Hispidulin Ameliorates Endotoxin-Induced Acute Kidney Injury in Mice
Abstract
:1. Introduction
2. Results
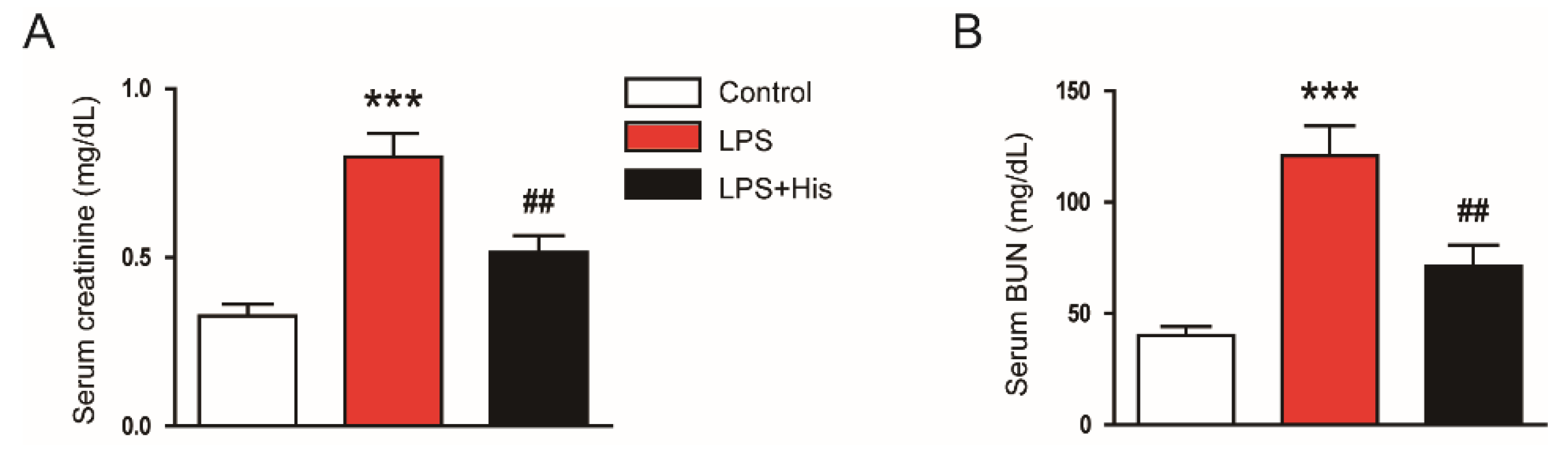
2.1. Hispidulin Ameliorated LPS-Induced Renal Dysfunction
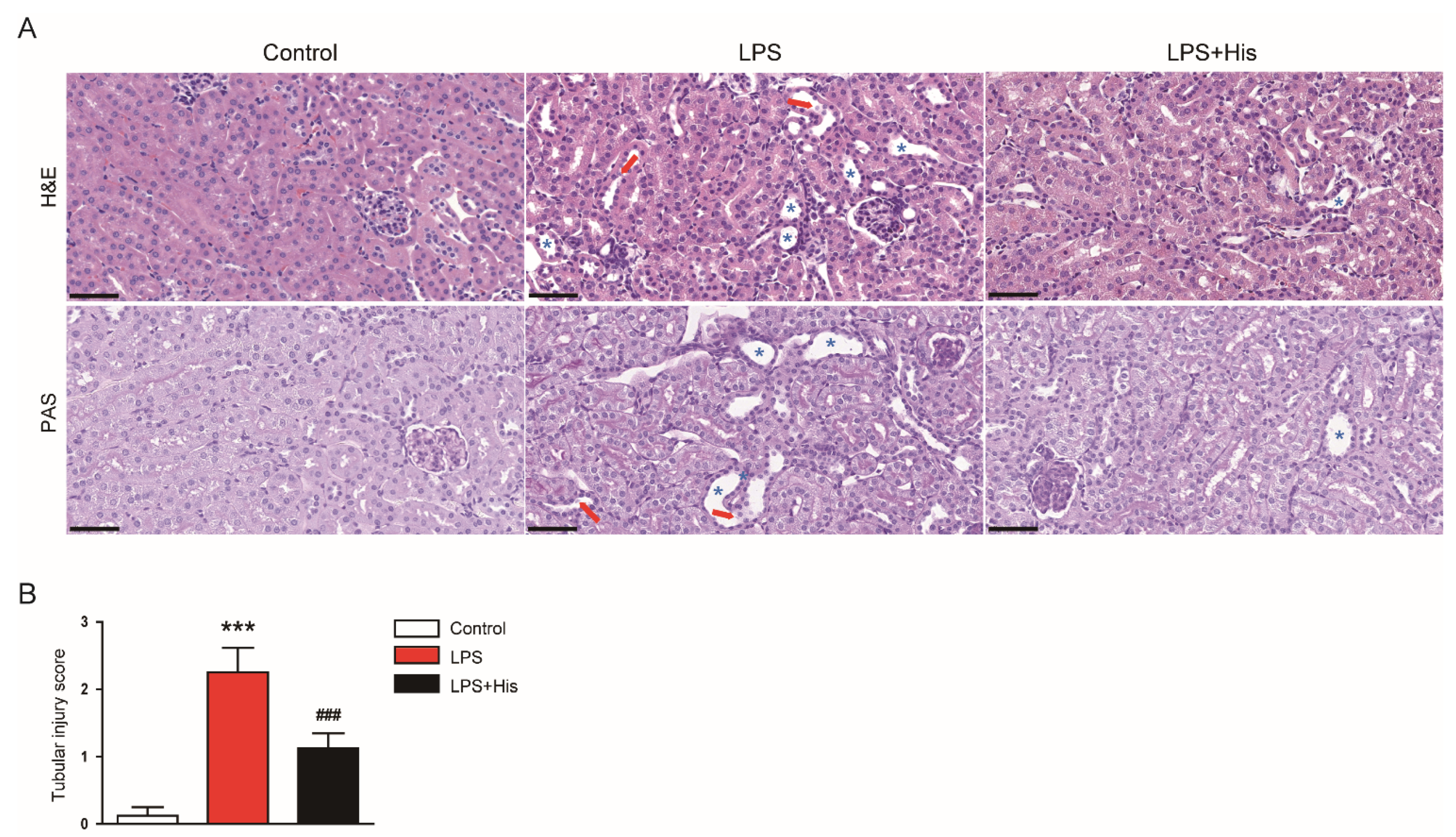
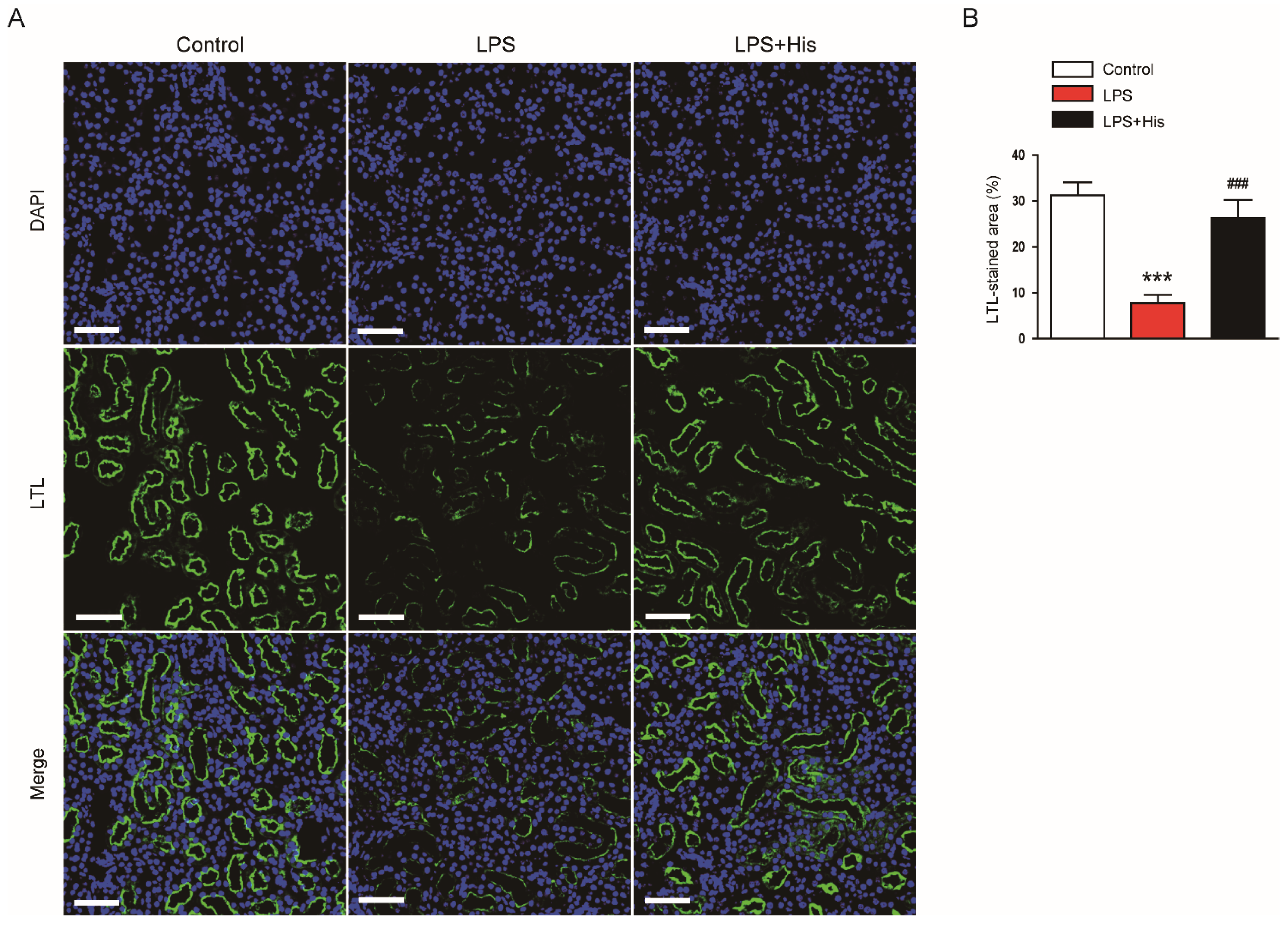
2.2. Hispidulin Alleviated LPS-Induced Histological Abnormalities
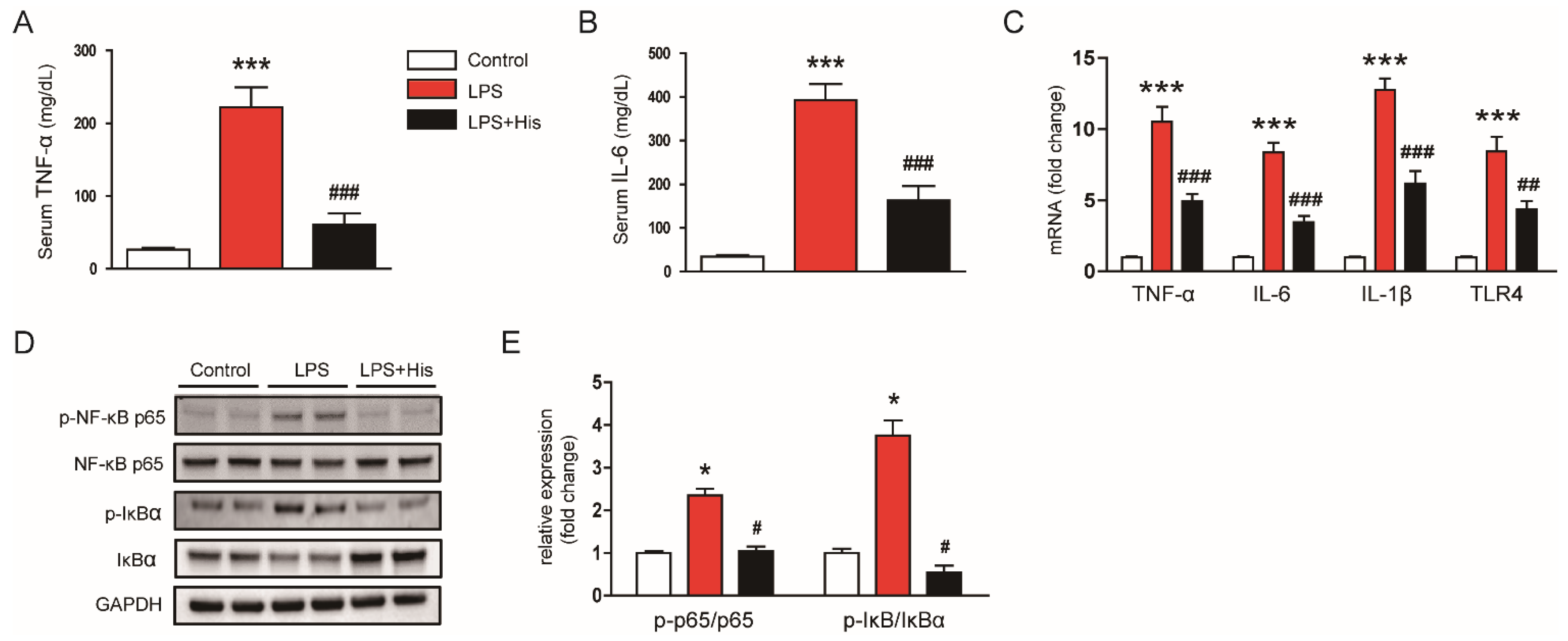
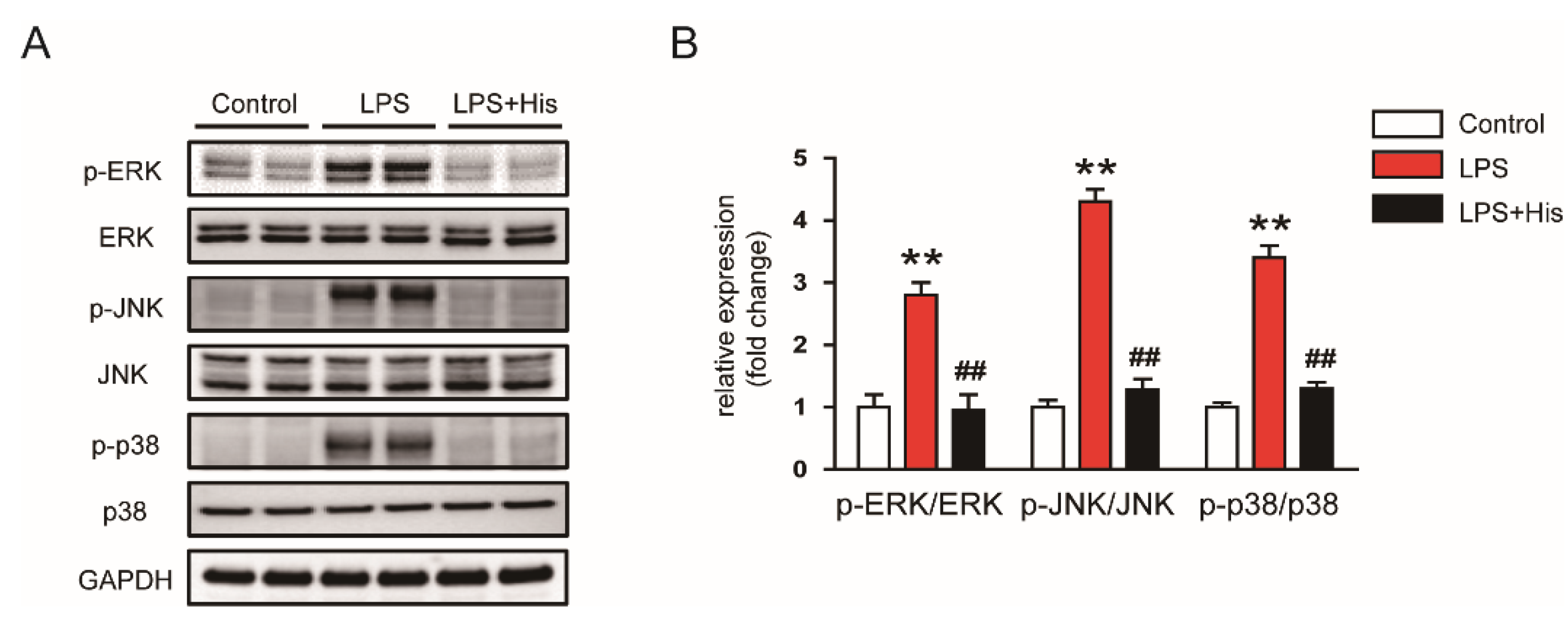
2.3. Hispidulin Inhibited LPS-Induced Inflammation
2.4. Hispidulin Suppressed LPS-Induced Oxidative Stress
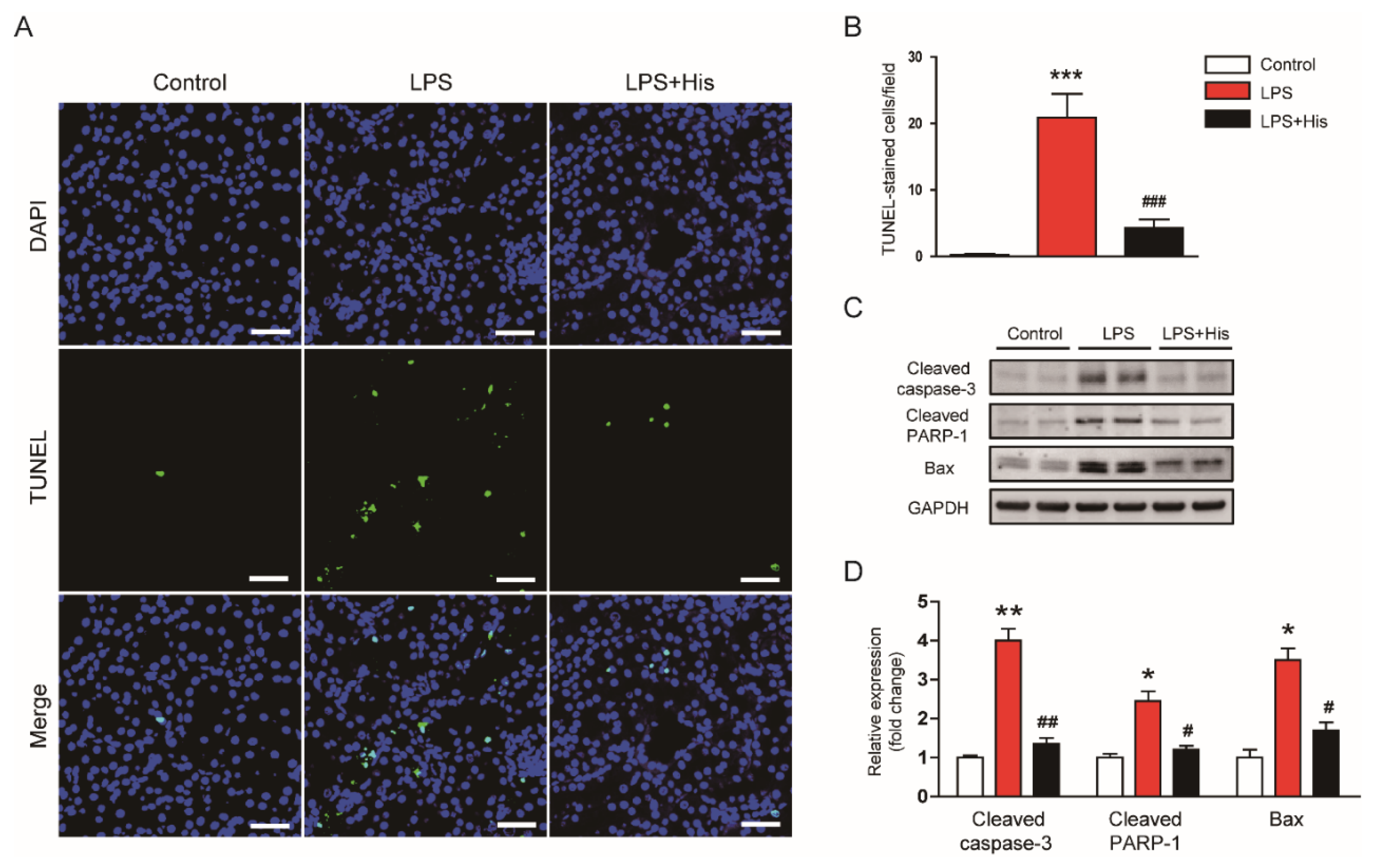
2.5. Hispidulin Attenuated LPS-Induced Apoptosis
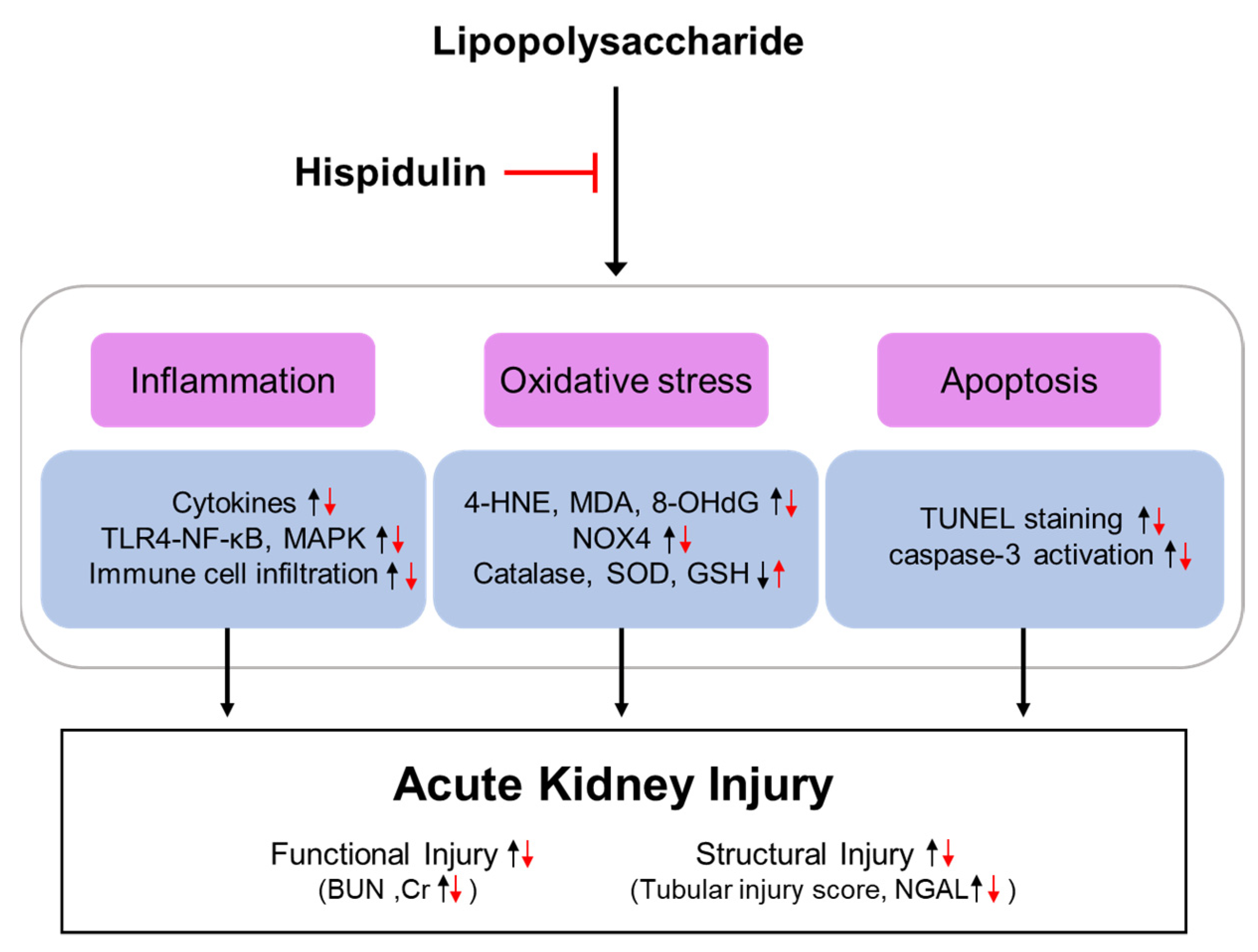
3. Discussion
4. Materials and Methods
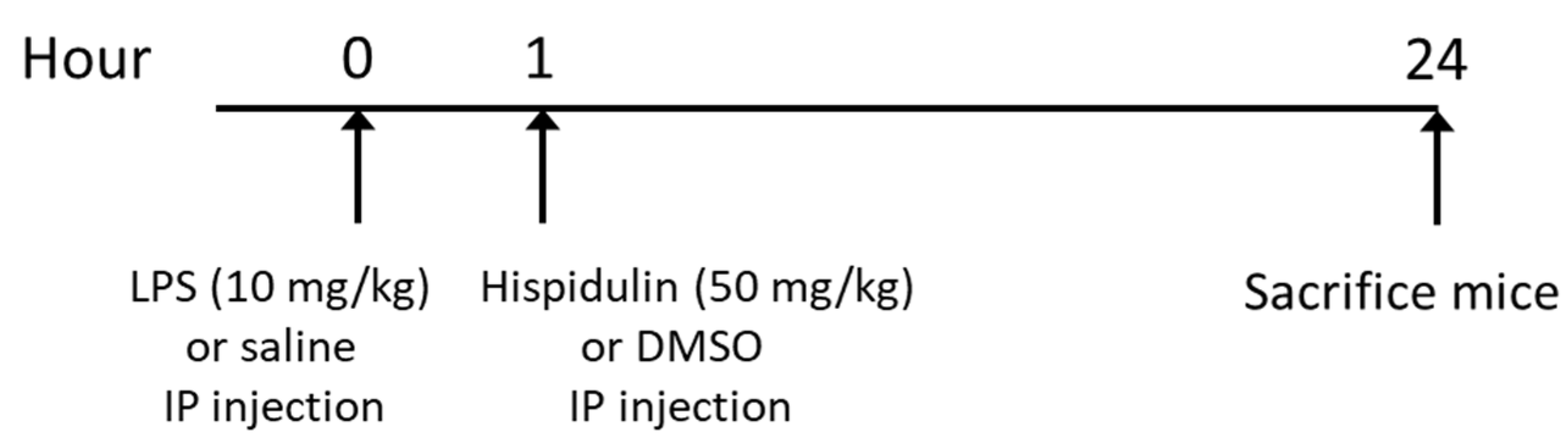
4.1. Animal Experiments
4.2. Biochemial Analyses of Serum and Kidney Tissue
4.3. Histological Examination, IHC, and IF Staining
