Reduction in Allergenicity and Induction of Oral Tolerance of Glycated Tropomyosin from Crab
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Human Sera
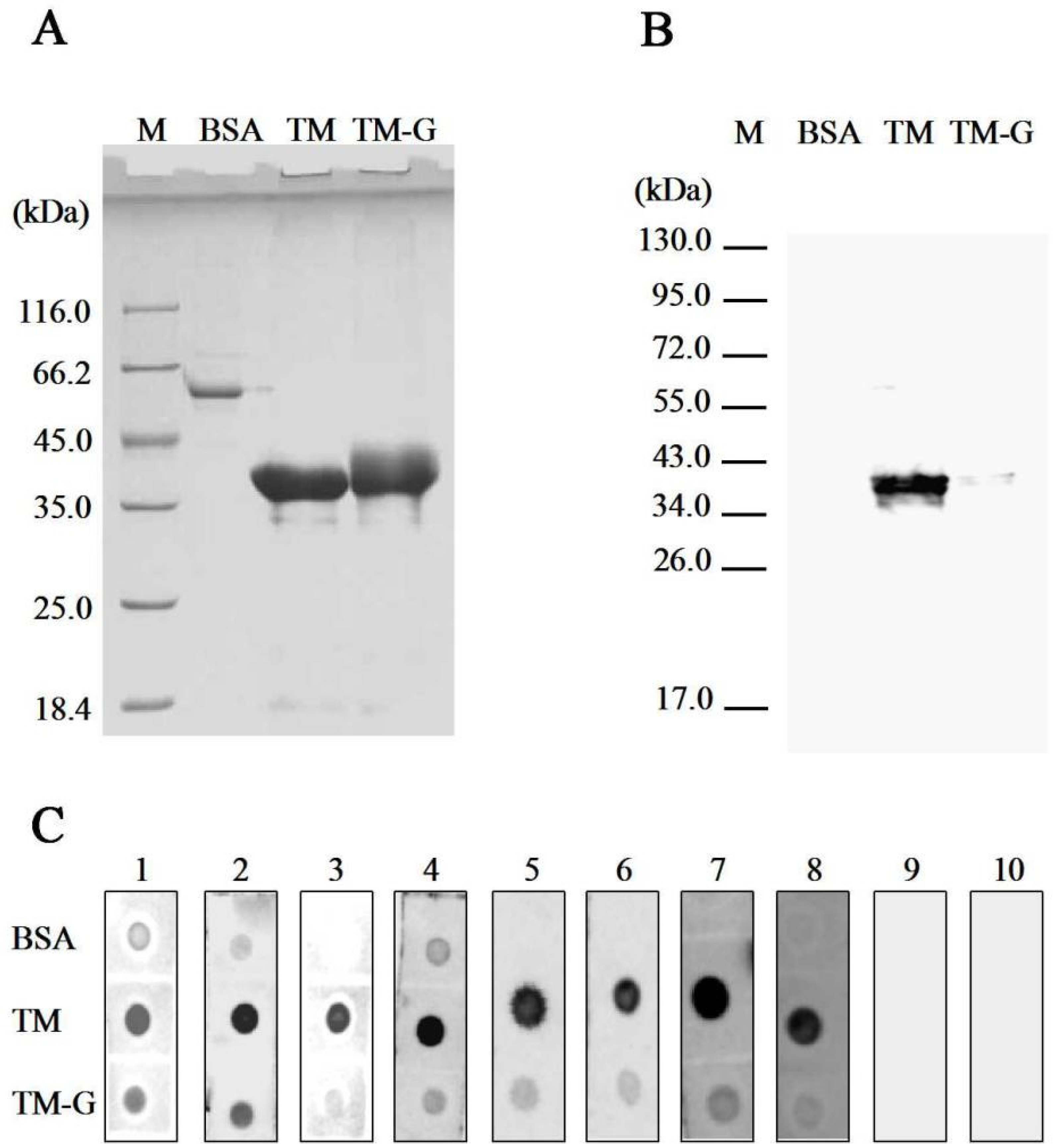
2.4. Preparation and Immunobinding Capacity Analysis of TM-G
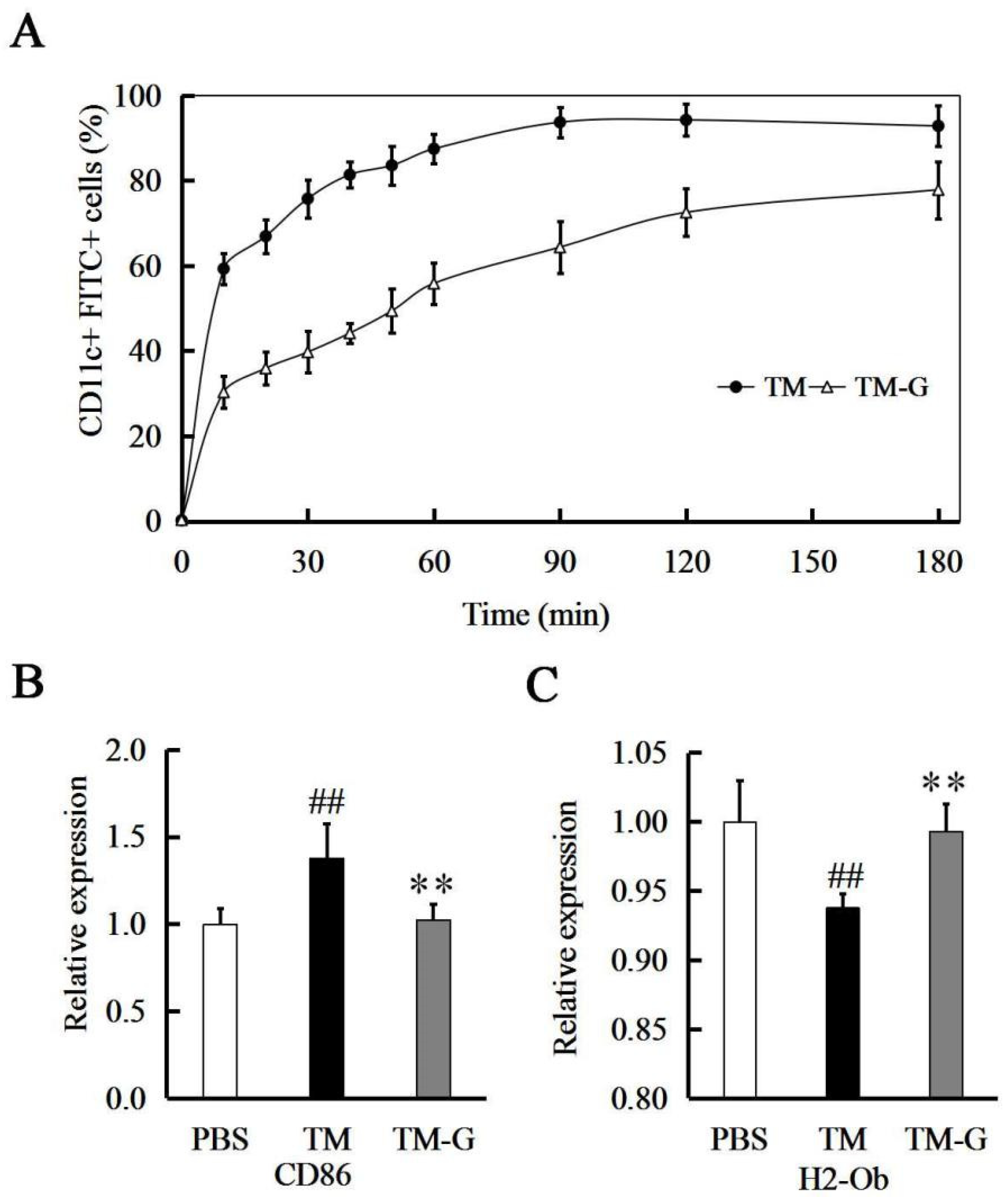
2.5. Allergen Uptake and Presentation by Mouse Bone Marrow-Derived Dendritic Cells
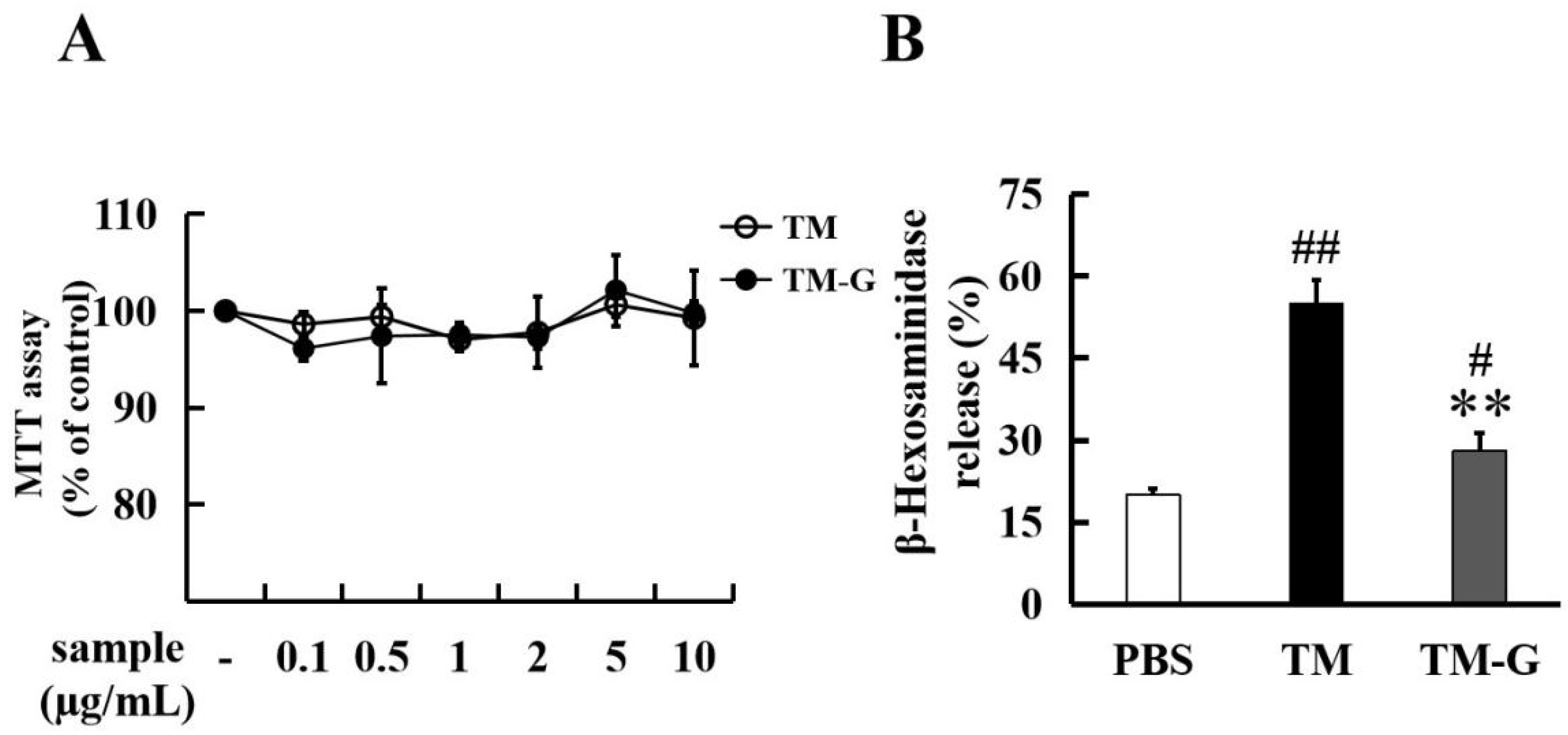
2.6. Degranulation Assay in RBL-2H3 Cells
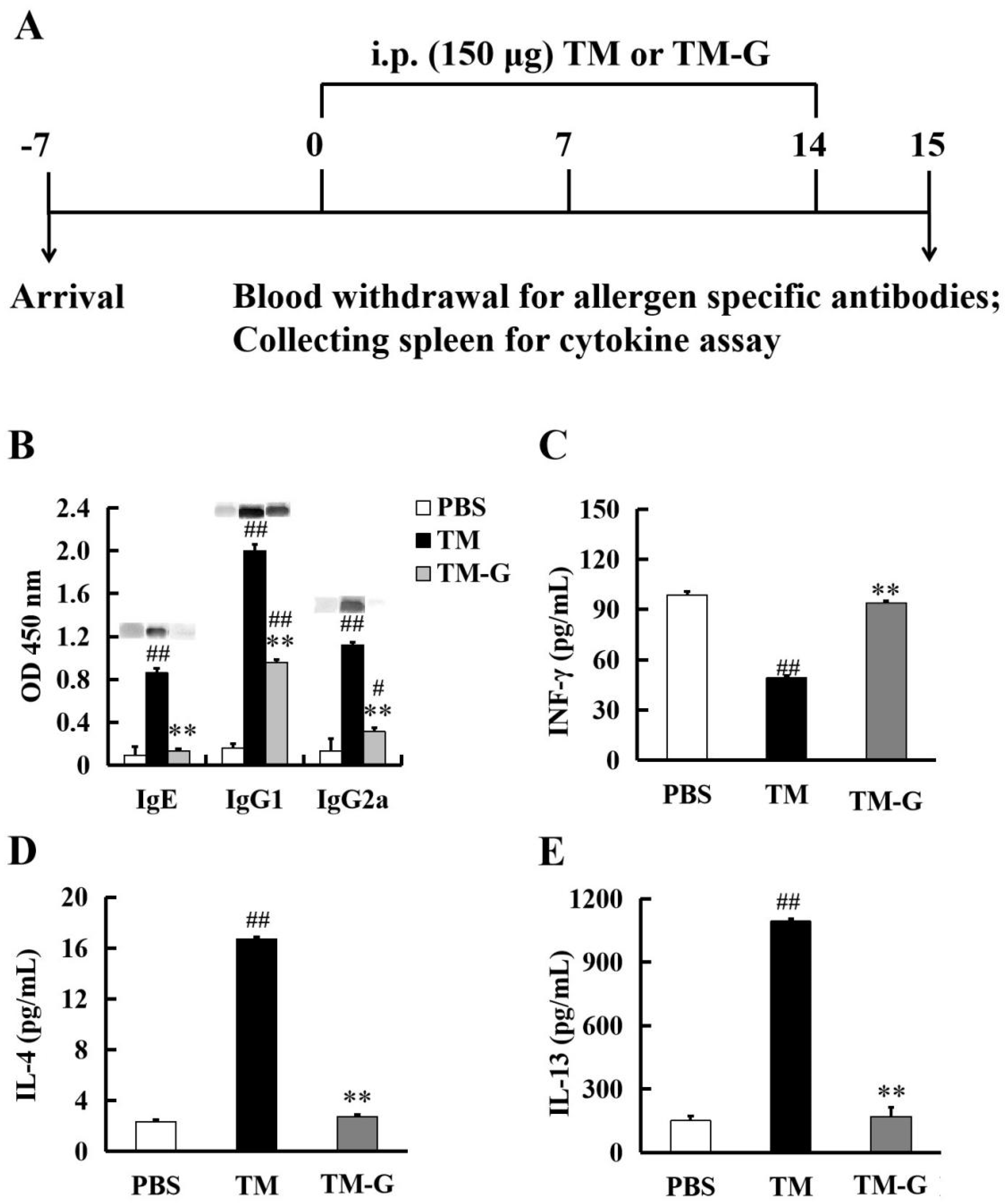
2.7. Animal Models of Injection Sensitization and Oral Tolerance to TM-G
2.8. Identification of Glycation Sites and Modification Analysis of the Epitopes
2.9. Statistical Analysis
3. Results
3.1. IgG/IgE-Binding Capacity Analysis of TM-G
3.2. TM-G Uptake by BMDCs
3.3. TM-G Presentation by BMDCs
3.4. Effect of TM-G on Cell Activity and Degranulation in RBL-2H3
3.5. Injection Sensitization to TM-G
3.6. Oral Tolerance to TM-G
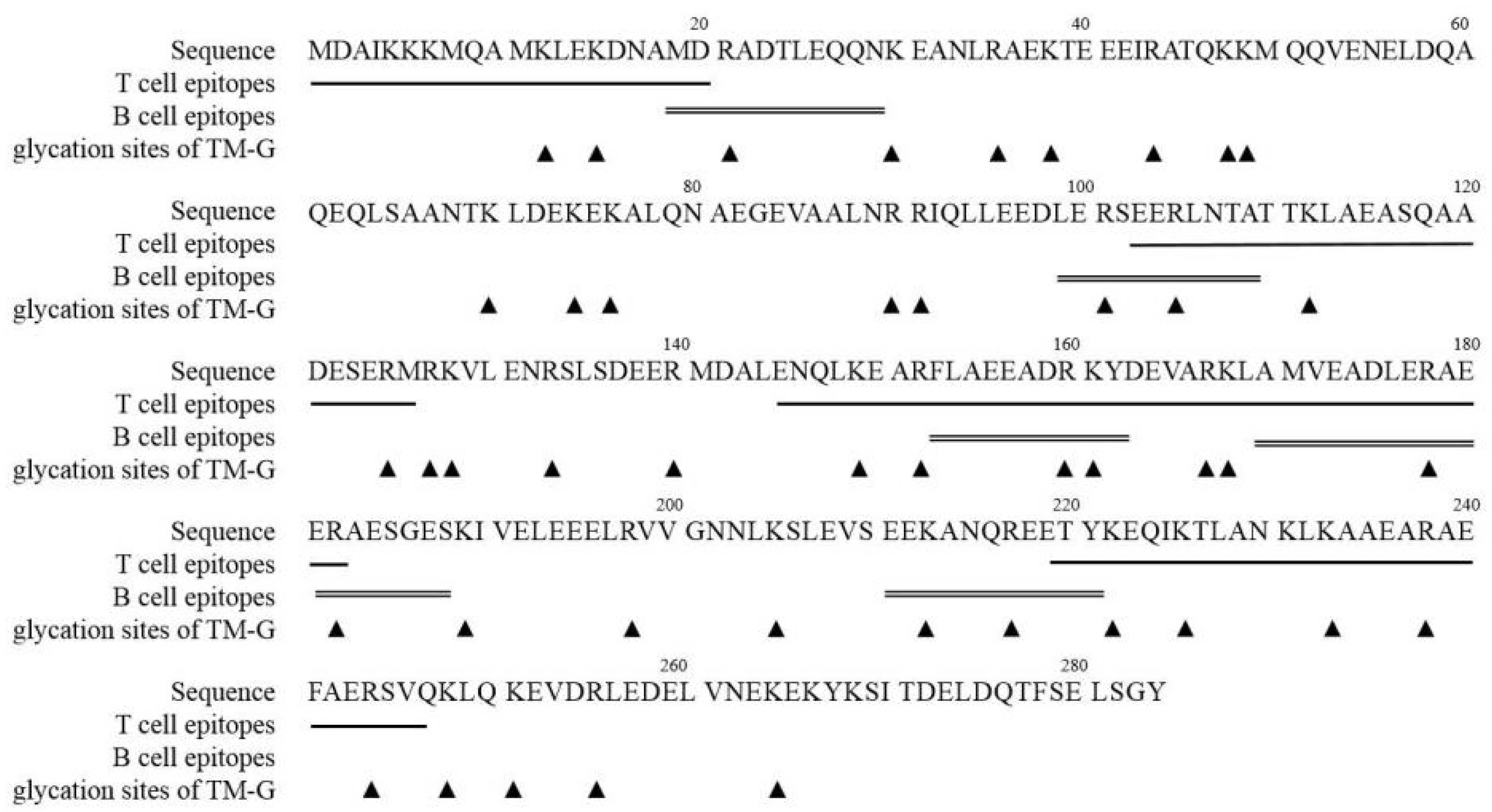
3.7. Modification of TM Epitopes via the MR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kattan, J. The Prevalence and Natural History of Food Allergy. Curr. Allergy Asthm. Rep. 2016, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tordesillas, L.; Berin, M.C.; Sampson, H.A. Immunology of Food Allergy. Immunity 2017, 47, 32–50. [Google Scholar] [CrossRef]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K. Food allergy. Nat. Rev. Dis. Primers 2018, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ruethers, T.; Taki, A.C.; Johnston, E.B.; Nugraha, R.; Le, T.T.K.; Kalic, T.; McLean, T.R.; Kamath, S.D.; Lopata, A.L. Seafood Allergy: A Comprehensive Review of Fish and Shellfish Allergens. Mol. Immunol. 