J. Natl. Cancer Inst. 1975, 54, 1457–1467. [Google Scholar] [CrossRef]
- Hata, K.; Mukaiyama, T.; Tsujimura, N.; Sato, Y.; Kosaka, Y.; Sakamoto, K.; Hori, K. Differentiation-inducing activity of lupane triterpenes on a mouse melanoma cell line. Cytotechnology 2006, 52, 151–158. [Google Scholar] [CrossRef][Green Version]
- Alesiani, D.; Cicconi, R.; Mattei, M.; Montesano, C.; Bei, R.; Canini, A. Cell cycle arrest and differentiation induction by 5,7-dimethoxycoumarin in melanoma cell lines. Int. J. Oncol. 2008, 32, 425–434. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Subramaniam, D.; Jensen, R.A.; Anant, S. Targeting cancer stem cells and signaling pathways by phytochemicals: Novel approach for breast cancer therapy. Semin. Cancer Biol. 2016, 40–41, 192–208. [Google Scholar] [CrossRef]
- Sudomova, M.; Berchova-Bimova, K.; Marzocco, S.; Liskova, A.; Kubatka, P.; Hassan, S.T.S. Berberine in Human Oncogenic Herpesvirus Infections and Their Linked Cancers. Viruses 2021, 13, 1014. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Ferrer, S.; Bonnet, D.; Steensma, D.P.; Hasserjian, R.P.; Ghobrial, I.M.; Gribben, J.G.; Andreeff, M.; Krause, D.S. Bone marrow niches in haematological malignancies. Nat. Rev. Cancer 2020, 20, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Girardi, T.; Vicente, C.; Cools, J.; De Keersmaecker, K. The genetics and molecular biology of T-ALL. Blood 2017, 129, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Hasanpourghadi, M.; Pandurangan, A.K.; Mustafa, M.R. Modulation of oncogenic transcription factors by bioactive natural products in breast cancer. Pharmacol. Res. 2018, 128, 376–388. [Google Scholar] [CrossRef]
- Tewary, P.; Gunatilaka, A.A.; Sayers, T.J. Using natural products to promote caspase-8-dependent cancer cell death. Cancer Immunol. Immunother. CII 2017, 66, 223–231. [Google Scholar] [CrossRef]
- Gupta, K.; Chakrabarti, A.; Rana, S.; Ramdeo, R.; Roth, B.L.; Agarwal, M.L.; Tse, W.; Agarwal, M.K.; Wald, D.N. Securinine, a myeloid differentiation agent with therapeutic potential for AML. PLoS ONE 2011, 6, e21203. [Google Scholar] [CrossRef]
- Sharma, J.; Pandey, A.; Sharma, S.; Dixit, A. Securinine Induces Differentiation of Human Promyelocytic Leukemic HL-60 Cells through JNK-Mediated Signaling Pathway. Nutr. Cancer 2022, 74, 1122–1137. [Google Scholar] [CrossRef]
- Han, S.; Zhang, G.; Li, M.; Chen, D.; Wang, Y.; Ye, W.; Ji, Z. L-securinine induces apoptosis in the human promyelocytic leukemia cell line HL-60 and influences the expression of genes involved in the PI3K/AKT/mTOR signaling pathway. Oncol. Rep. 2014, 31, 2245–2251. [Google Scholar] [CrossRef]
- Hou, W.; Wang, Z.Y.; Lin, J.; Chen, W.M. Induction of differentiation of the acute myeloid leukemia cell line (HL-60) by a securinine dimer. Cell Death Discov. 2020, 6, 123. [Google Scholar] [CrossRef]
- Sun, J.; Mu, H.; Yu, J.; Li, L.; Yan, H.; Li, G.; Tan, H.; Yang, N.; Yang, X.; Yi, L. Diallyl disulfide down-regulates calreticulin and promotes C/EBPalpha expression in differentiation of human leukaemia cells. J. Cell. Mol. Med. 2019, 23, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Shan, J.; Chen, X.; Li, G.; Li, L.; Tan, H.; Su, Q. Involvement of calreticulin in cell proliferation, invasion and differentiation in diallyl disulfide-treated HL-60 cells. Oncol. Lett. 2016, 12, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Ji, X.; Lei, Y.; Jia, Y.; Liu, F.; Xia, H.; Tan, H.; Zeng, X.; Yi, L.; He, J.; et al. Diallyl disulfide induces downregulation and inactivation of cofilin 1 differentiation via the Rac1/ROCK1/LIMK1 pathway in leukemia cells. Int. J. Oncol. 2020, 56, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Agassi, S.F.T.; Yeh, T.M.; Chang, C.D.; Hsu, J.L.; Shih, W.L. Potentiation of Differentiation and Apoptosis in a Human Promyelocytic Leukemia Cell Line by Garlic Essential Oil and Its Organosulfur Compounds. Anticancer Res. 2020, 40, 6345–6354. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, N.; Chen, H.; Wang, Z.; Zheng, Q. The critical role of redox homeostasis in shikonin-induced HL-60 cell differentiation via unique modulation of the Nrf2/ARE pathway. Oxid. Med. Cell. Longev. 2012, 2012, 781516. [Google Scholar] [CrossRef]
- Duan, D.; Zhang, B.; Yao, J.; Liu, Y.; Fang, J. Shikonin targets cytosolic thioredoxin reductase to induce ROS-mediated apoptosis in human promyelocytic leukemia HL-60 cells. Free Radic. Biol. Med. 2014, 70, 182–193. [Google Scholar] [CrossRef]
- Han, W.; Xie, J.; Fang, Y.; Wang, Z.; Pan, H. Nec-1 enhances shikonin-induced apoptosis in leukemia cells by inhibition of RIP-1 and ERK1/2. Int. J. Mol. Sci. 2012, 13, 7212–7225. [Google Scholar] [CrossRef]
- Ma, H.Y.; Wang, C.Q.; He, H.; Yu, Z.Y.; Tong, Y.; Liu, G.; Yang, Y.Q.; Li, L.; Pang, L.; Qi, H.Y. Ethyl acetate extract of Caesalpinia sappan L. inhibited acute myeloid leukemia via ROS-mediated apoptosis and differentiation. Phytomed. Int. J. Phytother. Phytopharm. 2020, 68, 153142. [Google Scholar] [CrossRef]
- Wang, Q.; Salman, H.; Danilenko, M.; Studzinski, G.P. Cooperation between antioxidants and 1,25-dihydroxyvitamin D3 in induction of leukemia HL60 cell differentiation through the JNK/AP-1/Egr-1 pathway. J. Cell. Physiol. 2005, 204, 964–974. [Google Scholar] [CrossRef]