4.4. Western Blot
4.5. Real-Time RT-PCR
4.6. TUNEL Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zarjou, A.; Agarwal, A. Sepsis and acute kidney injury. J. Am. Soc. Nephrol. 2011, 22, 999–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, K.; Nishida, O. Targeting cytokines, pathogen-associated molecular patterns, and damage-associated molecular patterns in sepsis via blood purification. Int. J. Mol. Sci. 2021, 22, 8882. [Google Scholar] [CrossRef]
- Gabarin, R.S.; Li, M.; Zimmel, P.A.; Marshall, J.C.; Li, Y.; Zhang, H. Intracellular and extracellular lipopolysaccharide signaling in sepsis: Avenues for novel therapeutic strategies. J. Innate Immun. 2021, 13, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Leelahavanichkul, A.; Yuen, P.S.T.; Star, R.A. Animal models of sepsis and sepsis-induced kidney injury. J. Clin. Investig. 2009, 119, 2868–2878. [Google Scholar] [CrossRef] [Green Version]
- Hato, T.; Zollman, A.; Plotkin, Z.; El-Achkar, T.M.; Maier, B.F.; Pay, S.L.; Dube, S.; Cabral, P.; Yoshimoto, M.; McClintick, J.; et al. Endotoxin preconditioning reprograms s1 tubules and macrophages to protect the kidney. J. Am. Soc. Nephrol. 2018, 29, 104–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wu, J.; Hu, Z.; Luo, C.; Wang, P.; Zhang, Y.; Li, H. Dexmedetomidine alleviates lipopolysaccharide-induced acute kidney injury by inhibiting p75NTR-mediated oxidative stress and apoptosis. Oxid. Med. Cell. Longev. 2020, 2020, 5454210. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zheng, Y.; Huang, J.; Peng, W.; Chen, X.; Kang, X.; Zeng, Q. UCP2 ameliorates mitochondrial dysfunction, inflammation, and oxidative stress in lipopolysaccharide-induced acute kidney injury. Int. Immunopharmacol. 