2018, 100, 28–57. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.L.; Cao, M.J.; Su, W.J.; Zhang, L.J.; Huang, Y.Y.; Liu, G.M. Identification and Characterisation of the Major Allergen of Chinese Mitten Crab (Eriocheir Sinensis). Food Chem. 2008, 111, 998–1003. [Google Scholar] [CrossRef]
- Iwan, M.; Vissers, Y.M.; Fiedorowicz, E.; Kostyra, H.; Kostyra, E.; Savelkoul, H.F.J.; Wichers, H.J. Impact of Maillard Reaction on Immunoreactivity and Allergenicity of the Hazelnut Allergen Cor a 11. J. Agric. Food Chem. 2011, 59, 7163–7171. [Google Scholar] [CrossRef]
- Patrick, G.; Stefan, V.; Andrea, W.; Jorg, N.; Hofmann, T. Maillard Reaction and Enzymatic Browning Affect the Allergenicity of Pru av 1, the Major Allergen from Cherry (Prunus avium). J. Agric. Food Chem. 2004, 52, 4002–4007. [Google Scholar]
- Sancho, A.I.; Rigby, N.M.; Zuidmeer, L.; Asero, R.; Mistrello, G.; Amato, S.; González-Mancebo, E.; Fernández-Rivas, M.; Ree, R.V.; Mills, E.N. The Effect of Thermal Processing on the IgE Reactivity of the Non-specific Lipid Transfer Protein from Apple, Mal D 3. Allergy 2005, 60, 1262–1268. [Google Scholar] [CrossRef]
- Nakamura, A.; Sasaki, F.; Watanabe, K.; Ojima, T.; Ahn, D.; Saeki, H. Changes in Allergenicity and Digestibility of Squid Tropomyosin During the Maillard Reaction with Ribose. J. Agric. Food Chem. 2006, 54, 9529–9534. [Google Scholar] [CrossRef]
