Identification of Non-Volatile Compounds Generated during Storage That Impact Flavor Stability of Ready-to-Drink Coffee
Abstract
1. Introduction
2. Results and Discussion
2.1. Sensory Evaluation of RTD Coffee Samples by Degree of Difference (DOD)
2.2. Multivariate Statistical Modeling
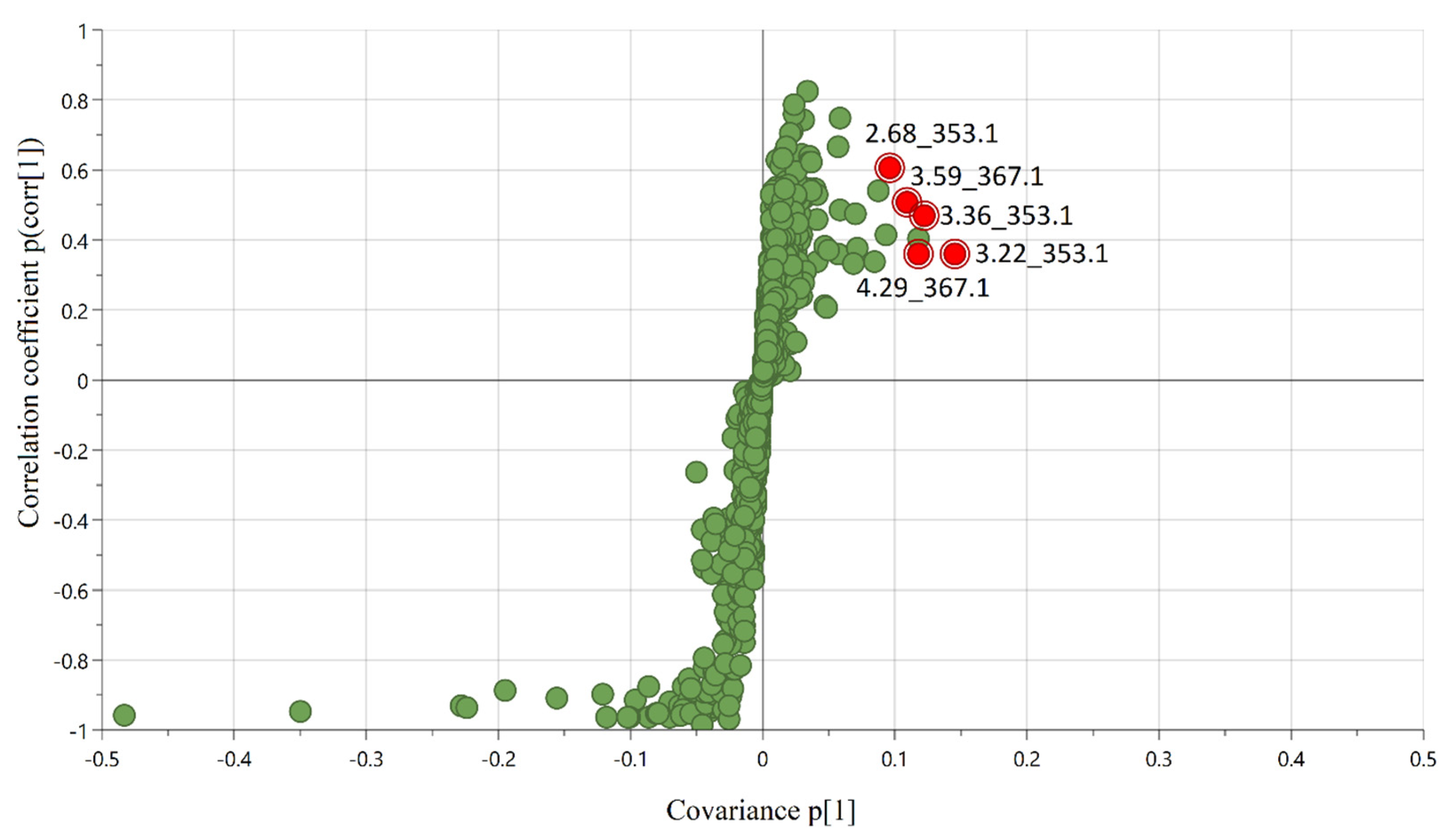
2.3. Identification of Positively Correlated Predictive Compounds
2.4. Targeted Analysis of Hydrophilic Coffee Acids
2.5. Quantification of Positive Correlated Predictive Compounds in RTD Coffee Samples
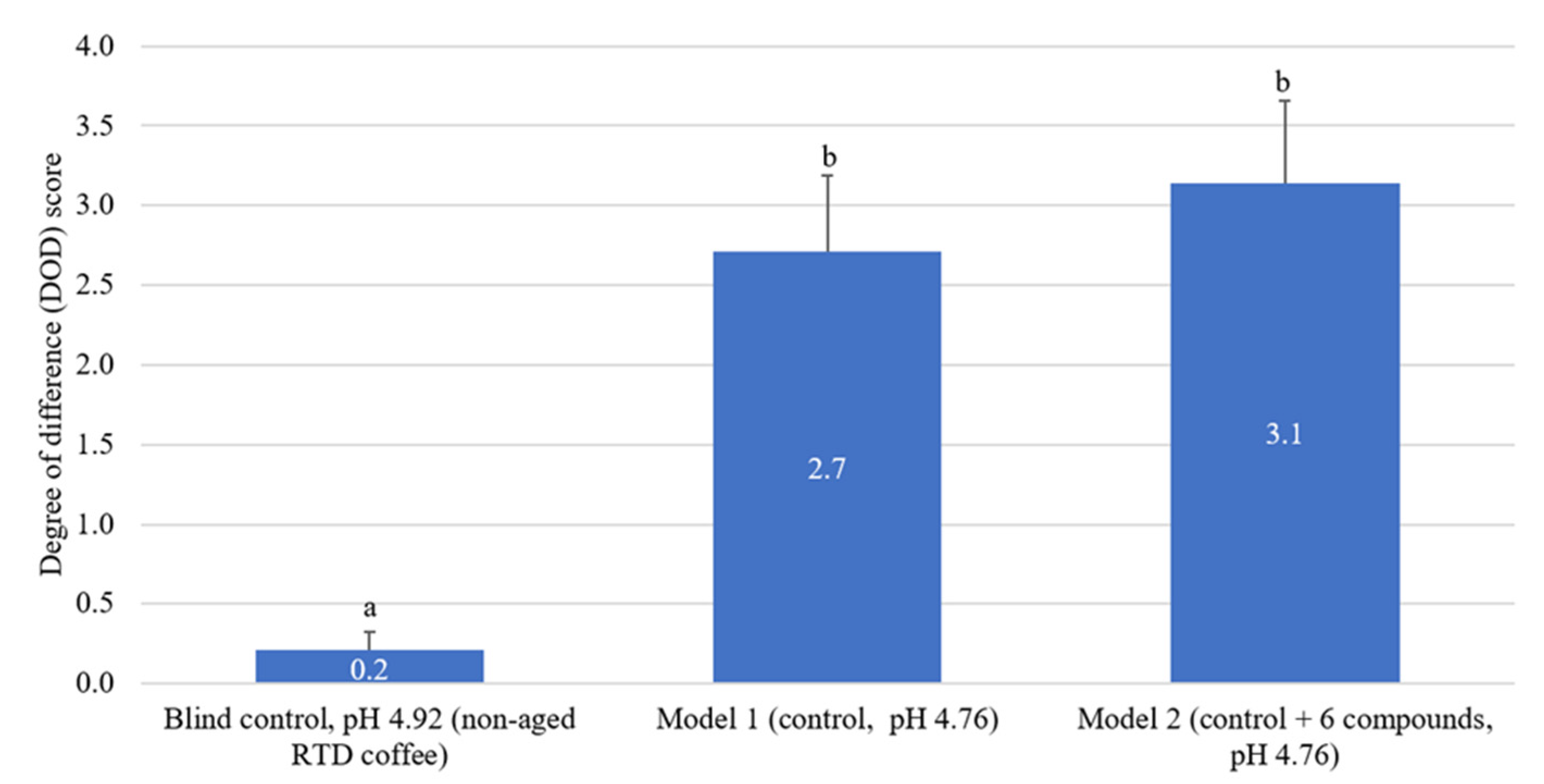
2.6. Sensory Recombination of Identified Acids in RTD Coffee Samples
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Ready-to-Drink (RTD) Coffee Samples
3.3. Sensory Evaluation by the Degree of Difference (DOD) Test
3.4. Ultra-Performance Liquid Chromatography-Mass Spectrometry (UPLC-MS) Chemical Profiling
3.5. Multivariate Statistical Analysis (MVA)
3.6. Off-Line Multidimensional Preparative-Liquid Chromatography/Mass Spectrometry (Prep-LC/MS) Fractionation
3.7. Quantification by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC–MS/MS)
3.8. Sensory Validation in RTD Coffee
3.9. Nuclear Magnetic Resonance (NMR)
3.10. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, Composition, and Application of Coffee and Its Industrial Residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- ICO. Coffee Development Report 2020; International Coffee Organization: London, UK, 2020. [Google Scholar]
- ICO. Coffee Development Report 2019; International Coffee Organization: London, UK, 2019. [Google Scholar]
- Failla, J. Coffee—US—July 2019; Mintel Group Ltd.: London, UK, 2019. [Google Scholar]
- Wang, E.S.-T.; Yu, J.-R. Effect of product attribute beliefs of ready-to-drink coffee beverages on consumer-perceived value and repurchase intention. Br. Food J. 2016, 118, 2963–2980. [Google Scholar] [CrossRef]