2019, 71, 336–349. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Fan, X.; Fan, Z.; Yang, Z.; Huang, T.; Tong, Y.; Yang, D.; Mao, X.; Yang, M. Flavonoids—Natural gifts to promote health and longevity. Int. J. Mol. Sci. 2022, 23, 2176. [Google Scholar] [CrossRef]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed. Pharmacother. 2020, 122, 109772. [Google Scholar] [CrossRef] [PubMed]
- Khajevand-Khazaei, M.; Mohseni-Moghaddam, P.; Hosseini, M.; Gholami, L.; Baluchnejadmojarad, T.; Roghani, M. Rutin, a quercetin glycoside, alleviates acute endotoxemic kidney injury in C57BL/6 mice via suppression of inflammation and up-regulation of antioxidants and SIRT1. Eur. J. Pharmacol. 2018, 833, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Fu, Y.; Lin, H. Baicalin inhibits renal cell apoptosis and protects against acute kidney injury in pediatric sepsis. Med. Sci. Monit. 2016, 22, 5109–5115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atif, M.; Ali, I.; Hussain, A.; Hyder, S.V.; Niaz, B.; Khan, F.A.; Maalik, A.; Farooq, U. Pharmacological assessment of hispidulin--a natural bioactive flavone. Acta Pol. Pharm. 2015, 72, 829–842. [Google Scholar]
- Patel, K.; Patel, D.K. Medicinal importance, pharmacological activities, and analytical aspects of hispidulin: A concise report. J. Tradit. Complement Med. 2016, 7, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Lee, S.; Kim, N.; Dhakal, H.; Choi, Y.-A.; Kwon, T.K.; Khang, D.; Kim, S.-H. Hispidulin alleviates 2,4-dinitrochlorobenzene and house dust mite extract-induced atopic dermatitis-like skin inflammation. Biomed. Pharmacother. 2021, 137, 111359. [Google Scholar] [CrossRef]
