Fruit Peels as a Sustainable Waste for the Biosorption of Heavy Metals in Wastewater: A Review
Abstract
:1. Introduction
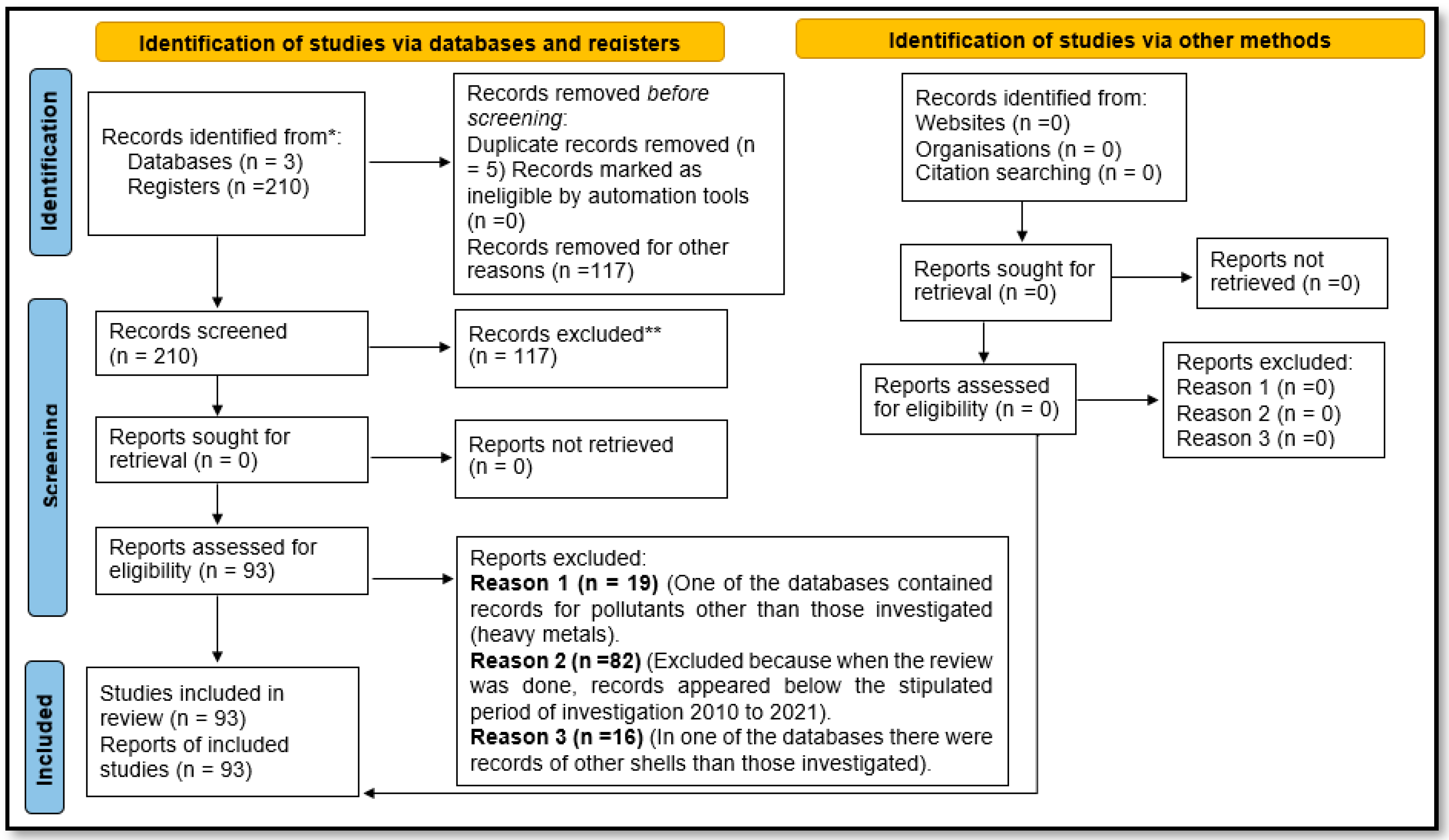
2. Materials and Methods
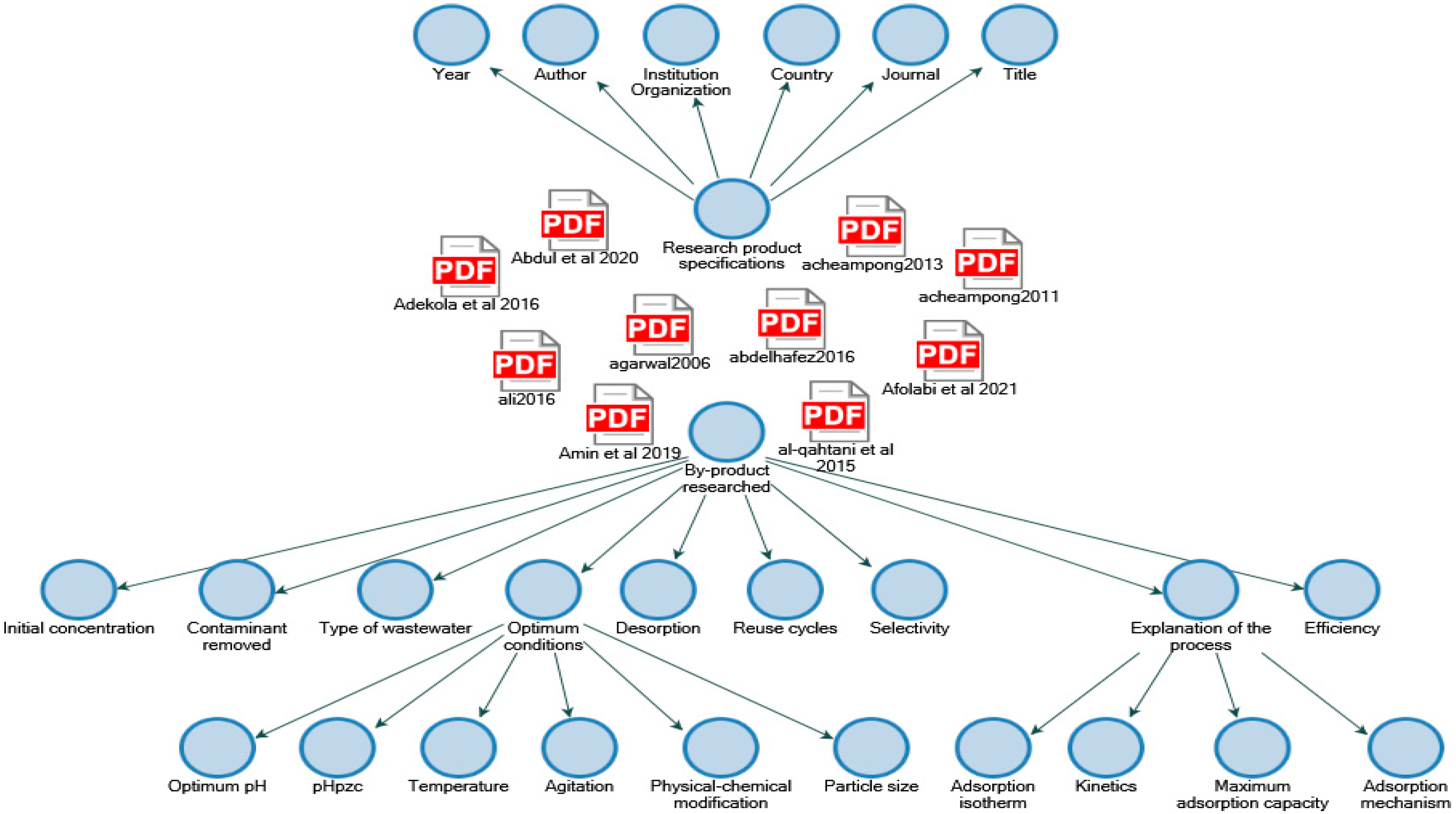
2.1. Documentary Analysis
2.2. Content Analysis
3. Results and Discussion
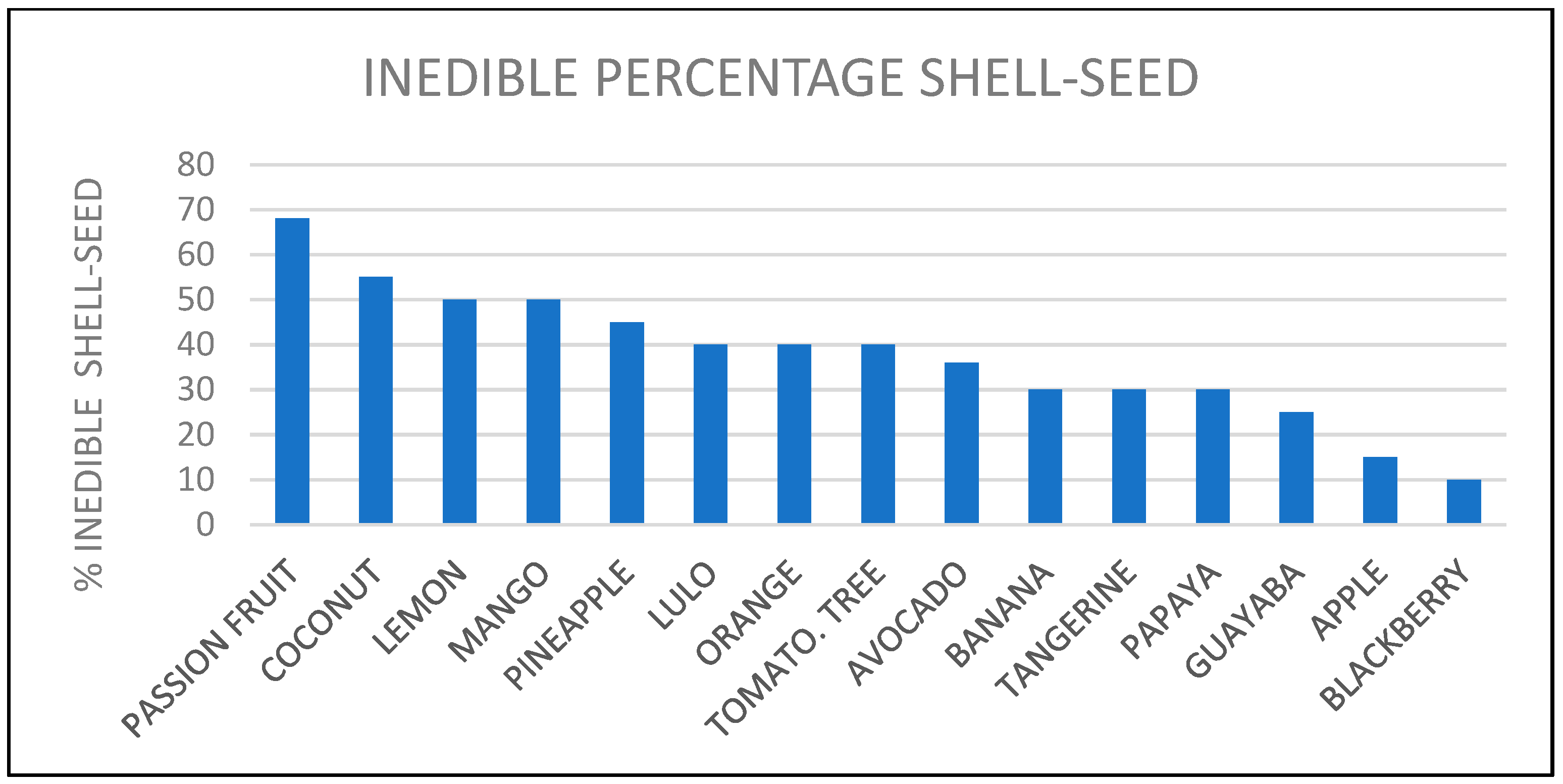
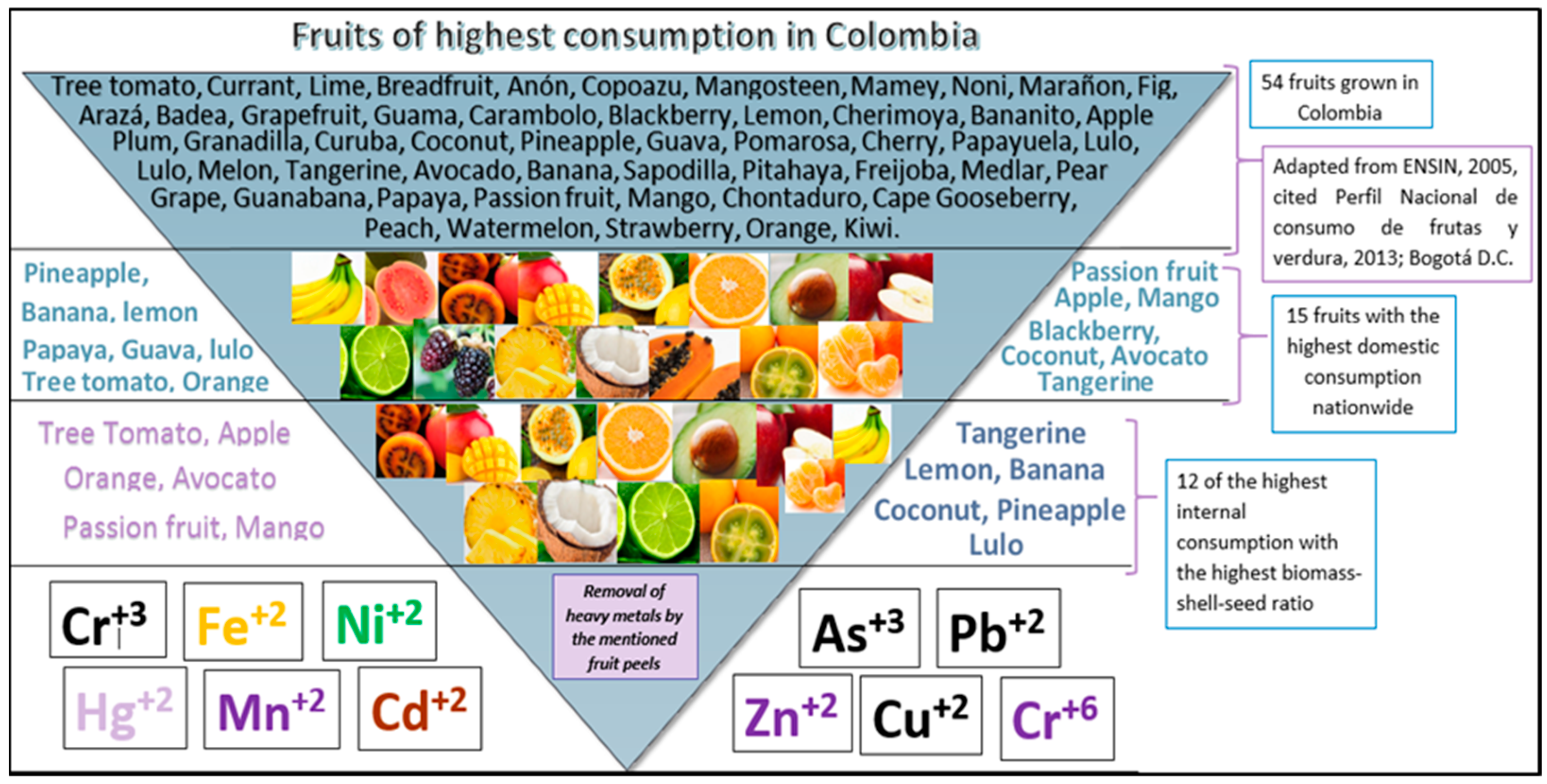
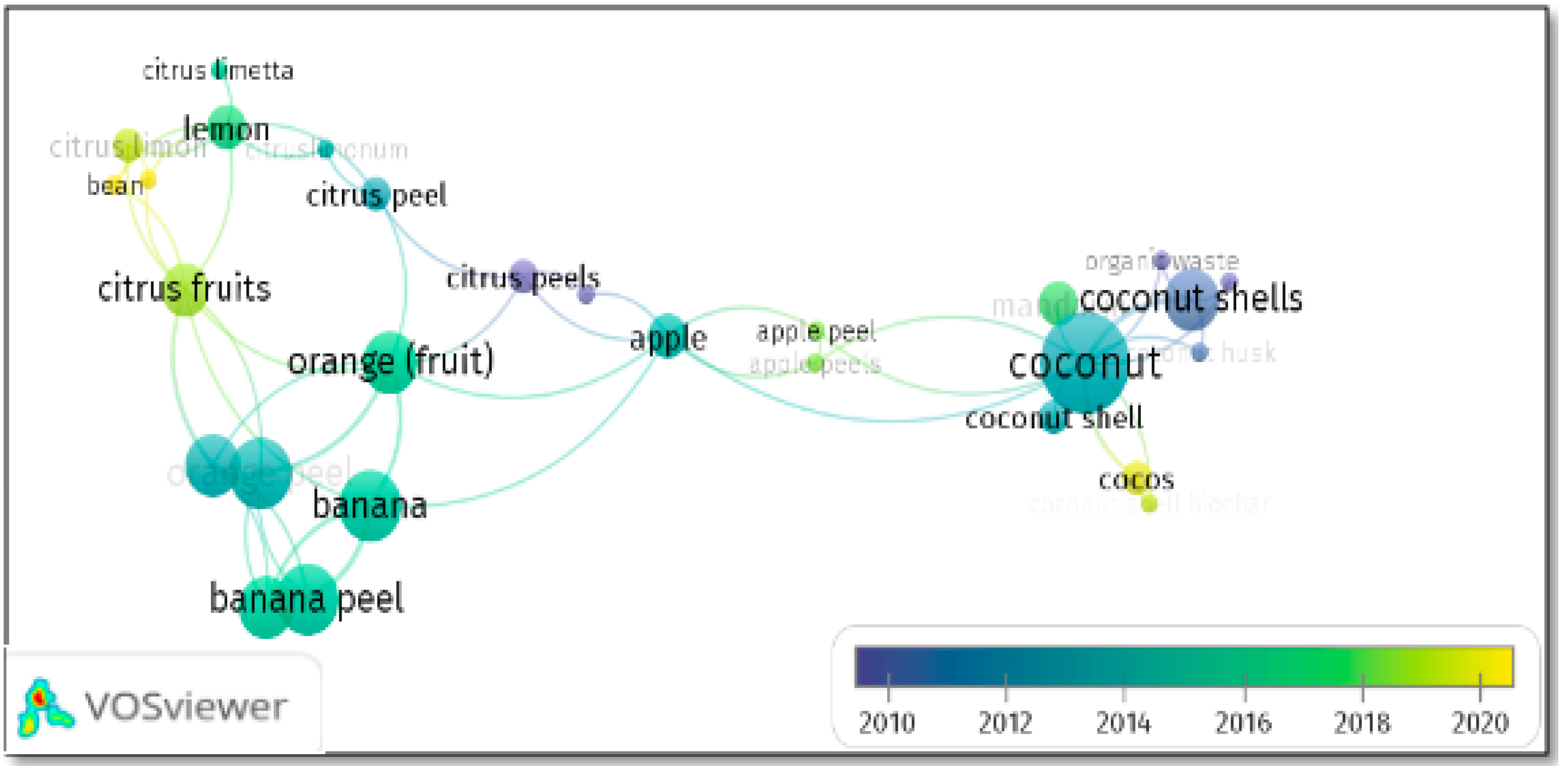
3.1. Selected Fruit Peels
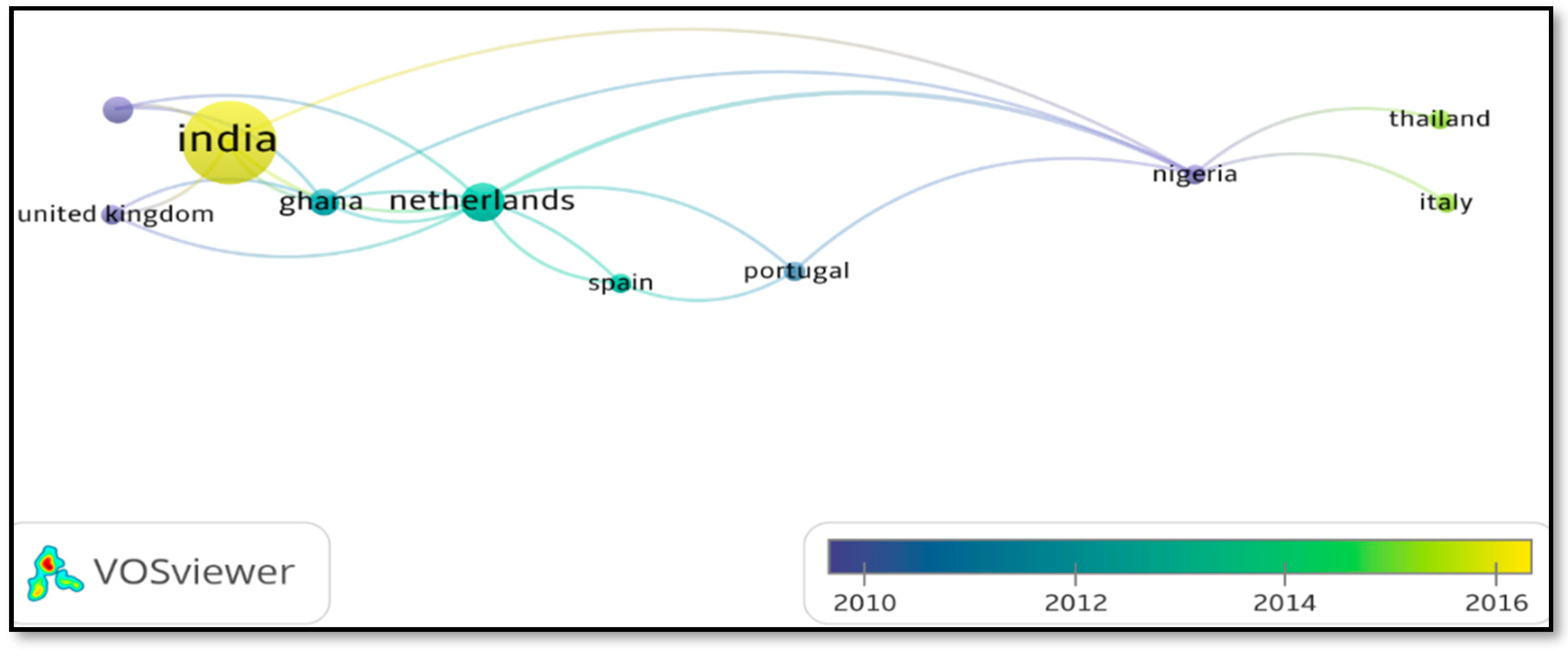
3.2. Generalities of the Articles Found and Selected
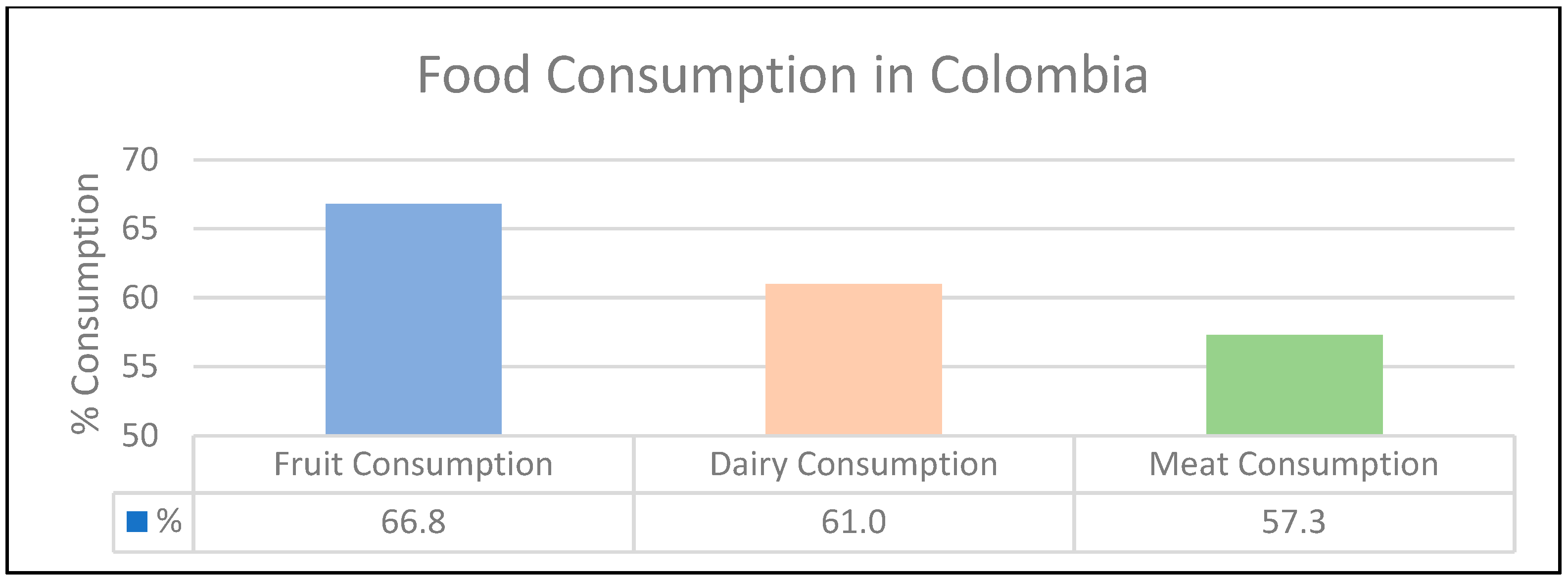