- Flament, I. Coffee Flavor Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Phan, U.T.X.; Chambers, E. Motivations for choosing various food groups based on individual foods. Appetite 2016, 105, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Samoggia, A.; Riedel, B. Coffee consumption and purchasing behavior review: Insights for further research. Appetite 2018, 129, 70–81. [Google Scholar] [CrossRef]
- Sunarharum, W.B.; Williams, D.J.; Smyth, H.E. Complexity of coffee flavor: A compositional and sensory perspective. Food Res. Int. 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Blank, I.; Sen, A.; Grosch, W. Potent odorants of the roasted powder and brew of Arabica coffee. Z. Lebensm.-Unters. Forsch. 1992, 195, 239–245. [Google Scholar] [CrossRef]
- Blank, I.; Sen, A.; Grosch, W. Aroma Impact Compounds of Arabica and Robusta Coffee. Qualitative and Quantitative Investigations. In 14th International Scientific Colloquium on Coffee; ASIC: San Francisco, CA, USA, 1991; pp. 117–129. [Google Scholar]
- Grosch, W.; Czerny, M.; Mayer, F.; Moors, A. Sensory Studies on the Key Odorants of Roasted Coffee. In Caffeinated Beverages; ACS Publications: Washington, DC, USA, 2000; Volume 754, pp. 202–209. [Google Scholar]
- Ginz, M.; Engelhardt, U.H. Identification of proline-based diketopiperazines in roasted coffee. J. Agric. Food Chem. 2000, 48, 3528–3532. [Google Scholar] [CrossRef]
- Frank, O.; Blumberg, S.; Kunert, C.; Zehentbauer, G.; Hofmann, T. Structure determination and sensory analysis of bitter-tasting 4-vinylcatechol oligomers and their identification in roasted coffee by means of LC-MS/MS. J. Agric. Food Chem. 2007, 55, 1945–1954. [Google Scholar] [CrossRef]
- Ikeda, M.; Akiyama, M.; Hirano, Y.; Miyaji, K.; Sugawara, Y.; Imayoshi, Y.; Iwabuchi, H.; Onodera, T.; Toko, K. Effects of Manufacturing Processing Conditions on Retronasal-Aroma Odorants from a Milk Coffee Drink. J. Food Sci. 2018, 83, 2733–2744. [Google Scholar] [CrossRef]
- Kumazawa, K. Flavor chemistry of tea and coffee drinks. Food Sci. Technol. Res. 2006, 12, 71–84. [Google Scholar] [CrossRef][Green Version]