- Perusko, M.; Roest, M.V.; Stanic-Vucinic, D.; Simons, P.J.; Pieters, R.H.H.; Velickovic, T.; Smit, J.J. Glycation of the Major Milk Allergen β-lactoglobulin Changes its Allergenicity by Alterations in Cellular Uptake and Degradation. Mol. Nutr. Food Res. 2018, 62, 1800341. [Google Scholar] [CrossRef]
- Humeniuk, P.; Dubiela, P.; Hoffmann-Sommergruber, K. Dendritic cells and Their Role in Allergy: Uptake, Proteolytic Processing and Presentation of Allergens. Int. J. Mol. Sci. 2017, 18, 1491. [Google Scholar] [CrossRef]
- Jensen, K.K.; Andreatta, M.; Marcatili, P.; Buus, S.; Greenbaum, J.A.; Yan, Z.; Sette, A.; Peters, B.; Nielsen, M. Improved Methods for Predicting Peptide Binding Affinity to MHC Class II Molecules. Immunology 2018, 154, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Man, A.L.; Bertelli, E.; Regoli, M.; Chambers, S.J.; Nicoletti, C. Antigen-specific T Cell-mediated Apoptosis of Dendritic Cells is Impaired in a Mouse Model of Food Allergy. Allergy Clin. Immunol. 2004, 113, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Saiz, R.; Bruton, K.; Koenig, J.F.E.; Waserman, S.; Jordana, M. The IgE Memory Reservoir in Food Allergy. Allergy Clin. Immunol. 2018, 142, 1441–1443. [Google Scholar] [CrossRef] [PubMed]
- Knol, E.F.; Mul, F.P.; Jansen, H.; Calafat, J.; Roos, D. Monitoring Human Basophil Activation Via CD63 Monoclonal Antibody 435. Allergy Clin. Immunol. 1991, 88, 328–338. [Google Scholar] [CrossRef]
- Tordesillas, L.; Berin, M.C. Mechanisms of Oral Tolerance. Clin. Rev. Allergy Immu. 2018, 55, 107–117. [Google Scholar] [CrossRef]