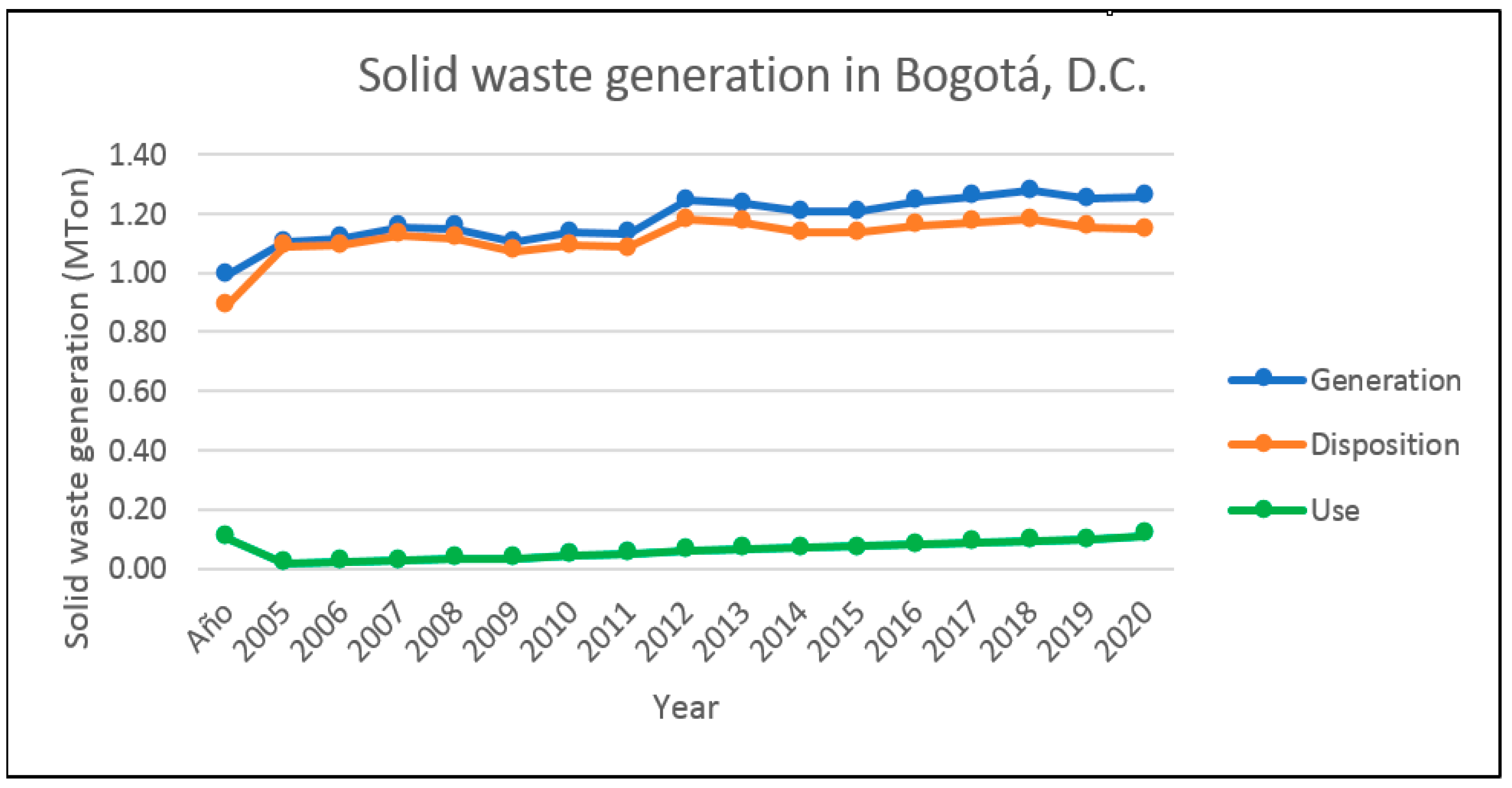
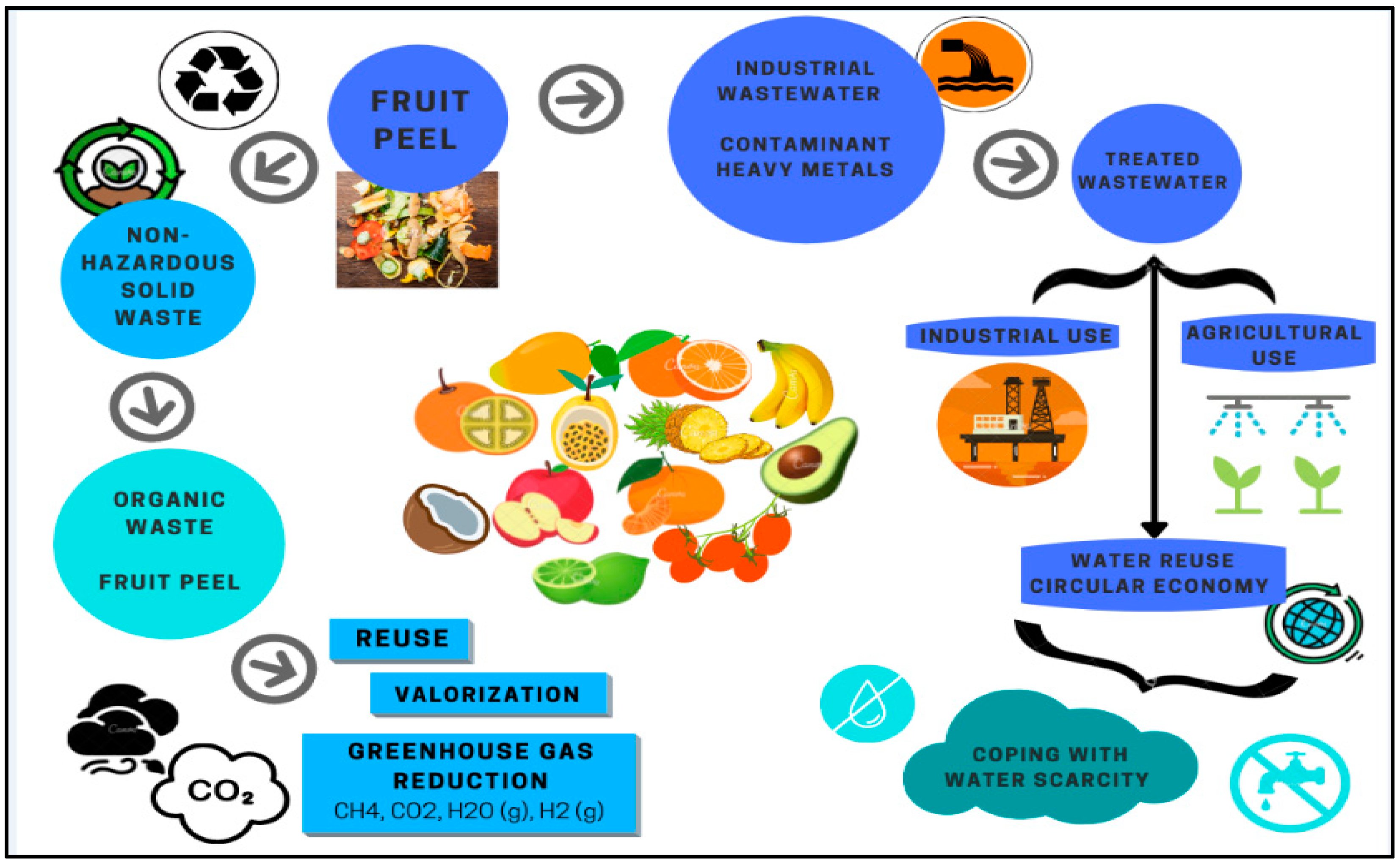
3.3. Colombian Solid Waste
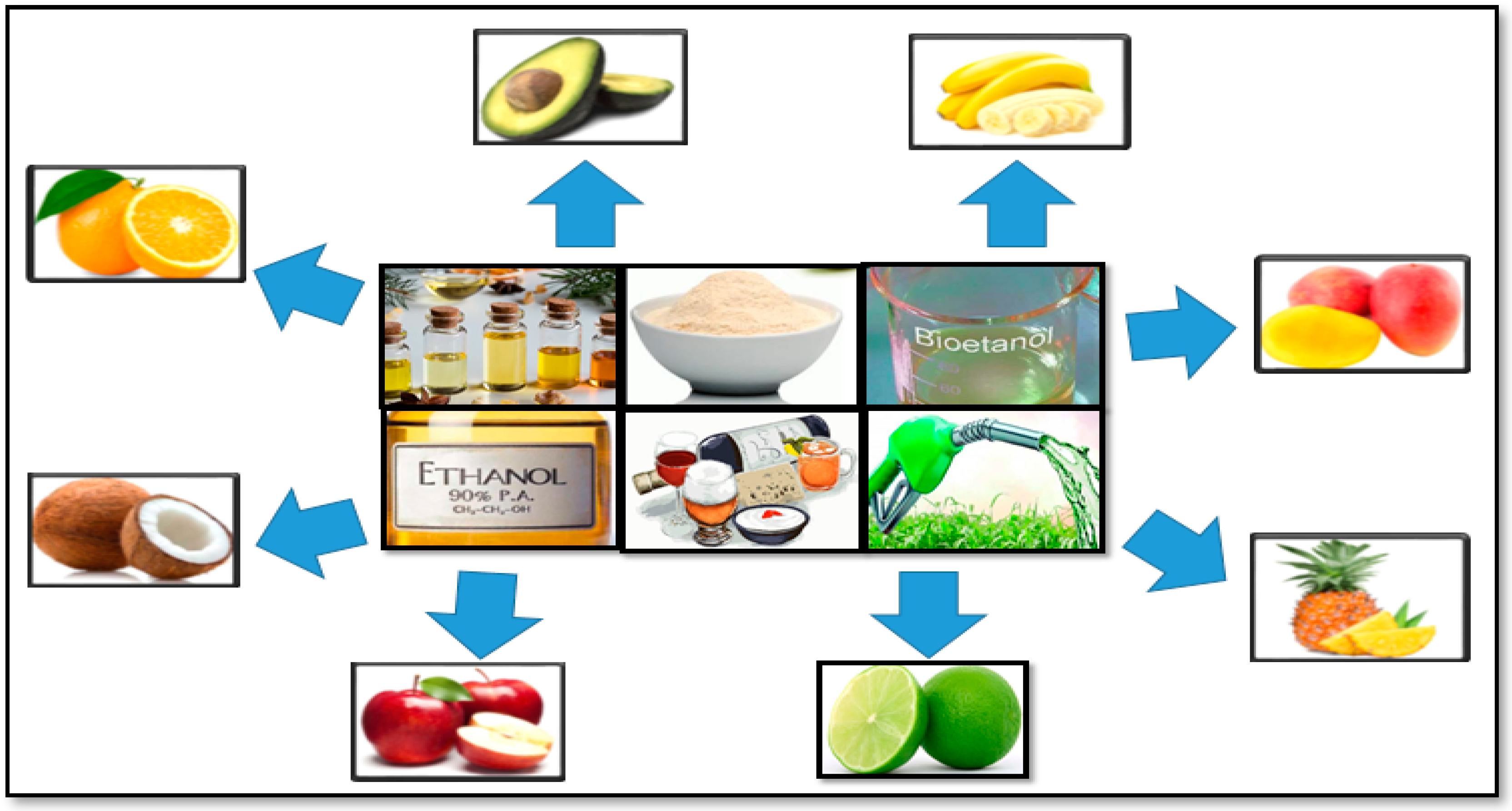
3.4. Biorefinery of Fruit Peels
4. Technoeconomic Aspects and Future Research
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barrantes, E.A.; Núñez, M.C. Eficacia del tratamiento de aguas residuales de la Universidad de Costa Rica en la Sede de Occidente, San Ramón, Costa Rica. Cuadernos de Investigación UNED 2017, 9, 193–197. [Google Scholar]
- WWAP. Informe Mundial de las Naciones Unidas sobre el Desarrollo de los Recursos Hídricos 2017; Aguas residuales: El Recurso Desaprovechado; UNESCO: Paris, France, 2017. [Google Scholar]
- UNESCO; ONU-Agua. Informe Mundial de las Naciones Unidas sobre el Desarrollo de los Recursos Hídricos 2020; Agua y Cambio Climático; UNESCO: Paris, France, 2020. [Google Scholar]
- Banco-Mundial. Un 70% de las Aguas Residuales de Latinoamérica Vuelven a los ríos sin ser Tratadas. 2013. Available online: https://www.bancomundial.org/es/news/feature/2014/01/02/rios-de-latinoamerica-contaminados.print (accessed on 16 June 2021).