- Liu, Z.H.; Li, J.; Xia, J.; Jiang, R.; Zuo, G.W.; Li, X.P.; Chen, Y.; Xiong, W.; Chen, D.L. Ginsenoside 20(s)-Rh2 as potent natural histone deacetylase inhibitors suppressing the growth of human leukemia cells. Chem.-Biol. Interact. 2015, 242, 227–234. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y. Ginsenoside Rh2 Mitigates Pediatric Leukemia Through Suppression of Bcl-2 in Leukemia Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 37, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, H.; Dou, G.; Li, J.; Zhang, X.; Jiang, M.; Li, P.; Huang, X.; Chen, H.; Li, L.; et al. Ginsenoside 20(S)-Rh2 Induces Apoptosis and Differentiation of Acute Myeloid Leukemia Cells: Role of Orphan Nuclear Receptor Nur77. J. Agric. Food Chem. 2017, 65, 7687–7697. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Yin, J.; Xu, C.; Mu, Y.; Lv, S. 20(S)-Ginsenoside Rh2 Induce the Apoptosis and Autophagy in U937 and K562 Cells. Nutrients 2018, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, L.; Wang, C.; He, H.; Liu, G.; Ma, H.; Pang, L.; Jiang, M.; Lu, Q.; Li, P.; et al. Cantharidin Induces Apoptosis and Promotes Differentiation of AML Cells Through Nuclear Receptor Nur77-Mediated Signaling Pathway. Front. Pharmacol. 2020, 11, 1321. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Chow, J.M.; Chien, M.H.; Lin, C.W.; Chen, H.Y.; Hsiao, P.C.; Yang, S.F. Cantharidic acid induces apoptosis of human leukemic HL-60 cells via c-Jun N-terminal kinase-regulated caspase-8/-9/-3 activation pathway. Environ. Toxicol. 2018, 33, 514–522. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Meng, Z.Z.; Yue, G.X.; Chen, J.X. Norcantharidin Induces HL-60 Cells Apoptosis In Vitro. Evid.-Based Complement. Altern. Med. Ecam 2012, 2012, 154271. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Q.; Li, Y.; Sun, P.; Kuek, V.; Yuan, J.; Yang, J.; Wen, L.; Wang, H.; Xu, J.; et al. Notopterol Attenuates Estrogen Deficiency-Induced Osteoporosis via Repressing RANKL Signaling and Reactive Oxygen Species. Front. Pharmacol. 2021, 12, 664836. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, X.; Yang, L.; Zhao, Y.; Chew, Z.; Xiao, J.; Liu, C.; Zheng, X.; Zheng, Y.; Shi, Q.; et al. The Natural Compound Notopterol Binds and Targets JAK2/3 to Ameliorate Inflammation and Arthritis. Cell Rep. 2020, 33, 108442. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, H.; Li, J.; Liu, W.; Wu, Q.; Xu, Z.; Qiao, Q.; Zhang, H.; Gao, H.; Zhao, Q. A natural BACE1 and GSK3beta dual inhibitor Notopterol effectively ameliorates the cognitive deficits in APP/PS1 Alzheimer’s mice by attenuating amyloid-beta and tau pathology. Clin. Transl. Med. 2020, 10, e50. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, L.; Ran, Q.; Wang, J.; Wang, C.; He, H.; Li, L.; Qi, H. Notopterol-induced apoptosis and differentiation in human acute myeloid leukemia HL-60 cells. Drug Des. Dev. Ther. 2019, 13, 1927–1940. [Google Scholar] [CrossRef]
- Khan, G.J.; Rizwan, M.; Abbas, M.; Naveed, M.; Boyang, Y.; Naeem, M.A.; Khan, S.; Yuan, S.; Baig, M.; Sun, L. Pharmacological effects and potential therapeutic targets of DT-13. Biomed. Pharmacother. 2018, 97, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Mao, T.; Li, S.; He, J.; Hou, X.; Li, H.; Zhan, M.; Yang, X.; Li, R.; Xiao, J.; et al. DT-13 inhibited the proliferation of colorectal cancer via glycolytic metabolism and AMPK/mTOR signaling pathway. Phytomed. Int. J. Phytother. Phytopharm. 2019, 54, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, Y.; Chen, X.; Yu, X.; Hou, X.; Li, H.; Zhan, M.; Lin, S.; Lu, L.; Yuan, S.; et al. DT-13 synergistically potentiates the sensitivity of gastric cancer cells to topotecan via cell cycle arrest in vitro and in vivo. Eur. J. Pharmacol. 2018, 818, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, H.; Liu, G.; Ma, H.; Li, L.; Jiang, M.; Lu, Q.; Li, P.; Qi, H. DT-13 induced apoptosis and promoted differentiation of acute myeloid leukemia cells by activating AMPK-KLF2 pathway. Pharmacol. Res. 2020, 158, 104864. [Google Scholar] [CrossRef]
- Samadian, N.; Hashemi, M. Effects of Apigenin and Apigenin- Loaded Nanogel on Induction of Apoptosis in Human Chronic Myeloid Leukemia Cells. Galen Med. J. 2018, 7, e1008. [Google Scholar] [CrossRef]
- Danisman Kalindemirtas, F.; Birman, H.; Candoken, E.; Bilgis Gazioglu, S.; Melikoglu, G.; Kuruca, S. Cytotoxic Effects of Some Flavonoids and Imatinib on the K562 Chronic Myeloid Leukemia Cell Line: Data Analysis Using the Combination Index Method. Balk. Med. J. 2019, 36, 96–105. [Google Scholar] [CrossRef]
- Tsolmon, S.; Nakazaki, E.; Han, J.; Isoda, H. Apigetrin induces erythroid differentiation of human leukemia cells K562: Proteomics approach. Mol. Nutr. Food Res. 2011, 55 (Suppl. S1), S93–S102. [Google Scholar] [CrossRef]
- Isoda, H.; Motojima, H.; Onaga, S.; Samet, I.; Villareal, M.O.; Han, J. Analysis of the erythroid differentiation effect of flavonoid apigenin on K562 human chronic leukemia cells. Chem. Biol. Interact. 2014, 220, 269–277. [Google Scholar] [CrossRef]