- Kumazawa, K.; Masuda, H. Investigation of the change in the flavor of a coffee drink during heat processing. J. Agric. Food Chem. 2003, 51, 2674–2678. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Akiyama, M.; Sumi, M.; Ikeda, M.; Iwatsuki, K.; Nishimura, O.; Kumazawa, K. Differences in flavor characteristics of coffee drinks originating from thermal sterilization process. Food Sci. Technol. Res. 2010, 16, 99–110. [Google Scholar] [CrossRef][Green Version]
- Pérez-Martínez, M.; Sopelana, P.; De Peña, M.P.; Cid, C. Effects of refrigeration and oxygen on the coffee brew composition. Eur. Food Res. Technol. 2008, 227, 1633–1640. [Google Scholar] [CrossRef]
- Pérez-Martínez, M.; Sopelana, P.; De Peña, M.P.; Cid, C. Changes in volatile compounds and overall aroma profile during storage of coffee brews at 4 and 25 C. J. Agric. Food Chem. 2008, 56, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
- Frank, O.; Ottinger, H.; Hofmann, T. Characterization of an intense bitter-tasting 1 H, 4 H-quinolizinium-7-olate by application of the taste dilution analysis, a novel bioassay for the screening and identification of taste-active compounds in foods. J. Agric. Food Chem. 2001, 49, 231–238. [Google Scholar] [CrossRef]
- Grosch, W. Detection of potent odorants in foods by aroma extract dilution analysis. Trends Food Sci. Technol. 1993, 4, 68–73. [Google Scholar] [CrossRef]
- Ottinger, H.; Bareth, A.; Hofmann, T. Characterization of natural “cooling” compounds formed from glucose and L-proline in dark malt by application of taste dilution analysis. J. Agric. Food Chem. 2001, 49, 1336–1344. [Google Scholar] [CrossRef]
- Andujar-Ortiz, I.; Peppard, T.L.; Reineccius, G. Flavoromics for determining markers of cooked and fermented flavor in strawberry juices. In The Chemical Sensory Informatics of Food: Measurement, Analysis, Integration; ACS Publications: Washington, DC, USA, 2015; pp. 293–312. [Google Scholar]
- Charve, J.; Manganiello, S.; Glabasnia, A. Analysis of umami taste compounds in a fermented corn sauce by means of sensory-guided fractionation. J. Agric. Food Chem. 2018, 66, 1863–1871. [Google Scholar] [CrossRef]
- Cong, W.; Schwartz, E.; Peterson, D.G. Identification of inhibitors of pinellic acid generation in whole wheat bread. Food Chem. 2021, 351, 129291. [Google Scholar] [CrossRef]