- Gu, P.; Gao, J.F.; D’Souza, C.A.; Kowalczyk, A.; Chou, K.Y.; Zhang, L. Trogocytosis of CD80 and CD86 by Induced Regulatory T Cells. Cell Mol. Immunol. 2011, 11, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; Aguirre, A.; Guzman, M.A.; Gonzalez, R.; Catalan, D.; Acuna-Castillo, C.; Larrondo, M.; López, M.; Pesce, B.; Rolland, J.; et al. Tolerogenic Dendritic Cells Derived from Donors with Natural Rubber Latex Allergy Modulate Allergen-specific T-cell Responses and IgE Production. PLoS ONE 2014, 9, e85930. [Google Scholar] [CrossRef][Green Version]
- Gouw, J.W.; Jo, J.; Meulenbroek, L.A.P.M.; Heijjer, T.S.; Kremer, E.; Sandalova, E.; Knulst, A.C.; Jeurink, P.V.; Garssen, J.; Rijnierse, A.; et al. Identification of Peptides with Tolerogenic Potential in a Hydrolysed Whey-based Infant Formula. Clin. Exp. Allergy 2018, 48, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.Y.Y.; Leung, N.Y.H.; Ho, M.H.; Gershwin, L.J.; Shu, S.A.; Leung, P.S.; Chu, K.H. Immunization with Hypoallergens of Shrimp Allergen Tropomyosin Inhibits Shrimp Tropomyosin Specific IgE Reactivity. PLoS ONE 2014, 9, e111649. [Google Scholar] [CrossRef]
- Li, X.; Miyakawa, T.; Takano, T.; Nakajima-Adachi, H.; Tanokura, M.; Hachimura, S. Induction of Oral Tolerance by Pepsin-digested Gliadin Retaining T Cell Reactivity in a MMouse Model of Wheat Allergy. Int. Arch. Allergy Imm. 2020, 181, 446–455. [Google Scholar] [CrossRef]