- Barleta, M.; Lima, R.A.; Costa, M.F. Distribution, sources and consequences of nutrients, persistent organic pollutants, metals and microplastics in South American estuaries. Sci. Total Environ. 2019, 651, 1199–1218. [Google Scholar] [CrossRef]
- Sánchez, J.M.; González, R.R.; Blancas, F.J.; Fonseca, A. Utilización de subproductos agroindustriales para la bioadsorción de metales pesados. Revista Especializada en Ciencias Químico-Biológicas 2020, 23, 1–6. [Google Scholar] [CrossRef]
- Marín, M.; González, V.H.; Lapo, B.; Molina, M.; Lemus, M. Niveles de mercurio en sedimentos de la zona costera de El Oro, Ecuador. Gayana 2016, 80, 147–153. [Google Scholar] [CrossRef]
- Fog, L. Es un hecho: La Bahía de Cartagena es un Paciente Terminal. 2021. Available online: https://www.elespectador.com/ambiente/es-un-hecho-la-bahia-de-cartagena-es-un-paciente-terminal/ (accessed on 17 November 2021).
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Comparative Sorption of Nickel from an Aqueous Solution Using Biochar Derived from Banana and Orange Peel Using a Batch System: Kinetic and Isotherm Models. Arab. J. Sci. Eng. 2019, 44, 10105–10116. [Google Scholar] [CrossRef]
- Caviedes, D.I.; Muñoz, R.A.; Perdomo, A.; Rodriguez, D.; Sandoval, I.J. Tratamientos para la Remoción de Metales Pesados Comúnmente Presentes en Aguas Residuales Industriales. Una Revisión Revista Ingeniería y Región 2015, 13, 73–90. [Google Scholar] [CrossRef]
- Concejo Nacional de Política Económica y Social República de Colombia Departamento Nacional de Planeacios (COMPES-4004). Economía Circular en la Gestión de Los Servicios de Agua Potable y Manejo de Aguas Residuals. 2020. Available online: https://santandercompetitivo.org/biblioteca-de-documentos/competitividad-en-colombia/conpes-4004-economa-circular-en-la-gestin-de-los-servicios-de-agua-potable-y-manejo-de-aguas-residua/ (accessed on 18 January 2022).
- Acuatecnica, S.A.S. ¿Cuántas Plantas De Tratamiento De Aguas Residuales Hay En Colombia? 2017. Available online: https://acuatecnica.com/cuantas-plantas-tratamiento-aguas-residuales-colombia/#comments (accessed on 20 November 2021).
- Mehdi, L.; Abdoul, N.R.; Abdelaziz, B.; Abdelrani, Y. Removal of Chromium Cr(VI) of Tanning Effluent with Activated Carbon from Tannery Solid Wastes. Am. J. Phys. Chem. 2017, 6, 103–109. [Google Scholar] [CrossRef]
- Ministerio de Ambiente y Desarrollo Sostenible. Resolución 1207 de 2014. Por la Cual se Adoptan Disposiciones Relacionadas con el uso de Aguas Residuales Tratadas. 25 de Septiembre de 2014, Bogotá, D.C., Colombia. Available online: http://www.leyex.info/leyes/Resolucionmads1207de2014.pdf (accessed on 18 January 2022).
- Consorcio; Caltiz-Aguas Residuales. Elaborar un Estudio que Contenga el Análisis y Documente las Técnicas de Reúso de Aguas Residuales Domésticas, Industriales, Agrícola y Aguas Lluvias, y Realice Recomendaciones para la Implementación y Reglamentación del Reúso de agua Residual en Colombia; Conosorcio N&V-Caltiz-Aguas Residuales: Bogotá, Colombia, 2019. [Google Scholar]
- Hernández, R.; Fernández, C.; Baptista, M.P. Metodología de la Investigación, 6a Edición Edificio Punta Santa Fe, Prolongación Paseo de la Reforma 1015, Torre A, Piso 17, Colonia Desarrollo Santa Fe, Delegación Álvaro Obregón, México D.F. 2014. Available online: https://www.uca.ac.cr/wp-content/uploads/2017/10/Investigacion.pdf (accessed on 18 January 2022).
- Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 327, n71. [Google Scholar] [CrossRef]
- ICBF, Instituto Colombiano de Bienestar Familiar. Encuesta Nacional de la Situación Nutricional en Colombia 2010 (ENSIN); ICBF: Bogotá, Colombia, 2010. [Google Scholar]
- Tafur, R.; Toro, J.C. Presente y Futuro de la fruticultura Colombiana. AGROSAV/A Corp. Colomb. Investig. Agropecu. 2007, 1, 9–22. [Google Scholar]
- Combariza, J.A. Perfil Nacional de Consumo de Frutas y Verduras Prosperidad Para Todos; Ministerio de Salud y Protección Social: Bogotá, Colombia, 2013. [Google Scholar]
- ICBF, Instituto Colombiano de Bienestar Familiar. Tabla de Composición de Alimentos Colombianos (TCAC); ICFB: Bogotá, Colombia, 2018. [Google Scholar]
- Tadepalli, S.; Murthy, K.S.; Rakesh, N.N. Modelado de regresión isotérmica y lineal de Cu (II) y Fe (II) utilizando piel de naranja como adsorbente en estudios por lotes. Revista Internacional de Investigación PharmTech 2016, 9, 197–210. [Google Scholar]
- Hussein, R.A. Evaluación de la eficacia de la piel de naranja (Citrus reticulata) en la recuperación de níquel de aguas residuales de galvanoplastia. Revista de la Asociación de Salud Pública de Egipto 2014, 89, 154–158. [Google Scholar]
- Izquierdo, M.; Marzal, P.; Lens, P.N. Efecto de los ligandos orgánicos sobre la eliminación de cobre (II) de las aguas residuales del revestimiento metálico mediante biosorbentes a base de piel de naranja. Contaminación del Agua, el Aire y el Suelo 2013, 224, 1–5. [Google Scholar]
- Liu, M.; Yuan, Q.; Jia, H.; Li, S.; Wang, X.; Wang, C. Cáscara de naranja modificada con cisteína para la eliminación de Cu (II) de soluciones acuosas. Ciencia y Tecnología del Agua 2013, 67, 2444–2450. [Google Scholar]
- Liang, S.; Guo, X.; Feng, N.; Tian, Q. Aplicación de xantato de piel de naranja para la adsorción de Pb2+ de soluciones acuosas. Revista de Materiales Peligrosos 2013, 170, 425–429. [Google Scholar]
- Pavithra, S.; Thandapani, G.; S, S.; PN, S.; Alkhamis, H.H.; Alrefaei, A.F.; Almutairi, M.H. Estudios de adsorción por lotes en un compuesto de hidrogel de quitosano/piel de naranja adaptado a la superficie para la eliminación de iones Cr (VI) y Cu (II) de aguas residuales sintéticas. Chemosphere 2021, 271, 1–5. [Google Scholar]
- Njikam, E.; Schiewer, S. Optimización y modelado cinético de la desorción de cadmio a partir de cáscaras de cítricos: Un proceso para la regeneración biosorbente. Revista de Materiales Peligrosos 2012, 213, 242–248. [Google Scholar]
- Schiewer, S.; Patil, S.B. Residuos de frutas ricos en pectina como biosorbentes para la eliminación de metales pesados: Equilibrio y cinética. Tecnología de Fuentes Biológicas 2008, 99, 1896–1903. [Google Scholar]
- Naji, M.; Kareem, S.; Nief, O.A. Corteza residual de frutas y agrícolas como adsorbentes de resinas naturales para la eliminación de iones metálicos pesados de las aguas residuales. Archivos de Plantas 2019, 19, 966–971. [Google Scholar]
- Afolabi, F.O.; Musonge, P.; Bakare, B.F. Aplicación de la metodología de superficie de respuesta en la eliminación de Cu2+ y Pb2+ de soluciones acuosas utilizando cáscaras de naranja. Sci. Afr. 2021, 13, 1–5. [Google Scholar]
- Romero, L.A.; García, H.; Gonzalez, L.V.; Baldenegro, L.A.; Carrasco, F. Adsorbentes funcionalizados preparados a partir de cáscaras de frutas: Estudios de equilibrio, cinéticos y termodinámicos para la adsorción de cobre en solución acuosa. J. Clean. Prod. 2017, 162, 195–204. [Google Scholar]
- Lasheen, M.R.; Ammar, N.S.; Ibrahim, H.S. Adsorción/desorción de Cd (II), Cu (II) y Pb (II) mediante cáscara de naranja modificada químicamente: Estudios cinéticos y de equilibrio. Solid State Sci. 2012, 14, 202–210. [Google Scholar]
- Feng, N.; Guo, X. Caracterización de la capacidad de adsorción y mecanismos de adsorción de cobre, plomo y zinc por piel de naranja modificada. Trans. Nonferrous Met. Soc. China 2012, 22, 1224–1231. [Google Scholar]
- Shah, J.; Jan, M.R.; Ul-Haq, A. Eliminación de plomo de medios acuosos mediante cáscara de naranja carbonizada y tratada con ácido. Tenside Surfactants Deterg. 2014, 51, 240–246. [Google Scholar]
- Khan, M.; Shah, J.; Jan, M.R. Eliminación de Ni (II) de muestras de agua utilizando un compuesto de nanopartículas magnéticas de piel de naranja (MNP-OP). Desalin. Water Treat. 2020, 1, 274–282. [Google Scholar]
- Muhammad, T.; Abdulrahman, A.; Muhammad, N. Absorción Comportamientos de cobre, plomo y arsénico en solución acuosa utilizando fibras de palmera datilera y piel de naranja: Cinética y termodinámica. Pol. J. Environ. Stud. 2017, 26, 543–557. [Google Scholar]
- Patiño, L.; Hernández, J.A.; Ardila, A.; Salazar, M.; Talavera, A.; Hernández, R. Capacidad de eliminación de Cr (III) en solución acuosa en relación con los grupos funcionales presentes en la piel de naranja (Citrus sinensis). Appl. Sci. 2021, 11, 6346. [Google Scholar]
- López, G.; Barrera, C.E.; Balderas, P.; Roa, G.; Bilyeu, B. Eliminación de cromo hexavalente en soluciones acuáticas mediante nanopartículas de hierro incrustadas en la médula de la piel de naranja. Chem. Eng. J. 2011, 173, 480–485. [Google Scholar]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Aplicación del biocarbón derivado de la piel de naranja para la biosorción efectiva de cobre y cadmio en estudios por lotes: Modelos de isotermas y estudios cinéticos. Arab. J. Geosci. 2019, 12, 1–6. [Google Scholar]
- Gupta, V.K.; Nayak, A. Eliminación y recuperación de cadmio de soluciones acuosas mediante nuevos adsorbentes preparados a partir de cáscara de naranja y nanopartículas de Fe2O3. Chem. Eng. J. 2012, 180, 81–90. [Google Scholar]
- El Nemr, A.; Aboughaly, R.M.; El Sikaily, A.; Ragab, S.; Masoud, M.S.; Ramadan, M.S. Carbón microporoso nanoactivado tipo I derivado de la piel de naranja y su aplicación para la eliminación de Cr (VI) del medio acuático. Biomass Convers. Biorefinery 2020, 122, 184–189. [Google Scholar]
- Saini, J.; Garg, V.K.; Gupta, R.K. Nanocompuestos de SiO2 @ OPW sintetizados en verde para una mejor eliminación del plomo (II) del agua. Arab. J. Chem. 2020, 13, 2496–2499. [Google Scholar]
- Abdelhafez, A.A.; Li, J. Eliminación de Pb (II) de una solución acuosa mediante el uso de biocarros derivados del bagazo de caña de azúcar y la piel de naranja. J. Taiwan Inst. Chem. Eng. 2016, 61, 367–375. [Google Scholar]
- Pakshirajan, K.; Worku, A.N.; Acheampong, M.A.; Lubberding, H.J.; Lens, P.N. Eliminación de Cr (III) y Cr (VI) de soluciones acuosas mediante residuos de frutas asequibles y biomasa de algas. Bioquímica y Biotecnología Aplicadas 2013, 170, 498–513. [Google Scholar]