- Reineccius, G. Flavoromics-the Next Frontier, Abstracts of Papers. In Proceedings of the 235th ACS National Meeting, New Orleans, LA, USA, 6–10 April 2008; ACS Publications: Washington, DC, USA, 2008; p. AGFD-061. [Google Scholar]
- Gao, C.; Tello, E.; Peterson, D.G. Identification of coffee compounds that suppress bitterness of brew. Food Chem. 2021, 350, 129225. [Google Scholar] [CrossRef]
- Sittipod, S.; Schwartz, E.; Paravisini, L.; Peterson, D.G. Identification of flavor modulating compounds that positively impact coffee quality. Food Chem. 2019, 301, 125250. [Google Scholar] [CrossRef] [PubMed]
- Ronningen, I.; Miller, M.; Xia, Y.; Peterson, D.G. Identification and validation of sensory-active compounds from data-driven research: A flavoromics approach. J. Agric. Food Chem. 2017, 66, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Borchgrevink, C.P.; Susskind, A.M.; Tarras, J.M. Consumer preferred hot beverage temperatures. Food Qual. Prefer. 1999, 10, 117–121. [Google Scholar] [CrossRef]
- Lee, H.-S.; O’Mahony, M. At what temperatures do consumers like to drink coffee?Mixing methods. J. Food Sci. 2002, 67, 2774–2777. [Google Scholar] [CrossRef]
- Yohannes, M.F.; Matsuda, T.; Sato, N. Substitution in Consumer Demand for Coffee Product Categories in Japan. J. Agric. Sci. 2016, 8, 50. [Google Scholar] [CrossRef][Green Version]
- Green, B.G.; Andrew, K. Stimulus-dependent effects of temperature on bitter taste in humans. Chem. Senses 2017, 42, 153–160. [Google Scholar] [CrossRef]
- Talavera, K.; Ninomiya, Y.; Winkel, C.; Voets, T.; Nilius, B. Influence of temperature on taste perception. Cell. Mol. Life Sci. 2007, 64, 377–381. [Google Scholar] [CrossRef]
- Clarke, R.J. Coffee: Volume 1: Chemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Rosa, M.D.; Barbanti, D.; Lerici, C.R. Changes in coffee brews in relation to storage temperature. J. Sci. Food Agric. 1990, 50, 227–235. [Google Scholar] [CrossRef]