- Dupont, C.; Couillerot, E.; Gillet, R.; Caron, C.; Zeches-Hanrot, M.; Riou, J.F.; Trentesaux, C. The benzophenanthridine alkaloid fagaronine induces erythroleukemic cell differentiation by gene activation. Planta Med. 2005, 71, 489–494. [Google Scholar] [CrossRef]
- Huang, B.; Liu, H.; Huang, D.; Mao, X.; Hu, X.; Jiang, C.; Pu, M.; Zhang, G.; Zeng, X. Apoptosis Induction and Imaging of Cadmium-Telluride Quantum Dots with Wogonin in Multidrug-Resistant Leukemia K562/A02 Cell. J. Nanosci. Nanotechnol. 2016, 16, 2499–2503. [Google Scholar] [CrossRef]
- Hu, C.; Xu, M.; Qin, R.; Chen, W.; Xu, X. Wogonin induces apoptosis and endoplasmic reticulum stress in HL-60 leukemia cells through inhibition of the PI3K-AKT signaling pathway. Oncol. Rep. 2015, 33, 3146–3154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boozari, M.; Mohammadi, A.; Asili, J.; Emami, S.A.; Tayarani-Najaran, Z. Growth inhibition and apoptosis induction by Scutellaria pinnatifida A. Ham. on HL-60 and K562 leukemic cell lines. Environ. Toxicol. Pharmacol. 2015, 39, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hui, H.; Wang, Q.; Li, H.; Zhao, K.; Zhou, Y.; Zhu, Y.; Wang, X.; You, Q.; Guo, Q.; et al. Correction: Wogonin induces cell cycle arrest and erythroid differentiation in imatinib-resistant K562 cells and primary CML cells. Oncotarget 2020, 11, 300–301. [Google Scholar] [CrossRef]
- Zhao, Y.; Bilal, M.; Raza, A.; Khan, M.I.; Mehmood, S.; Hayat, U.; Hassan, S.T.S.; Iqbal, H.M.N. Tyrosine kinase inhibitors and their unique therapeutic potentialities to combat cancer. Int. J. Biol. Macromol. 2021, 168, 22–37. [Google Scholar] [CrossRef]
- Shi, X.; Chen, X.; Li, X.; Lan, X.; Zhao, C.; Liu, S.; Huang, H.; Liu, N.; Liao, S.; Song, W.; et al. Gambogic acid induces apoptosis in imatinib-resistant chronic myeloid leukemia cells via inducing proteasome inhibition and caspase-dependent Bcr-Abl downregulation. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, M.; Zhang, Q.; Xu, J.; Ouyang, J. Gambogic acid induces death of K562 cells through autophagy and apoptosis mechanisms. Leuk. Lymphoma 2015, 56, 2953–2958. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.J. Research Advance on Reversing Multidrug Resistance of Chronic Myeloid Leukemia by Chinese Herbal Monomer–Review. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2020, 28, 1064–1068. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, S.R.; Chai, L.; Zhao, J.; Wang, Y.; Wang, Y. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Crit. Rev. Food Sci. Nutr. 2019, 59, S17–S29. [Google Scholar] [CrossRef]
- Liao, H.C.; Chou, Y.J.; Lin, C.C.; Liu, S.H.; Oswita, A.; Huang, Y.L.; Wang, Y.L.; Syu, J.L.; Sun, C.M.; Leu, C.M.; et al. Andrographolide and its potent derivative exhibit anticancer effects against imatinib-resistant chronic myeloid leukemia cells by downregulating the Bcr-Abl oncoprotein. Biochem. Pharmacol. 2019, 163, 308–320. [Google Scholar] [CrossRef]
- Liu, S.H.; Lin, C.H.; Liang, F.P.; Chen, P.F.; Kuo, C.D.; Alam, M.M.; Maiti, B.; Hung, S.K.; Chi, C.W.; Sun, C.M.; et al. Andrographolide downregulates the v-Src and Bcr-Abl oncoproteins and induces Hsp90 cleavage in the ROS-dependent suppression of cancer malignancy. Biochem. Pharmacol. 2014, 87, 229–242. [Google Scholar] [CrossRef]
- Mokenapelli, S.; Gutam, M.; Vadiyaala, N.; Yerrabelli, J.R.; Banerjee, S.; Roy, P.; Kancha, R.K.; Kunduru, B.R.; Sagurthi, S.R.; Chitneni, P.R. Synthesis and cytotoxicity of novel 14alpha-O-(1,4-disubstituted-1,2,3-triazolyl) ester derivatives of andrographolide. Nat. Prod. Res. 2021, 35, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.S.; Yen, J.H.; Wang, C.Y.; Chen, P.Y.; Hung, J.H.; Wu, M.J. 8-Hydroxydaidzein, an Isoflavone from Fermented Soybean, Induces Autophagy, Apoptosis, Differentiation, and Degradation of Oncoprotein BCR-ABL in K562 Cells. Biomedicines 2020, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.S.; Wang, C.Y.; Chen, P.S.; Hung, J.H.; Yen, J.H.; Wu, M.J. 8-Hydroxydaidzein Downregulates JAK/STAT, MMP, Oxidative Phosphorylation, and PI3K/AKT Pathways in K562 Cells. Biomedicines 2021, 9, 1907. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Lin, T.W.; Huang, Y.L.; Huang, R.L. Induction of apoptosis and differentiation by atractylenolide-1 isolated from Atractylodes macrocephala in human leukemia cells. Bioorg. Med. Chem. Lett. 2016, 26, 1905–1909. [Google Scholar] [CrossRef] [PubMed]
- Piechotta, V.; Jakob, T.; Langer, P.; Monsef, I.; Scheid, C.; Estcourt, L.J.; Ocheni, S.; Theurich, S.; Kuhr, K.; Scheckel, B.; et al. Multiple drug combinations of bortezomib, lenalidomide, and thalidomide for first-line treatment in adults with transplant-ineligible multiple myeloma: A network meta-analysis. Cochrane Database Syst. Rev. 2019, 2019, CD013487. [Google Scholar] [CrossRef]
- Carpenter, R.S.; Goodrich, L.R.; Frisbie, D.D.; Kisiday, J.D.; Carbone, B.; McIlwraith, C.W.; Centeno, C.J.; Hidaka, C. Osteoblastic differentiation of human and equine adult bone marrow-derived mesenchymal stem cells when BMP-2 or BMP-7 homodimer genetic modification is compared to BMP-2/7 heterodimer genetic modification in the presence and absence of dexamethasone. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2010, 28, 1330–1337. [Google Scholar] [CrossRef]