- Memon, J.R.; Memon, S.Q.; Bhanger, M.I.; El-Turki, A.; Hallam, K.R.; Allen, G.C. Cáscara de plátano: Absorbente verde y económico para la eliminación selectiva de Cr (VI) de las aguas residuales industriales. Coloides Superf. B Biointerfaces 2009, 70, 232–237. [Google Scholar]
- Kumar, M.; Majumder, C.B. Eliminación biológica de cromo (VI) de aguas residuales sintéticas mediante el uso de “piel de plátano tratada con ácido”. Revista de Investigación de Ciencias Farmacéuticas, Biológicas y Químicas 2014, 5, 662–679. [Google Scholar]
- Michael, N.X.; Ragupathi, T. Estudio experimental sobre reducción del contenido de cromo de efluentes industriales mediante cáscara de plátano. Revista Internacional de Investigación ChemTech 2015, 8, 725–731. [Google Scholar]
- Cheah, C.; Yue, C.S.; Ting, A.S. Efectos de los pretratamientos térmicos y químicos de las cáscaras de banano para la eliminación de metales en sistemas de un solo metal y multimetal. Contaminación del Agua, el Aire y el Suelo 2020, 232, 1–5. [Google Scholar]
- Zhou, N.; Chen, H.; Xi, J.; Yao, D.; Zhou, Z.; Tian, Y.; Lu, X. Biocarros con excelente propiedad de adsorción de Pb (II) producidos a partir de cáscaras de plátano frescas y deshidratadas mediante carbonización hidrotermal. Tecnología de Fuentes Biológicas 2017, 232, 204–210. [Google Scholar]
- Ajmi, R.N.; Sultan, M.; Hanno, S.H. Bioabsorbente de cromo, cadmio y plomo de aguas residuales industriales por planta de residuos. Revista de Investigación y Ciencias Farmacéuticas 2018, 10, 672–674. [Google Scholar]
- Ping, W.; Wu, Y.; Yang, C.; Jiang, X.; Liu, L. Propiedades y mecanismo de adsorción de Cd (II) de las aguas residuales mediante cáscara de plátano modificada. Trans. Soc. China Ing. Agríc. 2019, 35, 269–279. [Google Scholar]
- Lavanya, K.M.; Florence, J.A.; Vivekanandan, B.; Lakshmipathy, R. Investigaciones comparativas de la cáscara de plátano en bruto y libre de metales alcalinos como adsorbente para la eliminación de iones Hg2+. Mater. Today Proc. 2021, 1, 1–9. [Google Scholar]
- Liu, C.; Ngo, H.H.; Guo, W.; Tung, K.L. Condiciones óptimas para la preparación de cáscaras de plátano, bagazo de caña de azúcar y cáscara de sandía para eliminar el cobre del agua. Bioresour. Technol. 2012, 119, 349–354. [Google Scholar] [PubMed]
- Vilardi, G.; Di Palma, L.; Verdone, N. Adsorción de metales pesados por micropolvo de cáscara de plátano: Modelado de equilibrio por modelos no lineales. Chin. J. Chem. Eng. 2018, 26, 455–464. [Google Scholar]
- Hu, Z.T.; Ding, Y.; Shao, Y.; Cai, L.; Jin, Z.Y.; Liu, Z.; Zhao, J.; Li, F.; Pan, Z.; Li, X.; et al. Biocarbón de cáscara de plátano con estructura ensamblada en nanoflake para el tratamiento de la contaminación cruzada en el agua: Comportamientos de interacción entre el plomo y la tetraciclina. Chem. Eng. J. 2021, 420, 1–10. [Google Scholar]
- Al-Qahtani, K.M. Purificación de agua utilizando diferentes cortezas de frutos de desecho para la eliminación de metales pesados. J. Taibah Univ. Sci. 2015, 10, 700–708. [Google Scholar]
- Anwar, J.; Shafique, U.; Waheed-uz-Zaman, S.M.; Dar, A.; Anwar, S. Eliminación de Pb (II) y Cd (II) del agua por adsorción en cáscaras de banano. Bioresour. Technol. 2010, 101, 1752–1755. [Google Scholar]
- Ali, A.; Saeed, K.; Mabood, F. Eliminación de cromo (VI) del medio acuoso utilizando cáscaras de plátano modificadas químicamente como adsorbente eficiente de bajo costo. Alex. Eng. J. 2016, 55, 2933–2942. [Google Scholar]
- Mohd, S.R.; Khan, A.J.; Rayathulhan, R.; Yunus, K.; Sarkar, M.Z. Biosorción de Pb y Cu a partir de una solución acuosa con polvo de cáscara de plátano. Desalin. Water Treat. 2015, 1, 1–12. [Google Scholar]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Eliminación de cobre y plomo utilizando biocarbón de banano en sistemas de adsorción por lotes: Estudios de isotermas y cinética. Arab. J. Sci. Eng. 2017, 1, 1–5. [Google Scholar]
- Aziz, H.A.; Adlan, M.N.; Hui, C.S.; Zahari, M.S.; Hameed, B.H. Eliminación de Ni, Cd, Pb, Zn y color de una solución acuosa utilizando un adsorbente potencial de bajo costo. Revista India de Ingeniería y Ciencias de los Materiales 2006, 12, 248–258. [Google Scholar]
- Agarwal, G.S.; Bhuptawat, H.K.; Chaudhari, S. Biosorción de cromo acuoso (VI) por semillas de Tamarindus indica. Tecnología de Fuentes Biológicas 2006, 97, 949–956. [Google Scholar]
- Amuda, O.S.; Giwa, A.A.; Bello, I.A. Eliminación de metales pesados de aguas residuales industriales utilizando carbón de cáscara de coco activado modificado. Revista de Ingeniería Bioquímica 2007, 36, 174–181. [Google Scholar]
- Acheampong, M.A.; Pereira, J.P.; Meulepas, R.J.; Lens, P.N. Biosorción de Cu (II) en materiales agrícolas de regiones tropicales. Revista de Tecnología Química y Biotecnología 2011, 86, 1184–1194. [Google Scholar]
- Kumar, S.; Meikap, B.C. Eliminación de cromo (VI) de las aguas residuales mediante el uso de adsorbente preparado a partir de cáscara de coco verde. Desalinización y Tratamiento de Agua 2013, 52, 3122–3132. [Google Scholar]
- Priyadharshini, B.; Marykutty, A.; Varshaa, L.P.; Kavisri, M. Investigación experimental sobre la eliminación de cromo metal utilizando cáscara de coco. Revista Internacional de Ciencia y Tecnología Avanzadas 2020, 29, 103–109. [Google Scholar]
- Abdul-Rahim, A.R.; Mohsin, H.M.; Thanabalan, M.; Rabat, N.E.; Saman, N.; Mat, H.; Johari, K. Adsorbente de residuos de coco desecado carbonoso eficaz para la aplicación de captaciones de metales pesados por adsorción: Análisis de equilibrio, cinético y termodinámico. Biomass Bioenergy 2020, 142, 1–12. [Google Scholar]
- Shrestha, S.; Son, G.; Lee, S.H.; Lee, T.G. Estudios isotermos y termodinámicos de la adsorción de Zn (II) en lignito y fibra de carbón activado a base de cáscara de coco. Chemosphere 2013, 92, 1053–1061. [Google Scholar]
- Acheampong, M.A.; Pakshirajan, K.; Annachhatre, A.P.; Lens, P.N. Eliminación de Cu (II) por biosorción en cáscara de coco en sistemas de columnas de lecho fijo. J. Ind. Eng. Chem. 2013, 19, 841–848. [Google Scholar]
- Acheampong, M.A.; Dapcic, A.D.; Yeh, D.; Lens, P.N. Absorción cíclica y desorción de Cu (II) sobre cáscara de coco y arena recubierta de óxido de hierro. Sep. Sci. Technol. 2013, 48, 2786–2794. [Google Scholar]
- Song, C.; Wu, S.; Cheng, M.; Tao, P.; Shao, M.; Gao, G. Adsorption Studies of Coconut Shell Carbons Prepared by KOH Activation for Removal of Lead(II) from Aqueous Solutions. Sustainability 2014, 6, 86–98. [Google Scholar]
- Caccin, M.; Giorgi, M.; Giacobbo, F.; Da Ros, M.; Besozzi, L.; Mariani, M. Eliminación de plomo (II) de soluciones acuosas mediante adsorción en carbones activados preparados a partir de cáscara de coco. Desalin. Water Treat. 2015, 1, 1–19. [Google Scholar]
- Halder, G.; Dhawane, S.; Barai, P.K.; Das, A. Optimización de la adsorción de cromo (VI) sobre carbón granular activado por vapor sobrecalentado a través de la metodología de superficie de respuesta y la red neuronal artificial. Environ. Prog. Sustain. Energy 2014, 34, 638–647. [Google Scholar]
- Vijetha, P.; Kumaraswamy, K.; Dhananjaneyulu, B.V.; Satyasree, N.; Lalitha, K.B. Estudios Cinéticos de Biosorción de Cadmio y Níquel a partir de Soluciones Acuosas con Citrus limonum. Revista de Investigación Farmacéutica, Biológica y Química Ciencias. 2014, 5, 1006–1015. [Google Scholar]
- Ergüvenerler, F.; Targan, Ş.; Tirtom, V.N. Eliminación de plomo de soluciones acuosas mediante biosorbentes de bajo coste y residuos (cáscaras de limón, judía y alcachofa). Ciencia y Tecnología del Agua 2020, 81, 159–169. [Google Scholar]
- Mohammadi, S.Z.; Mofidinasab, N.; Karimi, M.A.; Mosazadeh, F. Eliminación rápida y eficaz del ion Pb (II) y del tinte verde de malaquita de las aguas residuales mediante el uso de nanopartículas magnéticas de carbón activado y cobalto. Ciencia y Tecnología del Agua 2020, 82, 829–842. [Google Scholar]
- Threepanich, A.; Praipipat, P. Materiales de hidróxido-óxido de hierro (III) dopados con cáscaras de limón en polvo y perlas para aplicaciones de eliminación de plomo: Estudios de síntesis, caracterizaciones y adsorción de plomo. J. Environ. Chem. Eng. 2021, 9, 1–12. [Google Scholar]
- Villen, M.; Cerrillo, M.M.; Paz, J.M.; Rodriguez, J.M.; Arhoun, B. Valorización de residuos de cáscara de limón como biosorbente para la eliminación simultánea de níquel y cadmio de efluentes industriales. Environ. Technol. Innov. 2021, 21, 101380. [Google Scholar]
- Herrera, A.; Bitar, N.; Villabona, Á.; Tejada, C.; González, Á.D. Adsorción de níquel a partir de una solución acuosa utilizando biomasa de piel de limón modificada químicamente con nanopartículas de TiO2. Sustain. Chem. Pharm. 2020, 17, 2–7. [Google Scholar]
- Villen, M.; Gutierrez, D.; Gomez, C.; Vereda, C.; Rodríguez, J.M.; Arhoun, B. Optimización de la biosorción de Ni (II) a partir de una solución acuosa en cáscara de limón modificada. Environ. Res. 2019, 1, 1–23. [Google Scholar]
- Bhatnagar, A.; Minocha, A.K.; Sillanpää, M. Eliminación por adsorción de cobalto de una solución acuosa mediante la utilización de cáscara de limón como biosorbente. Biochem. Eng. J. 2010, 48, 181–186. [Google Scholar]
- Poonam, M.; Kumar, N. Estudio experimental y cinético de eliminación de plomo (Pb2+) del efluente de la batería utilizando adsorbente de biocarbón de cáscara de limón dulce (Citrus limetta). Environ. Dev. Sustain. 2019, 1, 1–5. [Google Scholar]
- Šabanović, E.; Muhić-Šarac, T.; Nuhanović, M.; Memić, M. Biosorción de uranio (VI) a partir de solución acuosa mediante cáscaras de Citrus limon: Cinética, equlibrio y estudios por lotes. J. Radioanal. Nucl. Chem. 2019, 319, 425–435. [Google Scholar]
- Enniya, I.; Rghioui, L.; Jourani, A. Adsorción de cromo hexavalente en solución acuosa sobre carbón activado preparado a partir de cáscaras de manzana. Química y Farmacia Sostenibles 2018, 7, 9–16. [Google Scholar]
- Sartape, A.S.; Mandhare, A.M.; Salvi, P.P.; Pawar, D.K.; Kolekar, S.S. Estudios cinéticos y de equilibrio de la adsorción de Cd (II) a partir de soluciones acuosas por carbón activado de cáscara de manzano. Desalinización y Tratamiento de Agua. 2013, 51, 4638–4650. [Google Scholar]