- Anese, M.; Nicoli, M.C. Antioxidant properties of ready-to-drink coffee brews. J. Agric. Food Chem. 2003, 51, 942–946. [Google Scholar] [CrossRef]
- Galindo-Prieto, B.; Eriksson, L.; Trygg, J. Variable influence on projection (VIP) for orthogonal projections to latent structures (OPLS). J. Chemom. 2014, 28, 623–632. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MS n identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, N.; Jaiswal, R.; Matei, M.F.; Sovdat, T.; Deshpande, S. How to distinguish between feruloyl quinic acids and isoferuloyl quinic acids by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Dokli, I.; Navarini, L.; Hameršak, Z. Syntheses of 3-, 4-, and 5-O-feruloylquinic acids. Tetrahedron Asymmetry 2013, 24, 785–790. [Google Scholar] [CrossRef]
- Illy, A.; Viani, R. Espresso Coffee: The Science of Quality; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Clarke, R.; Vitzthum, O. Coffee: Recent Developments; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Clarke, R.J.; Macrae, R. Coffee: Physiolog; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1988; Volume 3. [Google Scholar]
- Schrader, K.; Kiehne, A.; Engelhardt, U.H.; Gerhard Maier, H. Determination of chlorogenic acids with lactones in roasted coffee. J. Sci. Food Agric. 1996, 71, 392–398. [Google Scholar] [CrossRef]
- Bennat, C.; Engelhardt, U.H.; Kiehne, A.; Wirries, F.-M.; Maier, H.G. HPLC analysis of chlorogenic acid lactones in roasted coffee. Z. Lebensm.-Unters. Forsch. 1994, 199, 17–21. [Google Scholar] [CrossRef]
- Kraehenbuehl, K.; Page-Zoerkler, N.; Mauroux, O.; Gartenmann, K.; Blank, I.; Bel-Rhlid, R. Selective enzymatic hydrolysis of chlorogenic acid lactones in a model system and in a coffee extract. Application to reduction of coffee bitterness. Food Chem. 2017, 218, 9–14. [Google Scholar] [CrossRef]
- Bekedam, E.K.; Schols, H.A.; Van Boekel, M.A.; Smit, G. Incorporation of chlorogenic acids in coffee brew melanoidins. J. Agric. Food Chem. 2008, 56, 2055–2063. [Google Scholar] [CrossRef]
- Bekedam, E.K.; Roos, E.; Schols, H.A.; Van Boekel, M.A.; Smit, G. Low molecular weight melanoidins in coffee brew. J. Agric. Food Chem. 2008, 56, 4060–4067. [Google Scholar] [CrossRef]