- Ema, H.; Morita, Y.; Suda, T. Heterogeneity and hierarchy of hematopoietic stem cells. Exp. Hematol. 2014, 42, 74–82.e2. [Google Scholar] [CrossRef]
- Ying, X.; Sun, L.; Chen, X.; Xu, H.; Guo, X.; Chen, H.; Hong, J.; Cheng, S.; Peng, L. Silibinin promotes osteoblast differentiation of human bone marrow stromal cells via bone morphogenetic protein signaling. Eur. J. Pharmacol. 2013, 721, 225–230. [Google Scholar] [CrossRef]
- Kihara, T.; Ichikawa, S.; Yonezawa, T.; Lee, J.W.; Akihisa, T.; Woo, J.T.; Michi, Y.; Amagasa, T.; Yamaguchi, A. Acerogenin A, a natural compound isolated from Acer nikoense Maxim, stimulates osteoblast differentiation through bone morphogenetic protein action. Biochem. Biophys. Res. Commun. 2011, 406, 211–217. [Google Scholar] [CrossRef]
- Yonezawa, T.; Lee, J.W.; Akazawa, H.; Inagaki, M.; Cha, B.Y.; Nagai, K.; Yagasaki, K.; Akihisa, T.; Woo, J.T. Osteogenic activity of diphenyl ether-type cyclic diarylheptanoids derived from Acer nikoense. Bioorg. Med. Chem. Lett. 2011, 21, 3248–3251. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, S.U.; Kim, Y.S.; Min, Y.K.; Kim, S.H. Baicalein stimulates osteoblast differentiation via coordinating activation of MAP kinases and transcription factors. J. Cell. Biochem. 2008, 104, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Li, Y.; Cheng, Z.J.; Wang, X.; Mao, W.; Zhang, Y.W. Active Components of Traditional Chinese Medicinal Material for Multiple Myeloma: Current Evidence and Future Directions. Front. Pharmacol. 2022, 13, 818179. [Google Scholar] [CrossRef] [PubMed]
- Banik, K.; Khatoon, E.; Harsha, C.; Rana, V.; Parama, D.; Thakur, K.K.; Bishayee, A.; Kunnumakkara, A.B. Wogonin and its analogs for the prevention and treatment of cancer: A systematic review. Phytother. Res. PTR 2022. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Tang, J.J.; Chen, J.; Zhang, P.; Wang, T.; Chen, T.Y.; Yan, B.; Huang, B.; Wang, L.; Huang, M.J.; et al. Regulation of bone formation by baicalein via the mTORC1 pathway. Drug Des. Dev. Ther. 2015, 9, 5169–5183. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, H.; Chen, Y.; Ao, X.; Chen, C.; Zhang, W.; He, F.; Liao, X.; Jiang, X.; Li, T.; et al. Baicalein Accelerates Tendon-Bone Healing via Activation of Wnt/beta-Catenin Signaling Pathway in Rats. BioMed Res. Int. 2018, 2018, 3849760. [Google Scholar] [CrossRef]
- Boissy, P.; Andersen, T.L.; Abdallah, B.M.; Kassem, M.; Plesner, T.; Delaisse, J.M. Resveratrol inhibits myeloma cell growth, prevents osteoclast formation, and promotes osteoblast differentiation. Cancer Res. 2005, 65, 9943–9952. [Google Scholar] [CrossRef]
- Kupisiewicz, K.; Boissy, P.; Abdallah, B.M.; Hansen, F.D.; Erben, R.G.; Savouret, J.F.; Soe, K.; Andersen, T.L.; Plesner, T.; Delaisse, J.M. Potential of resveratrol analogues as antagonists of osteoclasts and promoters of osteoblasts. Calcif. Tissue Int. 2010, 87, 437–449. [Google Scholar] [CrossRef]
- Ma, R.; Yu, D.; Peng, Y.; Yi, H.; Wang, Y.; Cheng, T.; Shi, B.; Yang, G.; Lai, W.; Wu, X.; et al. Resveratrol induces AMPK and mTOR signaling inhibition-mediated autophagy and apoptosis in multiple myeloma cells. Acta Biochim. Biophys. Sin. 2021, 53, 775–783. [Google Scholar] [CrossRef]
- Li, Q.; Yue, Y.; Chen, L.; Xu, C.; Wang, Y.; Du, L.; Xue, X.; Liu, Q.; Wang, Y.; Fan, F. Resveratrol Sensitizes Carfilzomib-Induced Apoptosis via Promoting Oxidative Stress in Multiple Myeloma Cells. Front. Pharmacol. 2018, 9, 334. [Google Scholar] [CrossRef]
- Yang, X.J.; Xi, Y.M.; Li, Z.J. Icaritin: A Novel Natural Candidate for Hematological Malignancies Therapy. BioMed Res. Int. 2019, 2019, 4860268. [Google Scholar] [CrossRef]
- Ma, X.N.; Ma, C.X.; Shi, W.G.; Zhou, J.; Ma, H.P.; Gao, Y.H.; Xian, C.J.; Chen, K.M. Primary cilium is required for the stimulating effect of icaritin on osteogenic differentiation and mineralization of osteoblasts in vitro. J. Endocrinol. Investig. 2017, 40, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Shu, T.; Kang, L.; Wu, J.; Xing, J.; Lu, Z.; Chen, S.; Lv, J. Icaritin, a novel plant-derived osteoinductive agent, enhances the osteogenic differentiation of human bone marrow- and human adipose tissue-derived mesenchymal stem cells. Int. J. Mol. Med. 2017, 39, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ou, L.; Wang, C.; Yang, L.; Wang, P.; Liu, H.; Xiong, Y.; Sun, K.; Zhang, R.; Zhu, X. Icaritin induces MC3T3-E1 subclone14 cell differentiation through estrogen receptor-mediated ERK1/2 and p38 signaling activation. Biomed. Pharmacother. 2017, 94, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, Z.; Zhang, G.; Meng, K.; Kuang, W.; Li, J.; Zhou, X.; Li, R.; Peng, H.; Dai, C.; et al. Icaritin shows potent anti-leukemia activity on chronic myeloid leukemia in vitro and in vivo by regulating MAPK/ERK/JNK and JAK2/STAT3 /AKT signalings. PLoS ONE 2011, 6, e23720. [Google Scholar] [CrossRef]
- Tang, N.; Song, W.X.; Luo, J.; Haydon, R.C.; He, T.C. Osteosarcoma development and stem cell differentiation. Clin. Orthop. Relat. Res. 2008, 466, 2114–2130. [Google Scholar] [CrossRef]