- Liu, G.Y.; Hu, M.J.; Sun, L.C.; Han, X.Y.; Liu, Q.M.; Alcocer, M.; Fei, D.X.; Cao, M.J.; Liu, G.M. Allergenicity and Oral Tolerance of Enzymatic Cross-linked Tropomyosin Evaluated Using Cell and Mouse Models. J. Agric. Food Chem. 2017, 65, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Wu, Z.; Zhang, Y.; Li, K.; Yuan, J.; Li, X.; Yang, A.; Tong, P.; Chen, H. Polyphenol-oxidase-catalyzed Cross-linking of Ara h 2: Reaction Sites and Effect on Structure and Allergenicity. J. Sci. Food Agric. 2020, 100, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Mangsbo, S.M.; Fletcher, E.A.K.; Maren, W.W.C.V.; Redeker, A.; Cordfunke, R.A.; Dillmann, I.; Dinkelaar, J.; Ouchaou, K.; Codee, J.D.C.; Marel, G.A.V.D. Linking T Cell Epitopes to a Common Linear B cell Epitope: A Targeting and Adjuvant Strategy to Improve T Cell Responses. Mol. Immunol. 2018, 93, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Hennecke, J.; Wiley, D.C. T Cell Receptor-MHC Interactions up Close. Cell 2001, 104, 1–4. [Google Scholar] [CrossRef]
- Liu, C.; Krishna, S.S. Food Allergen Epitope Mapping. J. Agric. Food Chem. 2018, 66, 7238–7248. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Chen, W.; Zhou, P. Conformation Stability, in Vitro Digestibility and Allergenicity of Tropomyosin from Shrimp (Exopalaemon modestus) as Affected by High Intensity Ultrasound. Food Chem. 2018, 245, 997–1009. [Google Scholar] [CrossRef]
- Han, X.Y.; Yang, H.; Rao, S.T.; Liu, G.Y.; Hu, M.J.; Zeng, B.C.; Cao, M.J.; Liu, G.M. The Maillard Reaction Reduced the Sensitization of Tropomyosin and Arginine Kinase from Scylla Paramamosain, Simultaneously. J. Agric. Food Chem. 2018, 66, 2934–2943. [Google Scholar] [CrossRef]