- Moreira, A.S.; Nunes, F.M.; Simoes, C.; Maciel, E.; Domingues, P.; Domingues, M.R.; Coimbra, M.A. Transglycosylation reactions, a main mechanism of phenolics incorporation in coffee melanoidins: Inhibition by Maillard reaction. Food Chem. 2017, 227, 422–431. [Google Scholar] [CrossRef]
- Moreira, A.S.; Coimbra, M.A.; Nunes, F.M.; Passos, C.P.; Santos, S.A.; Silvestre, A.J.; Silva, A.M.; Rangel, M.; Domingues, M.R. Chlorogenic acid-arabinose hybrid domains in coffee melanoidins: Evidences from a model system. Food Chem. 2015, 185, 135–144. [Google Scholar] [CrossRef]
- Leroy, T.; Ribeyre, F.; Bertrand, B.; Charmetant, P.; Dufour, M.; Montagnon, C.; Marraccini, P.; Pot, D. Genetics of coffee quality. Braz. J. Plant Physiol. 2006, 18, 229–242. [Google Scholar] [CrossRef]
- Rao, N.Z.; Fuller, M. Acidity and antioxidant activity of cold brew coffee. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Woodman, J. Carboxylic acids. In Coffee; Springer: Berlin/Heidelberg, Germany, 1985; pp. 266–289. [Google Scholar]
- Seninde, D.R.; Chambers, E. Coffee Flavor: A Review. Beverages 2020, 6, 44. [Google Scholar] [CrossRef]
- Buffo, R.A.; Cardelli-Freire, C. Coffeeflavour: An overview. Flavour Fragrance J. 2004, 19, 99–104. [Google Scholar] [CrossRef]
- Frank, O.; Zehentbauer, G.; Hofmann, T. Bioresponse-guided decomposition of roast coffee beverage and identification of key bitter taste compounds. Eur. Food Res. Technol. 2006, 222, 492–508. [Google Scholar] [CrossRef]
- Peleg, H.; Noble, A. Effect of viscosity, temperature and pH on astringency in cranberry juice. Food Qual. Prefer. 1999, 10, 343–347. [Google Scholar] [CrossRef]
- Marsh, K.B.; Friel, E.N.; Gunson, A.; Lund, C.; MacRae, E. Perception of flavour in standardised fruit pulps with additions of acids or sugars. Food Qual. Prefer. 2006, 17, 376–386. [Google Scholar] [CrossRef]
- Trugo, L.; Macrae, R. A study of the effect of roasting on the chlorogenic acid composition of coffee using HPLC. Food Chem. 1984, 15, 219–227. [Google Scholar] [CrossRef]