- Chand, P.; Shil, A.K.; Sharma, M.; Pakade, Y.B. Adsorción mejorada de iones de cadmio de una solución acuosa utilizando orujo de manzana modificado químicamente: Mecanismo, cinética y termodinámica. Int. Biodeterior. Biodegrad. 2014, 90, 8–16. [Google Scholar]
- Heraldy, E.; Lestari, W.W.; Permatasari, D.; Arimurti, D.D. Biosorbente de desechos de tomate y residuos de jugo de manzana para eliminar el plomo. J. Environ. Chem. Eng. 2018, 6, 1201–1208. [Google Scholar]
- Chand, P.; Pakade, Y.B. Síntesis y caracterización de nanopartículas de hidroxiapatita impregnadas en orujo de manzana para mejorar la adsorción de iones Pb (II), Cd (II) y Ni (II) de una solución acuosa. Environ. Sci. Pollut. Res. 2015, 22, 10919–10929. [Google Scholar]
- Mahmoud, M.E.; Mohamed, A.K. Novedoso hidrogel de pectina derivado de un compuesto de estructuras organometálicas a base de cáscara de mandarina para una eliminación mejorada de los iones Cr (VI) y Pb (II). Revista Internacional de Macromoléculas Biológicas 2020, 164, 920–931. [Google Scholar]
- Ehrampoush, M.H.; Miria, M.; Salmani, M.H.; Mahvi, A.H. Eliminación de cadmio de una solución acuosa mediante nanopartículas de óxido de hierro de síntesis verde con extracto de cáscara de mandarina. J. Environ. Health Sci. Eng. 2015, 13, 1–5. [Google Scholar]
- Unugul, T.; Nigiz, F.U. Síntesis de cáscara de mandarina carbonizada tratada con ácido para la purificación de cobre. Water Pract. Technol. 2020, 29, 2108–2113. [Google Scholar]
- Inagaki, C.S.; Caretta, T.O.; Alfaya, R.V. da S.; Alfaya, A.A. da S. Cáscara de mandarina mexicana (Citrus nobilis) como nuevo biosorbente para eliminar Cu (II), Cd (II) y Pb (II) del efluente industrial. Desalin. Water Treat. 2013, 1, 5537–5546. [Google Scholar]
- Iqbal, M.; Saeed, A.; Zafar, S.I. Espectrofotometría FTIR, modelado de isotermas de adsorción y cinética, intercambio iónico y análisis EDX para comprender el mecanismo de eliminación de Cd2+ y Pb2+ por residuos de cáscara de mango. J. Hazard. Mater. 2009, 164, 161–171. [Google Scholar] [PubMed]
- Farihahusnah, H.; Mohamed, K.; Małgorzata, S. Biocarbón derivado de subproductos de frutas mediante un proceso de pirólisis para la eliminación del ión Pb (II): Una revisión actualizada. Chemosphere 2021, 287, 1–10. [Google Scholar]
- Rai, M.K.; Shahi, G.; Meena, V.; Meena, R.; Chakraborty, S.; Singh, R.S.; Rai, B.N. Eliminación de cromo hexavalente Cr (VI) utilizando carbón activado preparado a partir de granos de mango activados con H3PO4. Resour.-Effic. Technol. 2016, 2, 63–70. [Google Scholar]
- Adekola, F.; Adegoke, H.; Ajikanle, R. Estudios cinéticos y de equilibrio de la adsorción de Pb (II) y Cd (II) en la cáscara de mango salvaje africano (irvingia gabonensis). Bull. Chem. Soc. Ethiop. 2016, 30, 185. [Google Scholar]
- Shakya, A.; Agarwal, T. Eliminación de Cr (VI) del agua utilizando biocarros derivados de la cáscara de piña: Potencial de adsorción y evaluación de la reutilización. J. Mol. Liq. 2019, 293, 111497. [Google Scholar]
- Lim, Z.E.; Thai, Q.B.; Le, D.K.; Luu, T.P.; Nguyen, P.T.; Do, N.H.; Le, P.K.; Phan-Thien, N.; Goh, X.T.; Doung, H.M. Aerogeles de piña funcionalizados para aplicaciones de adsorción de gas etileno y eliminación de iones de níquel (II). J. Environ. Chem. Eng. 2020, 8, 104524. [Google Scholar]
- Wang, C.; Gu, L.; Liu, X.; Zhang, X.; Cao, L.; Hu, X. Comportamiento de sorción del Cr (VI) en el biocarbón derivado de la piel de la piña y la influencia del pireno coexistente. Int. Biodeterior. Biodegrad. 2016, 111, 78–84. [Google Scholar]
- Phuengphai, P.; Singjanusong, T.; Kheangkhun, N.; Wattanakornsiri, A. Eliminación de cobre (II) de una solución acuosa utilizando cáscaras de frutas modificadas químicamente como biosorbentes eficientes y de bajo costo. Water Sci. Eng. 2021, 1, 1–12. [Google Scholar]
- Gerola, G.P.; Boas, N.V.; Caetano, J.; Tarley, C.R.; Gonçalves, A.C.; Dragunski, D.C. Utilización del subproducto de la piel de Maracuyá como biosorbente de iones de plomo (II). Water Air Soil Pollut. 2013, 224, 1–5. [Google Scholar]
- Aranda, E.; Cristiani, E. Efecto del pH sobre la eliminación de cromo hexavalente y total de soluciones acuosas por cáscara de aguacate utilizando sistemas continuos y por lotes. Environ. Sci. Pollut. Res. 2017, 1, 1–5. [Google Scholar]
- DANE. Cultivo de limón o lima Tahití (Citrus latifolia Tanaka) frente a los efectos de las condiciones climáticas adversas. Boletín Mensual, Insumos y Factores Asociados a la Producción Agropecuaria 2015, 41, 1–93. [Google Scholar]
- Carvajal, M.; Zuluaga, P.; Ocampo, O.; Duque, D. Las exportaciones de plátano como una estrategia de desarrollo rural en Colombia. Apuntes CENES 2018, 38, 113–148. [Google Scholar]
- DANE. El cultivo del mango, Magnifera indica, y su comportamiento frente a las comunidades ambientales y manejo. Boletín Mensual Insumos y Factores Asociados a la Producción Agropecuaria 2015, 31, 1–64. [Google Scholar]
- Cámara de comercio. Tomate de árbol, Programa de apoyo agrícola agroindustrial vicepresidencia de fortalecimiento empresarial. Núcleo Ambiental 2015, 1, 1–20. [Google Scholar]
- Garzón, J. Establecimiento y Manejo de un Cultivo de Piña en la Sede de la Asociación de Ingenieros Agrónomos del Llano en Villavicencio. Bachelor’s Thesis, Universidad de los llanos, Villavicencio, Colombia, 2016. [Google Scholar]
- Álvarez, E. Guía Técnica del Cultivo de la Maracuyá, Programa MAG-CENTA-FRUTALES; Centro Nacional de Tecnología Agropecuaria y Forestal: Ciudad Arce, El Salvador, 2010. [Google Scholar]
- Orduz, J.; Castiblanco, S.; Calderón, C.; Velasquéz, H. Potencial de rendimiento y calidad de 13 variedades e híbridos comerciales de cítricos en condiciones del piedemonte llanero de Colombia. Revista Colombiana de Ciencias Hortícolas 2011, 5, 171–185. [Google Scholar]
- Lizano, M. Guía Técnica del cultivo de Coco; Programa Nacional de Frutas del Salvador Ministerio De Agricultura Y Ganadería: Santa Tecla, El Salvador, 2015. [Google Scholar]
- FINANGRO. Ficha de Inteligencia del Aguacate. Fondo para el Financiamiento del Sector Agropecuario. Unidad de Gestión de Riesgos Agropecuarios–UGRA. Vicepresidencia de Garantías y Riesgos Agropecuario. 2018. Available online: https://www.finagro.com.co/productos-y-servicios/ISA/gesti%C3%B3n-de-riesgos-agropecuarios (accessed on 18 January 2022).
- Flores, M. Texto guía del Participante, Producción de Manzana. Fundación Educación para el Desarrollo FAUTAPO. 2014. Sucre-Bolivia. Available online: http://saludpublica.bvsp.org.bo/cc/bo40.1/documentos/704.pdf (accessed on 18 January 2022).
- Muñoz, J.; Rodríguez, L.; Bermúdez, L. Analysis of the competitiveness of the lulo production system (Solanum quitoense Lam.) in three municipalities of Nariño. Revista Colombiana de Ciencias Hortícolas 2013, 7, 173–185. [Google Scholar]
- Mateus, D.; Orduz, J. Mandarina Dancy: Una nueva alternativa para la citricultura del piedemonte llanero de Colombia. Revista Corpoica Cienc Tecnol Agropecu 2015, 16, 105–112. [Google Scholar]
- Noguera, K.; Oliveros, J. Los rellenos sanitarios en Latinoamérica. Caso Colombiano. Revista Académica Colombiana de Ciencia XXXIV 2010, 132, 347–356. [Google Scholar]
- Unidad Administrativa Especial de Servicios Públicos. Observación de Residuos Sólidos en Bogotá. 2020. Available online: https://www.uaesp.gov.co/content/observatorio-residuos-solidos (accessed on 5 January 2022).
- Bhatnagar, A.; Sillanpaa, M.; Witek-Krowiak, A. Agricultural waste peels as versatile biomass for water purification—A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar]
- Concejo Nacional de Política Económica y Social República de Colombia Departamento Nacional de Planeacios (COMPES-3874). Política Nacional Para la Gestión Integral de Residuos Sólidos. 2016. Available online: https://colaboracion.dnp.gov.co/CDT/Conpes/Econ%C3%B3micos/3874.pdf (accessed on 18 January 2022).
- Division for Sustainable Development. How Does the CSD Relate to the Johannesburg Summit? 2002. Available online: https://www.un.org/esa/dsd/dsd/dsd_faqs_csd.shtml#Q5 (accessed on 5 January 2022).
- Saval, S. Aprovechamiento de Residuos Agroindustriales: Pasado, Presente y Futuro. Biotecnología y Bioingeniería 2012, 16, 14–46. [Google Scholar]
- Huang, R.; Cao, M.; Guo, H.; Qi, W.; Su, R.; He, Z. Enhanced Ethanol Production from Pomelo Peel Waste by Integrated Hydrothermal Treatment, Multienzyme Formulation, and Fed-Batch Operation. J. Agric. Food Chem. 2014, 60, 4643–4651. [Google Scholar]
- Tejada, C.; Quiñones, E.; Tejada, L.; Marimón, W. Absorción de Cromo Hexavalente en soluciones acuosas por cascaras de naranja (Citrus sinensis). Prod. Limpia 2014, 10, 9–21. [Google Scholar]
- Mafra, M.; Igarashi, L.; Zuim, D.; Vasques, C.; Ferreira, M. Adsorption of remazol brilliant blue on an orange peel adsorbent. Braz. J. Chem. Eng. 2013, 30, 657–665. [Google Scholar]
- Pinzón, M.; Tamayo, M. Caracterización de la cáscara de naranja para su uso como material bioadsorbente. Rev. BISTUA 2008, 6, 28–37. [Google Scholar]
- Deveci, H.; Kar, Y. Adsorption of hexavalent chromium from aqueous solutions by bio-chars obtained during biomass pyrolysis. J. Ind. Eng. Chem. 2013, 19, 190–196. [Google Scholar]
- Lugo, V.; Barrera, C.; Ureña, F.; Bilyeu, B.; Linares, I. Biosorption of Cr (III) and Fe (III) in single and binary systems onto pretreated orange peel. J. Environ. Manag. 2012, 112, 120–127. [Google Scholar]
- Rodríguez, M.; Gamba, N.; Lozano, O.; Estévez de Sístiva, A.; Castillo, E.; Pedraza, E.; Moreno, C.; Orduz, J.; Fonseca, J.; Herrera, I. Desempeño Ambiental de la Tecnología en la Industria Colombiana, 1st ed.; Instituto de Hidrología, Meteorología y Estudios Ambiental, Ed.; El medio ambiente en Colombia (520); Editorial Santa fe de Bogotá: Bogotá, Colombia, 1998. Available online: http://documentacion.ideam.gov.co/openbiblio/bvirtual/005192/medioambiente/cap12.pdf (accessed on 18 January 2022).