- Longhi, A.; Errani, C.; De Paolis, M.; Mercuri, M.; Bacci, G. Primary bone osteosarcoma in the pediatric age: State of the art. Cancer Treat. Rev. 2006, 32, 423–436. [Google Scholar] [CrossRef]
- Ying, M.; Liu, G.; Shimada, H.; Ding, W.; May, W.A.; He, Q.; Adams, G.B.; Wu, L. Human osteosarcoma CD49f(-)CD133(+) cells: Impaired in osteogenic fate while gain of tumorigenicity. Oncogene 2013, 32, 4252–4263. [Google Scholar] [CrossRef][Green Version]
- Ferguson, W.S.; Goorin, A.M. Current treatment of osteosarcoma. Cancer Investig. 2001, 19, 292–315. [Google Scholar] [CrossRef]
- Liskova, A.; Samec, M.; Koklesova, L.; Brockmueller, A.; Zhai, K.; Abdellatif, B.; Siddiqui, M.; Biringer, K.; Kudela, E.; Pec, M.; et al. Flavonoids as an effective sensitizer for anti-cancer therapy: Insights into multi-faceted mechanisms and applicability towards individualized patient profiles. EPMA J. 2021, 12, 155–176. [Google Scholar] [CrossRef]
- Pang, X.G.; Cong, Y.; Bao, N.R.; Li, Y.G.; Zhao, J.N. Quercetin Stimulates Bone Marrow Mesenchymal Stem Cell Differentiation through an Estrogen Receptor-Mediated Pathway. BioMed Res. Int. 2018, 2018, 4178021. [Google Scholar] [CrossRef]
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. Anti-cancer properties of quercetin in osteosarcoma. Cancer Cell Int. 2021, 21, 349. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pei, Y.; Wang, W.; Liu, F.; Zheng, K.; Zhang, X. Quercetin suppresses the proliferation and metastasis of metastatic osteosarcoma cells by inhibiting parathyroid hormone receptor 1. Biomed. Pharmacother. 2019, 114, 108839. [Google Scholar] [CrossRef] [PubMed]
- Casado-Diaz, A.; Anter, J.; Dorado, G.; Quesada-Gomez, J.M. Effects of quercetin, a natural phenolic compound, in the differentiation of human mesenchymal stem cells (MSC) into adipocytes and osteoblasts. J. Nutr. Biochem. 2016, 32, 151–162. [Google Scholar] [CrossRef]
- Zhang, D.; Fong, C.; Jia, Z.; Cui, L.; Yao, X.; Yang, M. Icariin Stimulates Differentiation and Suppresses Adipocytic Transdifferentiation of Primary Osteoblasts Through Estrogen Receptor-Mediated Pathway. Calcif. Tissue Int. 2016, 99, 187–198. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, F.; Liu, Z. Inhibitory effect of icariin on osteosarcoma cell proliferation via the Wnt/beta-catenin signaling pathway. Oncol. Lett. 2018, 16, 1405–1410. [Google Scholar] [CrossRef]
- Wang, Z.D.; Wang, R.Z.; Xia, Y.Z.; Kong, L.Y.; Yang, L. Reversal of multidrug resistance by icaritin in doxorubicin-resistant human osteosarcoma cells. Chin. J. Nat. Med. 2018, 16, 20–28. [Google Scholar] [CrossRef]
- Sun, L.J.; Li, C.; Wen, X.H.; Guo, L.; Guo, Z.F.; Liao, L.Q.; Guo, Y. Icariin Stimulates hFOB 1.19 Osteoblast Proliferation and Differentiation via OPG/RANKL Mediated by the Estrogen Receptor. Curr. Pharm. Biotechnol. 2021, 22, 168–175. [Google Scholar] [CrossRef]
- Xu, Y.; Li, L.; Tang, Y.; Yang, J.; Jin, Y.; Ma, C. Icariin promotes osteogenic differentiation by suppressing Notch signaling. Eur. J. Pharmacol. 2019, 865, 172794. [Google Scholar] [CrossRef]
- Dai, J.; Li, Y.; Zhou, H.; Chen, J.; Chen, M.; Xiao, Z. Genistein promotion of osteogenic differentiation through BMP2/SMAD5/RUNX2 signaling. Int. J. Biol. Sci. 2013, 9, 1089–1098. [Google Scholar] [CrossRef]
- Engel, N.; Adamus, A.; Schauer, N.; Kuhn, J.; Nebe, B.; Seitz, G.; Kraft, K. Synergistic Action of Genistein and Calcitriol in Immature Osteosarcoma MG-63 Cells by SGPL1 Up-Regulation. PLoS ONE 2017, 12, e0169742. [Google Scholar] [CrossRef]
- Song, M.; Tian, X.; Lu, M.; Zhang, X.; Ma, K.; Lv, Z.; Wang, Z.; Hu, Y.; Xun, C.; Zhang, Z.; et al. Genistein exerts growth inhibition on human osteosarcoma MG-63 cells via PPARgamma pathway. Int. J. Oncol. 2015, 46, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.; Bose, S. Controlled release of soy isoflavones from multifunctional 3D printed bone tissue engineering scaffolds. Acta Biomater. 2020, 114, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.J.; Choi, R.C.; Zheng, K.Y.; Chen, V.P.; Dong, T.T.; Wang, Z.T.; Vollmer, G.; Lau, D.T.; Tsim, K.W. Kaempferol as a flavonoid induces osteoblastic differentiation via estrogen receptor signaling. Chin. Med. 2012, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.W.; Chiu, Y.J.; Fan, M.J.; Lu, H.F.; Yeh, H.F.; Li, K.H.; Chen, P.Y.; Chung, J.G.; Yang, J.S. Kaempferol induced apoptosis via endoplasmic reticulum stress and mitochondria-dependent pathway in human osteosarcoma U-2 OS cells. Mol. Nutr. Food Res. 2010, 54, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Teng, J.; Ma, L.; Tong, H.; Ren, B.; Wang, L.; Li, W. Flavonoids Isolated From the Flowers of Limonium bicolor and their In vitro Antitumor Evaluation. Pharmacogn. Mag. 2017, 13, 222–225. [Google Scholar] [CrossRef]
- Lee, C.H.; Huang, Y.L.; Liao, J.F.; Chiou, W.F. Ugonin K-stimulated osteogenesis involves estrogen receptor-dependent activation of non-classical Src signaling pathway and classical pathway. Eur. J. Pharmacol. 2012, 676, 26–33. [Google Scholar] [CrossRef]
- Lee, C.H.; Huang, Y.L.; Liao, J.F.; Chiou, W.F. Ugonin K promotes osteoblastic differentiation and mineralization by activation of p38 MAPK- and ERK-mediated expression of Runx2 and osterix. Eur. J. Pharmacol. 2011, 668, 383–389. [Google Scholar] [CrossRef]