- Variyar, P.S.; Ahmad, R.; Bhat, R.; Niyas, Z.; Sharma, A. Flavoring components of raw monsooned arabica coffee and their changes during radiation processing. J. Agric. Food Chem. 2003, 51, 7945–7950. [Google Scholar] [CrossRef]
- Campa, C.; Doulbeau, S.; Dussert, S.; Hamon, S.; Noirot, M. Qualitative relationship between caffeine and chlorogenic acid contents among wild Coffea species. Food Chem. 2005, 93, 135–139. [Google Scholar] [CrossRef]
- Wei, F.; Furihata, K.; Miyakawa, T.; Tanokura, M. A pilot study of NMR-based sensory prediction of roasted coffee bean extracts. Food Chem. 2014, 152, 363–369. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Scholz, M.B.; Kitzberger, C.S.G.; Durand, N.; Rakocevic, M. From the field to coffee cup: Impact of planting design on chlorogenic acid isomers and other compounds in coffee beans and sensory attributes of coffee beverage. Eur. Food Res. Technol. 2018, 244, 1793–1802. [Google Scholar] [CrossRef]
- Borsato, D.; Pina, M.V.R.; Spacino, K.R.; dos Santos Scholz, M.B.; Androcioli Filho, A. Application of artificial neural networks in the geographical identification of coffee samples. Eur. Food Res. Technol. 2011, 233, 533–543. [Google Scholar] [CrossRef]
- Kumazawa, K.; Masuda, H. Effect of pH on the thermal stability of potent roasty odorants, 3-mercapto-3-methylbutyl esters, in coffee drink. J. Agric. Food Chem. 2003, 51, 8032–8035. [Google Scholar] [CrossRef] [PubMed]
- Aust, L.; Beard, S.; II, R.W. Degree of difference test method in sensory evaluation of heterogeneous product types. J. Food Sci. 1985, 50, 511–513. [Google Scholar] [CrossRef]
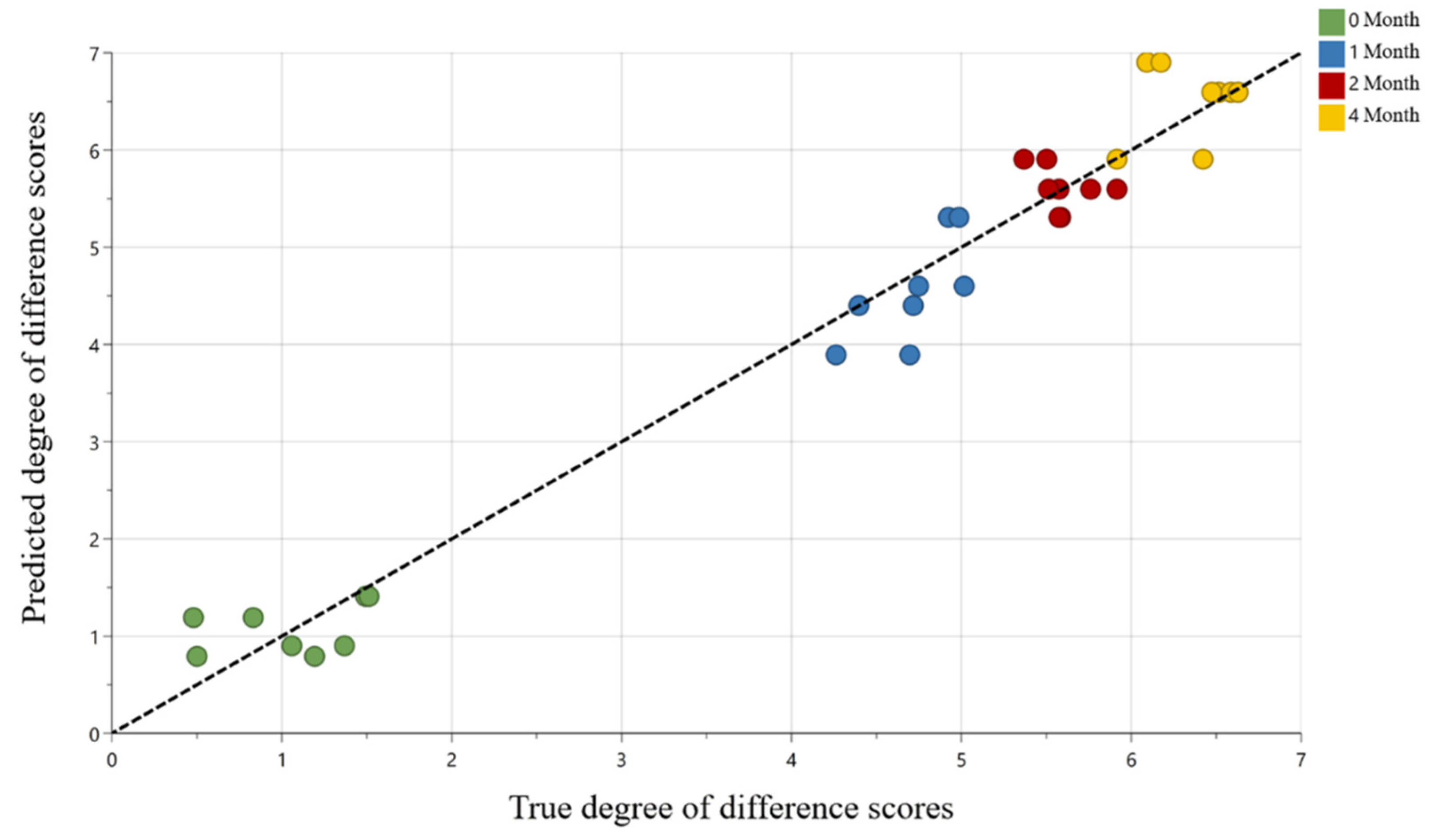
| Sample 2 | Blind Control, Non-Aged | 1 Month | 2 Month | 4 Month |
|---|---|---|---|---|
| (mean ± standard error) | ||||
| Arb AHS | 0.8 ± 0.3 a | 4.6 ± 0.6 b | 5.6 ± 0.3 bc | 6.6 ± 0.6 c |
| Arb N2F | 1.2 ± 0.3 d | 4.4 ± 0.7 e | 5.6 ± 0.5 ef | 6.6 ± 0.5 f |
| Rob AHS | 0.9 ± 0.2 g | 3.9 ± 0.5 h | 5.9 ± 0.6 i | 5.9 ± 0.5 i |
| Rob N2F | 1.4 ± 0.2 j | 5.3 ± 0.5 k | 5.3 ± 0.5 k | 6.9 ± 0.6 l |