- Liu, Q.M.; Zhou, Y.; Gao, Y.Y.; Shu, Z.D.; Zhang, J.; Liu, H.; Cao, M.J.; Liu, G.M. Degraded Porphyra Haitanensis Sulfated Polysaccharide Relieves Ovalbumin-induced Food Allergic Response by Restoring the Balance of T Helper Cell Differentiation. Food Func. 2021, 12, 4707–4719. [Google Scholar] [CrossRef]
- Wang, P.; Sidney, J.; Dow, C.; Mothe, B.; Sette, A.; Peters, B. A Systematic Assessment of MHC Class II Peptide Binding Predictions and Evaluation of a Consensus Approach. PLoS Comput. Biol. 2008, 4, e1000048. [Google Scholar] [CrossRef]
- Wang, P.; Sidney, J.; Kim, Y.; Sette, A.; Lund, O.; Nielsen, M.; Peters, B. Peptide Binding Predictions for HLA DR, DP and DQ Molecules. BMC Bioinform. 2010, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huan, F.; Li, M.S.; Han, T.J.; Xia, F.; Yang, Y.; Liu, Q.M.; Chen, G.F.; Cao, M.J.; Liu, G.M. Mapping and IgE-binding Capacity Analysis of Heat/digested Stable Epitopes of Mud Crab Allergens. Food Chem. 2021, 344, 128735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Xiao, H.; Zhang, X.F.; Zhou, P. Conformation, Allergenicity and Human Cell Allergy Sensitization of Tropomyosin from Exopalaemon modestus: Effects of Deglycosylation and Maillard reaction. Food Chem. 2019, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Denzin, L.K.; Khan, A.A.; Virdis, F.; Wilks, J.; Kane, M.; Beilinson, H.A.; Dikiy, S.; Case, L.K.; Roopenian, D.; Witkowski, M.; et al. Neutralizing Antibody Responses to Viral Infections are Linked Tothe