- Liu, C.; Ma, M.; Zhang, J.; Gui, S.; Zhang, X.; Xue, S. Galangin inhibits human osteosarcoma cells growth by inducing transforming growth factor-beta1-dependent osteogenic differentiation. Biomed. Pharmacother. 2017, 89, 1415–1421. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Han, W.; Lu, X.; Jin, S.; Yang, W.; Li, J.; He, W.; Qian, Y. Galangin suppresses human osteosarcoma cells: An exploration of its underlying mechanism. Oncol. Rep. 2017, 37, 435–441. [Google Scholar] [CrossRef]
- Huh, J.E.; Jung, I.T.; Choi, J.; Baek, Y.H.; Lee, J.D.; Park, D.S.; Choi, D.Y. The natural flavonoid galangin inhibits osteoclastic bone destruction and osteoclastogenesis by suppressing NF-kappaB in collagen-induced arthritis and bone marrow-derived macrophages. Eur. J. Pharmacol. 2013, 698, 57–66. [Google Scholar] [CrossRef]
- Zhang, N.; Ying, M.D.; Wu, Y.P.; Zhou, Z.H.; Ye, Z.M.; Li, H.; Lin, D.S. Hyperoside, a flavonoid compound, inhibits proliferation and stimulates osteogenic differentiation of human osteosarcoma cells. PLoS ONE 2014, 9, e98973. [Google Scholar] [CrossRef]
- Qi, X.C.; Li, B.; Wu, W.L.; Liu, H.C.; Jiang, Y.P. Protective effect of hyperoside against hydrogen peroxide-induced dysfunction and oxidative stress in osteoblastic MC3T3-E1 cells. Artif. Cells Nanomed. Biotechnol. 2020, 48, 377–383. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X.F. Hyperoside decreases the apoptosis and autophagy rates of osteoblast MC3T3E1 cells by regulating TNFlike weak inducer of apoptosis and the p38mitogen activated protein kinase pathway. Mol. Med. Rep. 2019, 19, 41–50. [Google Scholar] [CrossRef]
- Geng, S.; Sun, B.; Lu, R.; Wang, J. Coleusin factor, a novel anticancer diterpenoid, inhibits osteosarcoma growth by inducing bone morphogenetic protein-2-dependent differentiation. Mol. Cancer Ther. 2014, 13, 1431–1441. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kommagani, R.; Whitlatch, A.; Leonard, M.K.; Kadakia, M.P. p73 is essential for vitamin D-mediated osteoblastic differentiation. Cell Death Differ. 2010, 17, 398–407. [Google Scholar] [CrossRef]
- Wang, D.; Song, J.; Ma, H. An in vitro Experimental Insight into the Osteoblast Responses to Vitamin D3 and Its Metabolites. Pharmacology 2018, 101, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, S.; Na, S.; Rathnachalam, R. Noncalcemic actions of vitamin D receptor ligands. Endocr. Rev. 2005, 26, 662–687. [Google Scholar] [CrossRef] [PubMed]
- Tahbazlahafi, B.; Paknejad, M.; Khaghani, S.; Sadegh-Nejadi, S.; Khalili, E. Vitamin D Represses the Aggressive Potential of Osteosarcoma. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1312–1318. [Google Scholar] [CrossRef]
- Samuel, S.; Sitrin, M.D. Vitamin D’s role in cell proliferation and differentiation. Nutr. Rev. 2008, 66, S116–S124. [Google Scholar] [CrossRef]
- Gombart, A.F.; Luong, Q.T.; Koeffler, H.P. Vitamin D compounds: Activity against microbes and cancer. Anticancer Res. 2006, 26, 2531–2542. [Google Scholar]
- Cordella, M.; Tabolacci, C.; Senatore, C.; Rossi, S.; Mueller, S.; Lintas, C.; Eramo, A.; D’Arcangelo, D.; Valitutti, S.; Facchiano, A.; et al. Theophylline induces differentiation and modulates cytoskeleton dynamics and cytokines secretion in human melanoma-initiating cells. Life Sci. 2019, 230, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Yen, H.; Lu, J.Y.; Chang, T.M.; Hii, C.H. Theophylline enhances melanogenesis in B16F10 murine melanoma cells through the activation of the MEK 1/2, and Wnt/beta-catenin signaling pathways. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 137, 111165. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Nakashima, S.; Nakamura, S.; Wang, T.; Yoshikawa, M.; Matsuda, H. Inhibition of melanin production by anthracenone dimer glycosides isolated from Cassia auriculata seeds. J. Nat. Med. 2019, 73, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Zeng, H.; Xia, F.; Ji, Q.; Xue, J.; Ren, R.; Que, F.; Zhou, B. The dietary flavonoid isoliquiritigenin induced apoptosis and suppressed metastasis in melanoma cells: An in vitro and in vivo study. Life Sci. 2021, 264, 118598. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Chen, H.; Luo, X.; An, B.; Wu, W.; Cao, S.; Ruan, S.; Wang, Z.; Weng, L.; Zhu, H.; et al. Isoliquiritigenin suppresses human melanoma growth by targeting miR-301b/LRIG1 signaling. J. Exp. Clin. Cancer Res. CR 2018, 37, 184. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Ren, H.H.; Wang, D.; Chen, Y.; Qu, C.J.; Pan, Z.H.; Liu, X.N.; Hao, W.J.; Xu, W.J.; Wang, K.J.; et al. Isoliquiritigenin Induces Mitochondrial Dysfunction and Apoptosis by Inhibiting mitoNEET in a Reactive Oxygen Species-Dependent Manner in A375 Human Melanoma Cells. Oxid. Med. Cell. Longev. 2019, 2019, 9817576. [Google Scholar] [CrossRef] [PubMed]
- Qiang, D.; Ci, C.; Liu, W.; Wang, J.; He, C.; Ji, B.; Shao, X. Inhibitory effect of kaempferol on mouse melanoma cell line B16 in vivo and in vitro. Postepy Dermatol. I Alergol. 2021, 38, 498–504. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, P.; Sun, J.; Guo, L. Anticancer effects of kaempferol in A375 human malignant melanoma cells are mediated via induction of apoptosis, cell cycle arrest, inhibition of cell migration and downregulation of m-TOR/PI3K/AKT pathway. J. BUON Off. J. Balk. Union Oncol. 2018, 23, 218–223. [Google Scholar]