- Vargas, M.; Figueroa, H.; Tamayo, J.; Toledo, V.; Moo, V. Aprovechamiento de cáscaras de frutas: Análisis nutricional y compuestos bioactivos. Ciencias Naturales y Agropecuarias 2018, 26, 1–12. [Google Scholar]
- Feng, N.; Guo, X.; Liang, S. Adsorption study of copper(II) by chemically modified orange peel. J. Hazard. Mater. 2009, 164, 1286–1292. [Google Scholar]
- Shan, W.; Fang, D.; Zhao, Z.; Shuang, Y.; Ning, L.; Xing, Z.; Xiong, Y. Application of orange peel for adsorption separation of molybdenum(VI) from re-containing industrial effluent. Biomass Bioenergy 2012, 37, 289–297. [Google Scholar]
- Ali Khan Rao, R.; Rehman, F.; Kashifuddin, M. Removal of Cr (VI) from electroplating wastewater using fruit peel of Leechi (Litchi chinensis). Desalin. Water Treat. 2012, 49, 136–146. [Google Scholar]
- Monroy, F.; Echavarría, M.; Gómez, D. Design and validation of a system for hexavalent chromium adsorption in tannery effluents using orange peel and wheat bran. Tecnología y Ciencias del Agua 2021, 12, 1–31. [Google Scholar]
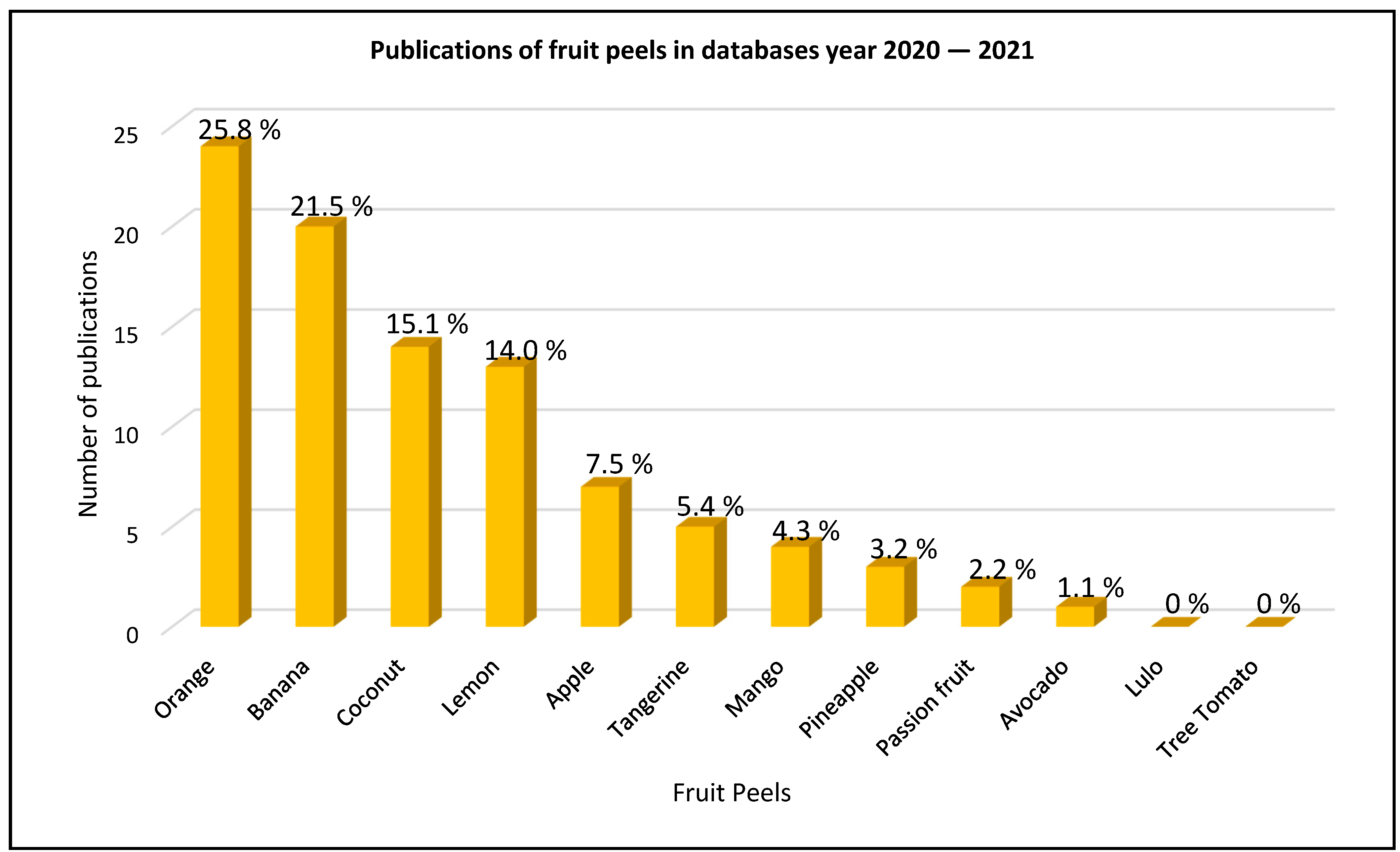

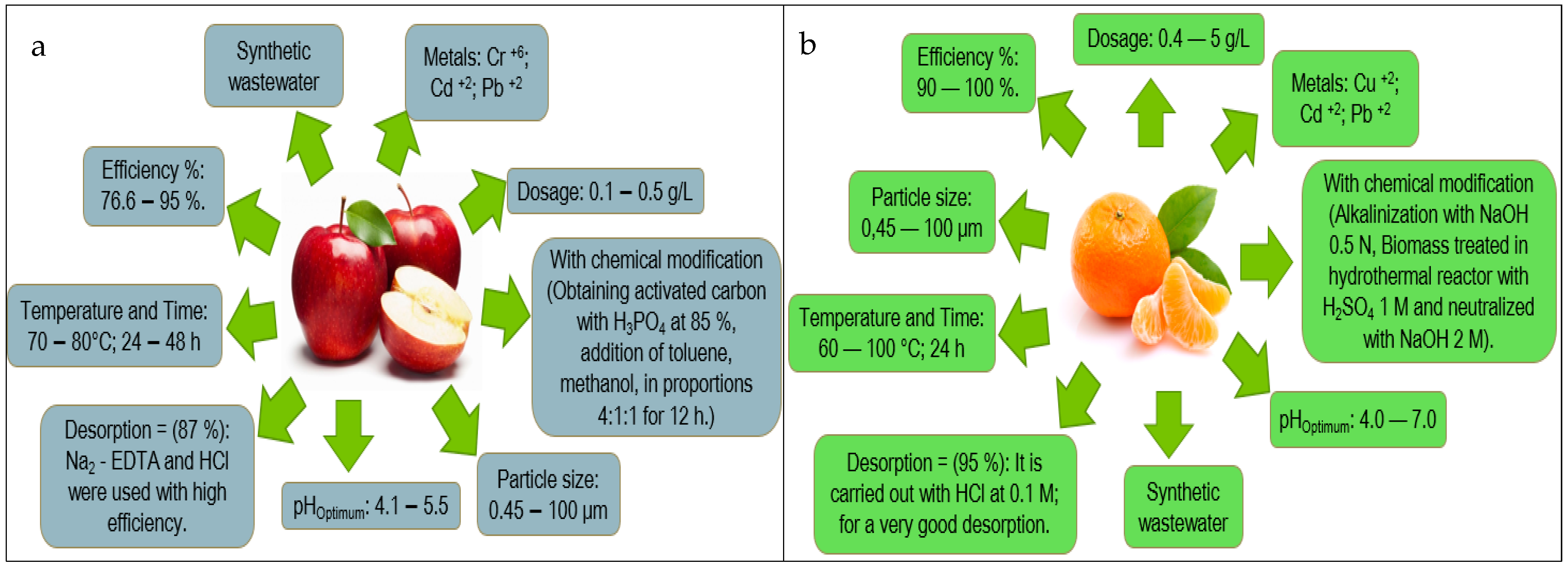
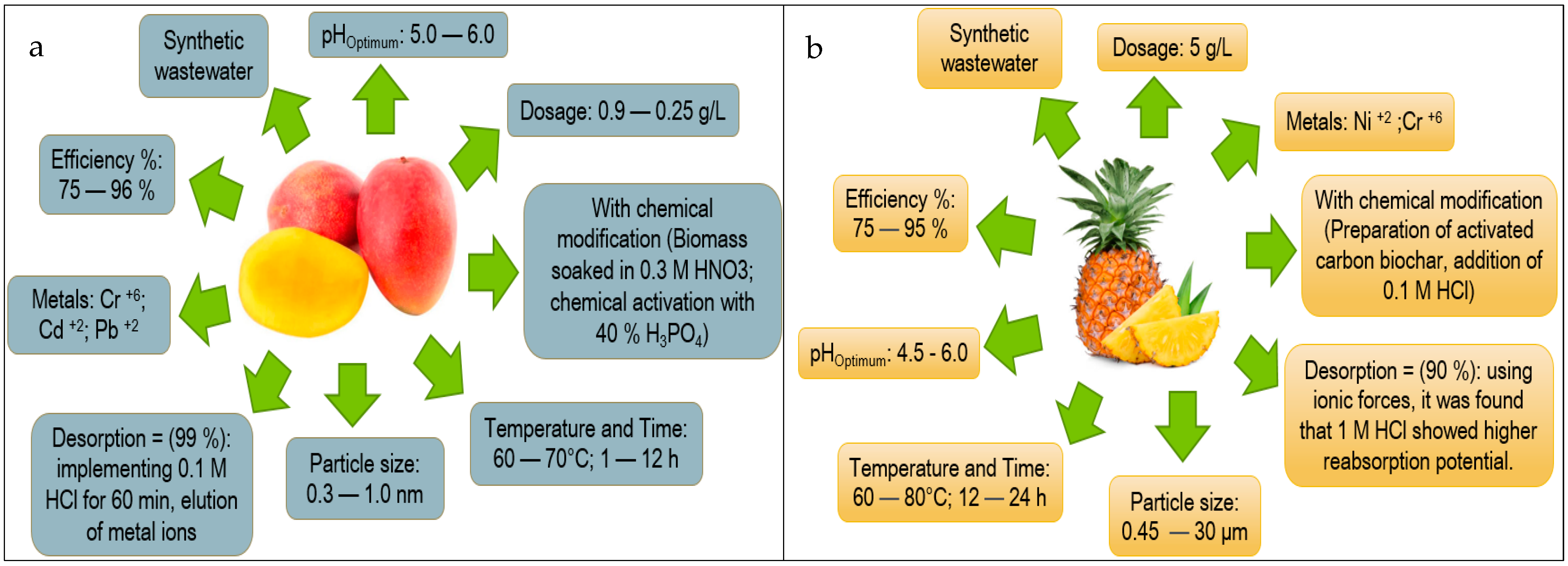
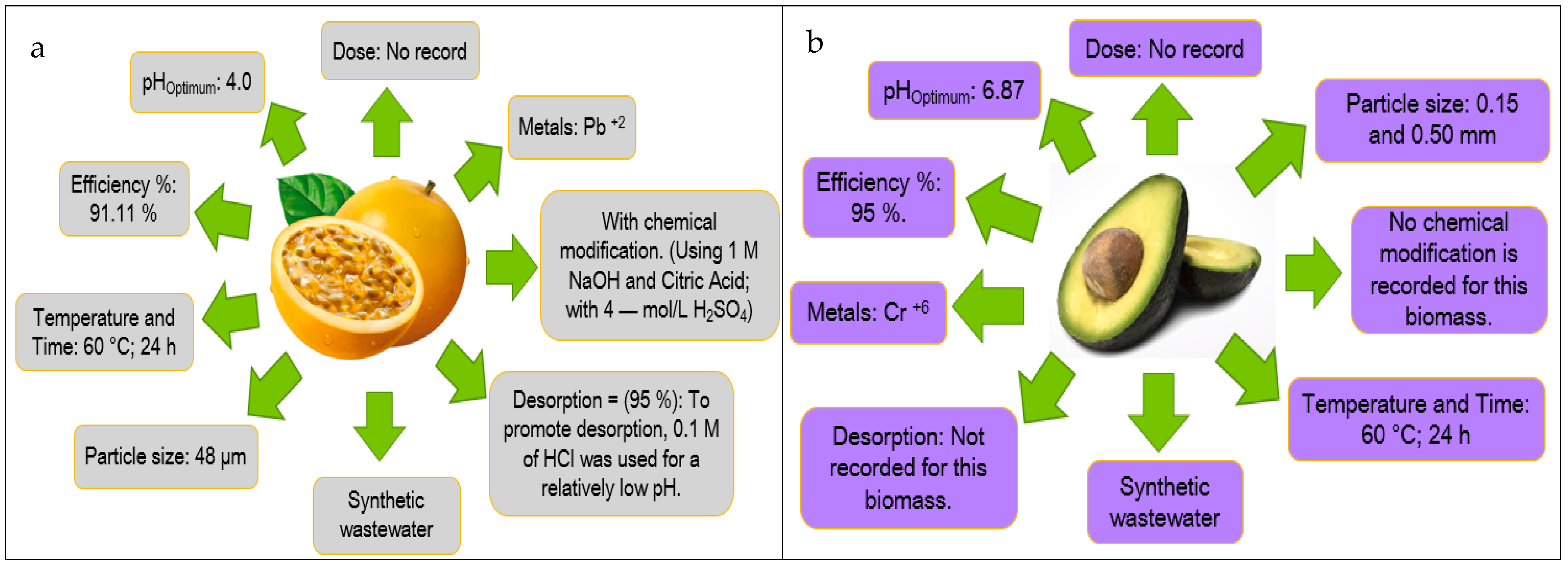
| Fruit Peel | Scopus (Number of Articles) | References Scopus | Science Direct (Number of Articles) | References Science Direct | Web of Science (Number of Articles) | References Web of Science |
|---|---|---|---|---|---|---|
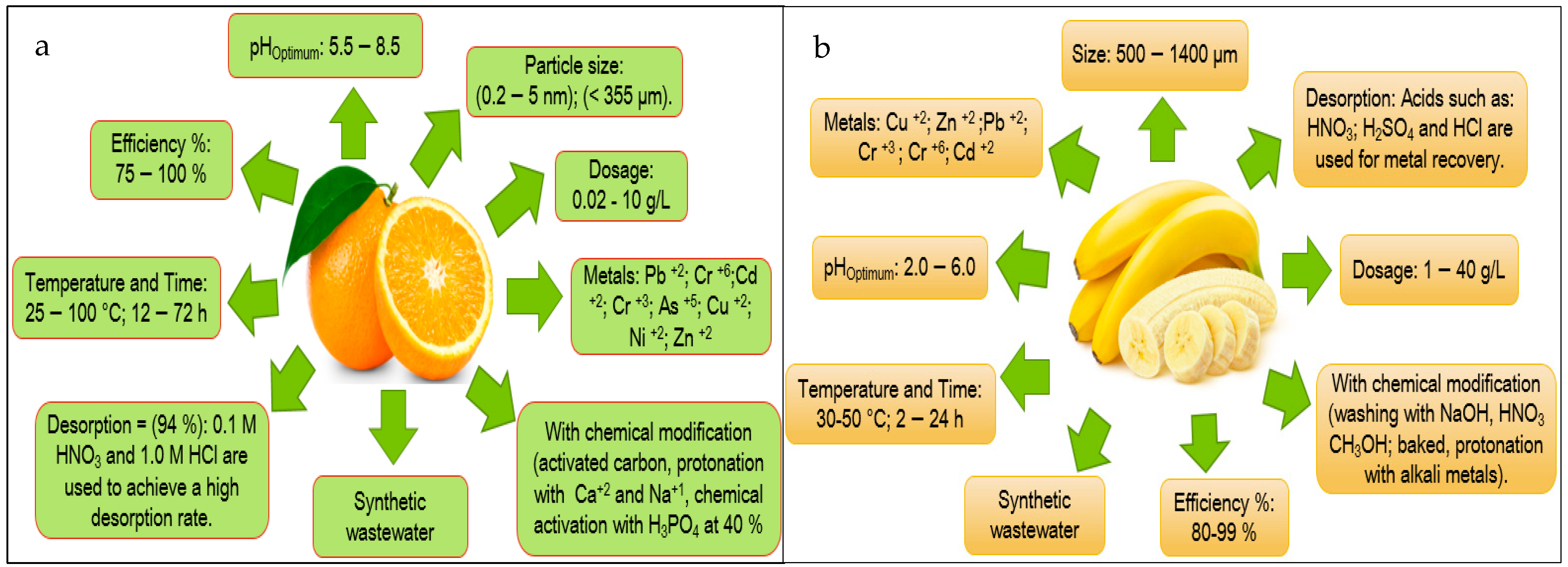
| Orange | 9 | [22,23,24,25,26,27,28,29,30] | 2 | [31,32] | 13 | [9,33,34,35,36,37,38,39,40,41,42,43,44] |
| Banana | 8 | [45,46,47,48,49,50,51,52] | 7 | [53,54,55,56,57,58,59] | 5 | [9,50,55,60,61] |
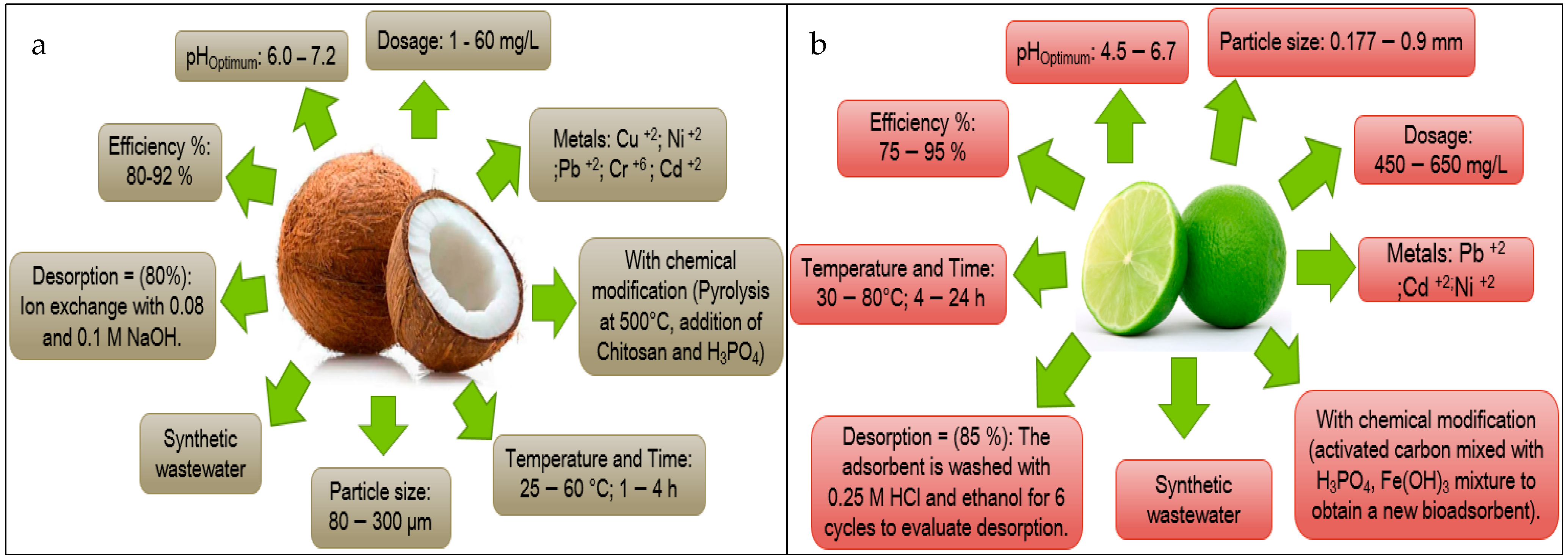
| Coconut | 6 | [62,63,64,65,66,67] | 2 | [68,69] | 6 | [66,70,71,72,73,74] |
| Lemon | 5 | [29,30,75,76,77] | 5 | [78,79,80,81,82] | 3 | [82,83,84] |
| Apple | 3 | [29,85,86] | 3 | [85,87,88] | 1 | [89] |
| Tangerine | 1 | [90] | 1 | [57] | 3 | [91,92,93] |
| Mango | 0 | - | 3 | [94,95,96] | 1 | [97] |
| Pineapple | 0 | - | 3 | [98,99,100] | 0 | - |
| Passion fruit | 0 | - | 1 | [101] | 1 | [102] |
| Avocado | 0 | - | 0 | - | 1 | [103] |
| Lulo | 0 | - | 0 | - | 0 | - |
| Tree Tomato | 0 | - | 0 | - | 0 | - |
| General Information on Selected Fruits in Colombia | |||
|---|---|---|---|
| Species | Origin/Year | Type of Fruit | References |
| Lemon | China/1941 | Major perennials | [104] |
| Banana | Asia/NE | Transient fruits | [105] |
| Mango * | India/NE | Major perennials | [106] |
| Tree tomato | Peru/NE | Transient Fruits | [107] |
| Pineapple | Southeast Brazil and Paraguay/NE | Transient fruits | [108] |
| Passion fruit | Brazil/NE | Transient fruits | [109] |
| Orange * | China/NE | Major perennials | [110] |
| Coconut | Malaysia/NE | Major perennials | [111] |
| Avocado * | Mexico and Guatemala/NE | Major perennials | [112] |
| Apple * | Central Asia/NE | - | [113] |
| Lulo | Colombia/NE | Transient fruits | [114] |
| Tangerine * | Asia/NE | Major perennials | [115] |
| Parameter | Value (%) | Analytical Method of Quantification | Reference |
|---|---|---|---|
| Carbon | 42.04 | AOAC 949.14 | [123] |
| Hydrogen | 5.44 | AOAC 949.14 | |
| Nitrogen | 0.70 | AOA 984.13 Kjeldahl | |
| Pectin | 10.98 | Acid digestion and Thermogravimetry | |
| Lignin | 6.51 | Photocolorimetry | |
| Cellulose | 13.08 | digestion and Thermogravimetry | |