Non-classical MHC Class II Gene H2-Ob. Immunity 2017, 47, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Rupa, P.; Nakamura, S.; Katayama, S.; Mine, Y. Effects of Ovalbumin Glycoconjugates on Alleviation of Orally Induced Egg Allergy in Mice Via Dendritic-cell Maturation and T-cell Activation. Mol. Nutr. Food Res. 2014, 58, 405–417. [Google Scholar] [CrossRef]
- Akkaya, B.; Oya, Y.; Akkaya, M.; Souz, J.A.; Holstein, A.H.; Kamenyeva, O.; Kabat, J.; Matsumura, R.; Dorward, D.W.; Glass, D.D.; et al. Regulatory T cells Mediate Specific Suppression by Depleting Peptide-MHC Class II from Dendritic Cells. Nat. Immunol. 2019, 20, 218–231. [Google Scholar] [CrossRef]
- Barthels, C.; Ogrinc, A.; Steyer, V.; Meier, S.; Simon, F.; Wimmer, M.; Blutke, A.; Straub, T.; Zimber-Strobl, U.; Lutgens, E.; et al. CD40-signalling Abrogates Induction of RORγt(+) Treg Cells by Intestinal CD103(+) DCs and Causes Fatal Colitis. Nat. Commun. 2017, 8, 1–12. [Google Scholar]
- Mueller, G.A.; Maleki, S.J.; Johnson, K.; Hurlburt, B.K.; Cheng, H.; Ruan, S.; Nesbit, J.B.; Pomés, A.; Edwards, L.L.; Schorzman, A.; et al. Identification of Maillard Reaction Products on Peanut Allergens that Influence Binding to the Receptor for Advanced Glycation End Products. Allergy 2013, 68, 1546–1554. [Google Scholar] [CrossRef]
- Bluemchen, K.; Eiwegger, T. Oral Peanut Immunotherapy How Much is too Much? How Much is Enough? Allergy 2019, 74, 220–222. [Google Scholar] [CrossRef]
- Scurlock, A.M. Oral and Sublingual Immunotherapy for Treatment of IgE-mediated Food Allergy. Clin. Rev. Allergy Immu. 2018, 55, 139–152. [Google Scholar] [CrossRef]