- Bouhlel Chatti, I.; Ben Toumia, I.; Krichen, Y.; Maatouk, M.; Chekir Ghedira, L.; Krifa, M. Assessment of Rhamnus alaternus Leaves Extract: Phytochemical Characterization and Antimelanoma Activity. J. Med. Food 2021. [Google Scholar] [CrossRef]
- Sabitov, A.; Gawel-Beben, K.; Sakipova, Z.; Strzepek-Gomolka, M.; Hoian, U.; Satbayeva, E.; Glowniak, K.; Ludwiczuk, A. Rosa platyacantha Schrenk from Kazakhstan-Natural Source of Bioactive Compounds with Cosmetic Significance. Molecules 2021, 26, 2578. [Google Scholar] [CrossRef]
- Heriniaina, R.M.; Dong, J.; Kalavagunta, P.K.; Wu, H.L.; Yan, D.S.; Shang, J. Effects of six compounds with different chemical structures on melanogenesis. Chin. J. Nat. Med. 2018, 16, 766–773. [Google Scholar] [CrossRef]
- Cui, S.; Wang, J.; Wu, Q.; Qian, J.; Yang, C.; Bo, P. Genistein inhibits the growth and regulates the migration and invasion abilities of melanoma cells via the FAK/paxillin and MAPK pathways. Oncotarget 2017, 8, 21674–21691. [Google Scholar] [CrossRef] [PubMed]
- Venza, I.; Visalli, M.; Oteri, R.; Beninati, C.; Teti, D.; Venza, M. Genistein reduces proliferation of EP3-expressing melanoma cells through inhibition of PGE2-induced IL-8 expression. Int. Immunopharmacol. 2018, 62, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.M.; Chang, Y.J.; Wu, J.Y.; Chang, T.S. Production and Anti-Melanoma Activity of Methoxyisoflavones from the Biotransformation of Genistein by Two Recombinant Escherichia coli Strains. Molecules 2017, 22, 87. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Yu, M.; Xu, Y. PTEN/Akt Signaling-Mediated Activation of the Mitochondrial Pathway Contributes to the 3,3′-Diindolylmethane-Mediated Antitumor Effect in Malignant Melanoma Cells. J. Med. Food 2020, 23, 1248–1258. [Google Scholar] [CrossRef]
- Maciejewska, D.; Rasztawicka, M.; Wolska, I.; Anuszewska, E.; Gruber, B. Novel 3,3′-diindolylmethane derivatives: Synthesis and cytotoxicity, structural characterization in solid state. Eur. J. Med. Chem. 2009, 44, 4136–4147. [Google Scholar] [CrossRef]
- Heo, J.R.; Lee, G.A.; Kim, G.S.; Hwang, K.A.; Choi, K.C. Phytochemical-induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis and differentiation in malignant melanoma cells. Phytomed. Int. J. Phytother. Phytopharm. 2018, 39, 100–110. [Google Scholar] [CrossRef]
- Colon, N.C.; Chung, D.H. Neuroblastoma. Adv. Pediatr. 2011, 58, 297–311. [Google Scholar] [CrossRef]
- Abdullah, A.; Talwar, P.; d’Hellencourt, C.L.; Ravanan, P. IRE1alpha is critical for Kaempferol-induced neuroblastoma differentiation. FEBS J. 2019, 286, 1375–1392. [Google Scholar] [CrossRef]
- Pham, H.N.T.; Sakoff, J.A.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. Comparative cytotoxic activity between kaempferol and gallic acid against various cancer cell lines. Data Brief 2018, 21, 1033–1036. [Google Scholar] [CrossRef]
- Lee, W.J.; Chen, L.C.; Lin, J.H.; Cheng, T.C.; Kuo, C.C.; Wu, C.H.; Chang, H.W.; Tu, S.H.; Ho, Y.S. Melatonin promotes neuroblastoma cell differentiation by activating hyaluronan synthase 3-induced mitophagy. Cancer Med. 2019, 8, 4821–4835. [Google Scholar] [CrossRef] [PubMed]
- Pourhanifeh, M.H.; Kamali, M.; Mehrzadi, S.; Hosseinzadeh, A. Melatonin and neuroblastoma: A novel therapeutic approach. Mol. Biol. Rep. 2021, 48, 4659–4665. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.; Chinchalongporn, V.; Govitrapong, P. Melatonin Prevents Neddylation Dysfunction in Abeta42-Exposed SH-SY5Y Neuroblastoma Cells by Regulating the Amyloid Precursor Protein- Binding Protein 1 Pathway. Curr. Alzheimer Res. 2020, 17, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Yun, J.H.; Jeong, M.S.; Kim, K.N.; Shin, T.; Kim, H.C.; Wie, M.B. Inhibitors of Lipoxygenase and Cyclooxygenase-2 Attenuate Trimethyltin-Induced Neurotoxicity through Regulating Oxidative Stress and Pro-Inflammatory Cytokines in Human Neuroblastoma SH-SY5Y Cells. Brain Sci. 2021, 11, 1116. [Google Scholar] [CrossRef]
- Nopparat, C.; Chaopae, W.; Boontem, P.; Sopha, P.; Wongchitrat, P.; Govitrapong, P. Melatonin Attenuates High Glucose-Induced Changes in Beta Amyloid Precursor Protein Processing in Human Neuroblastoma Cells. Neurochem. Res. 2021, 1–12. [Google Scholar] [CrossRef]
- Mishra, R.; Kaur, G. Tinospora cordifolia Induces Differentiation and Senescence Pathways in Neuroblastoma Cells. Mol. Neurobiol. 2015, 52, 719–733. [Google Scholar] [CrossRef]
- Sharma, A.; Saggu, S.K.; Mishra, R.; Kaur, G. Anti-brain cancer activity of chloroform and hexane extracts of Tinospora cordifolia Miers: An in vitro perspective. Ann. Neurosci. 2019, 26, 10–20. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.L.; Xue, N.N.; Li, C.; Guo, H.H.; Ren, T.K.; Zhan, Y.; Li, W.B.; Zhang, J.; Chen, X.G.; et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef]