| Hemicellulose | 6.47 | digestion and Thermogravimetry |
| Characteristics | Values | Reference |
|---|---|---|
| CaO | 1.42 | [124] |
| K2O | 0.18 | |
| SO3 | 0.14 | |
| MgO | 0.12 | |
| Fe2O3 | 0.11 | |
| SiO2 | 0.08 | |
| P2O5 | 0.05 | |
| BaO | 0.02 | |
| SrO | 0.01 | |
| Al2O3 | 0.01 | |
| NiO | 0.01 | |
| WO3 | Not detected | |
| ZnO | Not detected | |
| Mn | Not detected |
| Functional Group | Displacement cm−1 | References |
|---|---|---|
| Hydroxyl groups (OH) ranging from 3340 to 3600 cm−1. | 3441 | [125,126,127] |
| (C-H) methyl, methylene, and methoxy groups. | 2923.78 | |
| Carbonyl (C=O), indicating the vibration of the carboxyl groups of pectin. | 1748.15 | |
| Stretching of (C=C), as a consequence of the presence of aromatic rings. | 1636.17 | |
| Presence of (C-H), aliphatic and aromatic, groups in a deformation plane, methyl, methylene, and methoxy groups. | 1444.43 | |
| The range corresponds to the (C-O) group of alcohols and carboxylic acids. | 1333.24–1022 |
| Industry | Metals Generated by the Activity | Contamination By-Products |
|---|---|---|
| Ferrous metal mining | Cd, Cu, Ni, Cr, Co, Zn | Acid mine drainage, tailings, tailings dumps, ferrous metals and steel mills, chemical industry. |
| Ore extraction | As, Cd, Cu, Ni, Pb, Zn | Presence in ores as well as in by-products. |
| Smelting | As, Cd, Pb, Ti | Ore processing to obtain metals. |
| Metallurgy | Cr, Cu, Mn, Pb, Sb, Zn | Thermal processing of metals. |
| Alloys and steels | Pb, Mo, Ni, Cu, Cd, As, Te, U, Zn | Metal fabrication, disposal and recycling Tailings. |
| Waste management | Zn, Cu, Cd, Pb, Ni, Cr, Hg, Mn | Waste incineration or in leachates. |
| Metal corrosion | Fe, Cr, Pb, Ni, Co, Zn | Instability of metals exposed to the environment. |
| Electroplating | Cr, Ni, Zn, Cu | Liquid effluents from coating processes. |
| Paints and pigments | Pb, Cr, As, Ti, Ba, Zn | Aqueous waste from the manufacture and deterioration of old paint. |
| Batteries | Pb, Sb, Zn, Cd, Ni, Hg | Waste pile fluid, soil and groundwater contamination. |
| Electronics | Pb, Cd, Hg, Pt, Au, Cr, As, Ni, Mn | Aqueous and solid metal waste from the manufacturing and recycling process. |
| Agriculture and livestock | Cd, Cr, Mo, Pb, U, V, Zn, As, Mn, Cu | Contamination of runoff, surface and ground water, plant bioaccumulation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Aguilar, D.L.; Rodríguez-Miranda, J.P.; Salcedo-Parra, O.J. Fruit Peels as a Sustainable Waste for the Biosorption of Heavy Metals in Wastewater: A Review. Molecules 2022, 27, 2124. https://doi.org/10.3390/molecules27072124
Gómez-Aguilar DL, Rodríguez-Miranda JP, Salcedo-Parra OJ. Fruit Peels as a Sustainable Waste for the Biosorption of Heavy Metals in Wastewater: A Review. Molecules. 2022; 27(7):2124. https://doi.org/10.3390/molecules27072124
Chicago/Turabian StyleGómez-Aguilar, Dora Luz, Juan Pablo Rodríguez-Miranda, and Octavio José Salcedo-Parra. 2022. "Fruit Peels as a Sustainable Waste for the Biosorption of Heavy Metals in Wastewater: A Review" Molecules 27, no. 7: 2124. https://doi.org/10.3390/molecules27072124
APA StyleGómez-Aguilar, D. L., Rodríguez-Miranda, J. P., & Salcedo-Parra, O. J. (2022). Fruit Peels as a Sustainable Waste for the Biosorption of Heavy Metals in Wastewater: A Review. Molecules, 27(7), 2124. https://doi.org/10.3390/molecules27072124