| Compound No. | Chemical Feature (RT_m/z) | LC/MS Profiling Method | OPLS VIPpred Score | MRM Transition (Collision Energy) | Compound Identity | Compound Concentration (mg/L) i | Sample Concentration Difference (mg/L) | % Change (Concentration) | |
|---|---|---|---|---|---|---|---|---|---|
| Non-aged RTD coffee | 4-month aged RTD coffee | ||||||||
| 1 | 2.68_353.1 | Untargeted | 3.7 | 353.1 → 191.1 (20) | 3-caffeoylquinic acid | 172.9 a | 254.4 b | 81.5 | 47.1 |
| 2 | 3.22_353.1 | Untargeted | 5.6 | 353.1 → 179.0 (18) | 5-caffeoylquinic acid | 207.8 a | 266.6 b | 58.8 | 28.3 |
| 3 | 3.36_353.1 | Untargeted | 4.7 | 353.1 → 191.1 (18) | 4-caffeoylquinic acid | 165.2 a | 209.5 b | 44.3 | 26.8 |
| 4 | 3.59_367.1 | Untargeted | 4.1 | 367.1 → 134.0 (34) | 3-O-feruloylquinic acid | 87.8 a | 106.3 b | 18.5 | 21.1 |
| 5 | 4.29_367.1 | Untargeted | 4.5 | 367.1 → 191.0 (16) | 5-O-feruloylquinic acid | 56.9 a | 66.2 b | 9.3 | 16.3 |
| 6 | n/a ii | Targeted | n/a ii | 191.1 → 84.9 (22) | Quinic acid | 1258.9 a | 1427.8 b | 168.9 | 13.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Tello, E.; Simons, C.T.; Peterson, D.G. Identification of Non-Volatile Compounds Generated during Storage That Impact Flavor Stability of Ready-to-Drink Coffee. Molecules 2022, 27, 2120. https://doi.org/10.3390/molecules27072120
Lin H, Tello E, Simons CT, Peterson DG. Identification of Non-Volatile Compounds Generated during Storage That Impact Flavor Stability of Ready-to-Drink Coffee. Molecules. 2022; 27(7):2120. https://doi.org/10.3390/molecules27072120
Chicago/Turabian StyleLin, Hao, Edisson Tello, Christopher T. Simons, and Devin G. Peterson. 2022. "Identification of Non-Volatile Compounds Generated during Storage That Impact Flavor Stability of Ready-to-Drink Coffee" Molecules 27, no. 7: 2120. https://doi.org/10.3390/molecules27072120
APA StyleLin, H., Tello, E., Simons, C. T., & Peterson, D. G. (2022). Identification of Non-Volatile Compounds Generated during Storage That Impact Flavor Stability of Ready-to-Drink Coffee. Molecules, 27(7), 2120. https://doi.org/10.3390/molecules27072120