- Kawamoto, N.; Kaneko, H.; Kawamoto, M.; Ohnishi, H.; Matsui, E.; Teramoto, T.; Kato, Z.; Fukao, T.; Ueno, H.M.; Nakano, T.; et al. Oral Immunotherapy with Antigenicity-modified Casein Induces Desensitization in Cow’s Milk Allergy. Allergy 2020, 75, 197–200. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.-Y.; Bai, T.-L.; Yang, H.; Lin, Y.-C.; Ji, N.-R.; Wang, Y.-B.; Fu, L.-L.; Cao, M.-J.; Liu, J.-W.; Liu, G.-M. Reduction in Allergenicity and Induction of Oral Tolerance of Glycated Tropomyosin from Crab. Molecules 2022, 27, 2027. https://doi.org/10.3390/molecules27062027
Han X-Y, Bai T-L, Yang H, Lin Y-C, Ji N-R, Wang Y-B, Fu L-L, Cao M-J, Liu J-W, Liu G-M. Reduction in Allergenicity and Induction of Oral Tolerance of Glycated Tropomyosin from Crab. Molecules. 2022; 27(6):2027. https://doi.org/10.3390/molecules27062027
Chicago/Turabian StyleHan, Xin-Yu, Tian-Liang Bai, Huang Yang, Yi-Chen Lin, Nai-Ru Ji, Yan-Bo Wang, Ling-Lin Fu, Min-Jie Cao, Jing-Wen Liu, and Guang-Ming Liu. 2022. "Reduction in Allergenicity and Induction of Oral Tolerance of Glycated Tropomyosin from Crab" Molecules 27, no. 6: 2027. https://doi.org/10.3390/molecules27062027
APA StyleHan, X.-Y., Bai, T.-L., Yang, H., Lin, Y.-C., Ji, N.-R., Wang, Y.-B., Fu, L.-L., Cao, M.-J., Liu, J.-W., & Liu, G.-M. (2022). Reduction in Allergenicity and Induction of Oral Tolerance of Glycated Tropomyosin from Crab. Molecules, 27(6), 2027. https://doi.org/10.3390/molecules27062027