- Hall, S.; Anoopkumar-Dukie, S.; Grant, G.D.; Desbrow, B.; Lai, R.; Arora, D.; Hong, Y. Modulation of chemotherapy-induced cytotoxicity in SH-SY5Y neuroblastoma cells by caffeine and chlorogenic acid. Toxicol. Mech. Methods 2017, 27, 363–369. [Google Scholar] [CrossRef]
- Prommaban, A.; Utama-Ang, N.; Chaikitwattana, A.; Uthaipibull, C.; Porter, J.B.; Srichairatanakool, S. Phytosterol, Lipid and Phenolic Composition, and Biological Activities of Guava Seed Oil. Molecules 2020, 25, 2474. [Google Scholar] [CrossRef]
- Zhao, Z.; Bo, Z.; Gong, W.; Guo, Y. Inhibitor of Differentiation 1 (Id1) in Cancer and Cancer Therapy. Int. J. Med. Sci. 2020, 17, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Okada, M.; Shibuya, K.; Watanabe, E.; Seino, S.; Suzuki, K.; Narita, Y.; Shibui, S.; Kayama, T.; Kitanaka, C. Resveratrol promotes proteasome-dependent degradation of Nanog via p53 activation and induces differentiation of glioma stem cells. Stem Cell Res. 2013, 11, 601–610. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Jiao, Y.; Guo, A.; Xu, X.; Qu, X.; Wang, S.; Zhao, J.; Li, Y.; Cao, Y. Resveratrol sensitizes glioblastoma-initiating cells to temozolomide by inducing cell apoptosis and promoting differentiation. Oncol. Rep. 2016, 35, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Castino, R.; Pucer, A.; Veneroni, R.; Morani, F.; Peracchio, C.; Lah, T.T.; Isidoro, C. Resveratrol reduces the invasive growth and promotes the acquisition of a long-lasting differentiated phenotype in human glioblastoma cells. J. Agric. Food Chem. 2011, 59, 4264–4272. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ochoa, C.O.; Lopez-Arellano, M.E.; Roblero-Bartolon, G.; Diaz-Chavez, J.; Moreno-Banda, G.L.; Reyna-Figueroa, J.; Munguia-Moreno, J.A.; Madrid-Marina, V.; Lagunas-Martinez, A. Molecular mechanisms of cell death induced in glioblastoma by experimental and antineoplastic drugs: New and old drugs induce apoptosis in glioblastoma. Hum. Exp. Toxicol. 2020, 39, 464–476. [Google Scholar] [CrossRef]
- Tovilovic-Kovacevic, G.; Krstic-Milosevic, D.; Vinterhalter, B.; Toljic, M.; Perovic, V.; Trajkovic, V.; Harhaji-Trajkovic, L.; Zogovic, N. Xanthone-rich extract from Gentiana dinarica transformed roots and its active component norswertianin induce autophagy and ROS-dependent differentiation of human glioblastoma cell line. Phytomed. Int. J. Phytother. Phytopharm. 2018, 47, 151–160. [Google Scholar] [CrossRef]
- Krstic-Milosevic, D.; Banjac, N.; Jankovic, T.; Eler, K.; Vinterhalter, B. Gentiana clusii Perr.&Song.: Enhanced production of secondary metabolites by in vitro propagation. Plant Physiol. Biochem. PPB 2020, 154, 735–744. [Google Scholar] [CrossRef]
- Benameur, T.; Giacomucci, G.; Panaro, M.A.; Ruggiero, M.; Trotta, T.; Monda, V.; Pizzolorusso, I.; Lofrumento, D.D.; Porro, C.; Messina, G. New Promising Therapeutic Avenues of Curcumin in Brain Diseases. Molecules 2021, 27, 236. [Google Scholar] [CrossRef]
- Ryskalin, L.; Biagioni, F.; Busceti, C.L.; Lazzeri, G.; Frati, A.; Fornai, F. The Multi-Faceted Effect of Curcumin in Glioblastoma from Rescuing Cell Clearance to Autophagy-Independent Effects. Molecules 2020, 25, 4839. [Google Scholar] [CrossRef]
- Zhuang, W.; Long, L.; Zheng, B.; Ji, W.; Yang, N.; Zhang, Q.; Liang, Z. Curcumin promotes differentiation of glioma-initiating cells by inducing autophagy. Cancer Sci. 2012, 103, 684–690. [Google Scholar] [CrossRef]
- Kim, T.J.; Kwon, H.S.; Kang, M.; Leem, H.H.; Lee, K.H.; Kim, D.Y. The Antitumor Natural Compound Falcarindiol Disrupts Neural Stem Cell Homeostasis by Suppressing Notch Pathway. Int. J. Mol. Sci. 2018, 19, 3432. [Google Scholar] [CrossRef] [PubMed]
- Reiss, M.; Gamba-Vitalo, C.; Sartorelli, A.C. Induction of tumor cell differentiation as a therapeutic approach: Preclinical models for hematopoietic and solid neoplasms. Cancer Treat. Rep. 1986, 70, 201–218. [Google Scholar] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Li, C.Q.; Lei, H.M.; Hu, Q.Y.; Li, G.H.; Zhao, P.J. Recent Advances in the Synthetic Biology of Natural Drugs. Front. Bioeng. Biotechnol. 2021, 9, 691152. [Google Scholar] [CrossRef] [PubMed]
- Robles-Fernandez, I.; Rodriguez-Serrano, F.; Alvarez, P.J.; Ortiz, R.; Rama, A.R.; Prados, J.; Melguizo, C.; Alvarez-Manzaneda, E.; Aranega, A. Antitumor properties of natural compounds and related molecules. Recent Pat. Anti-Cancer Drug Discov. 2013, 8, 203–215. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Liao, Y.; Liu, J.; Sun, S. Research Progress of Natural Small-Molecule Compounds Related to Tumor Differentiation. Molecules 2022, 27, 2128. https://doi.org/10.3390/molecules27072128
He X, Liao Y, Liu J, Sun S. Research Progress of Natural Small-Molecule Compounds Related to Tumor Differentiation. Molecules. 2022; 27(7):2128. https://doi.org/10.3390/molecules27072128
Chicago/Turabian StyleHe, Xiaoli, Yongkang Liao, Jing Liu, and Shuming Sun. 2022. "Research Progress of Natural Small-Molecule Compounds Related to Tumor Differentiation" Molecules 27, no. 7: 2128. https://doi.org/10.3390/molecules27072128
APA StyleHe, X., Liao, Y., Liu, J., & Sun, S. (2022). Research Progress of Natural Small-Molecule Compounds Related to Tumor Differentiation. Molecules, 27(7), 2128. https://doi.org/10.3390/molecules27072128