- Kim, N.; Lee, S.; Kang, J.; Choi, Y.-A.; Lee, B.; Kwon, T.K.; Jang, Y.H.; Kim, S.-H. Hispidulin alleviates imiquimod-induced psoriasis-like skin inflammation by inhibiting splenic Th1/Th17 cell population and keratinocyte activation. Int. Immunopharmacol. 2020, 87, 106767. [Google Scholar] [CrossRef]
- An, P.; Xie, J.; Qiu, S.; Liu, Y.; Wang, J.; Xiu, X.; Li, L.; Tang, M. Hispidulin exhibits neuroprotective activities against cerebral ischemia reperfusion injury through suppressing NLRP3-mediated pyroptosis. Life Sci. 2019, 232, 116599. [Google Scholar] [CrossRef]
- An, P.; Wu, T.; Yu, H.; Fang, K.; Ren, Z.; Tang, M. Hispidulin protects against focal cerebral ischemia reperfusion injury in rats. J. Mol. Neurosci. 2018, 65, 203–212. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Z.; Jiang, N.; Wu, Z.; Xue, R.; Dong, B.; Fan, W.; Dai, G.; Chen, C.; Li, J.; et al. Hispidulin attenuates cardiac hypertrophy by improving mitochondrial dysfunction. Front. Cardiovasc. Med. 2020, 7, 582890. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, Z.; Ma, C. Hispidulin exerts anti-osteoporotic activity in ovariectomized mice via activating AMPK signaling pathway. Cell Biochem. Biophys. 2014, 69, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Nepal, M.; Choi, H.J.; Choi, B.-Y.; Yang, M.-S.; Chae, J.-I.; Li, L.; Soh, Y. Hispidulin attenuates bone resorption and osteoclastogenesis via the RANKL-induced NF-κB and NFATc1 pathways. Eur. J. Pharmacol. 2013, 715, 96–104. [Google Scholar] [CrossRef]
- Ferrándiz, M.L.; Bustos, G.; Payá, M.; Gunasegaran, R.; Alcaraz, M.J. Hispidulin protection against hepatotoxicity induced by bromobenzene in mice. Life Sci. 1994, 55, PL145–PL150. [Google Scholar] [PubMed]
- Yu, C.-I.; Cheng, C.-I.; Kang, Y.-F.; Chang, P.-C.; Lin, I.-P.; Kuo, Y.-H.; Jhou, A.-J.; Lin, M.-Y.; Chen, C.-Y.; Lee, C.-H. Hispidulin inhibits neuroinflammation in lipopolysaccharide-activated BV2 microglia and attenuates the activation of Akt, NF-κB, and STAT3 pathway. Neurotox. Res. 2020, 38, 163–174. [Google Scholar] [CrossRef]
- Gwon, M.-G.; Gu, H.; Leem, J.; Park, K.-K. Protective effects of 6-shogaol, an active compound of ginger, in a murine model of cisplatin-induced acute kidney injury. Molecules 2021, 26, 5931. [Google Scholar] [PubMed]
- Kim, J.-Y.; Hong, H.-L.; Kim, G.M.; Leem, J.; Kwon, H.H. Protective effects of carnosic acid on lipopolysaccharide-induced acute kidney injury in mice. Molecules 2021, 26, 7589. [Google Scholar] [CrossRef]
- Pak, E.S.; Uddin, M.J.; Ha, H. Inhibition of Src family kinases ameliorates LPS-induced acute kidney injury and mitochondrial dysfunction in mice. Int. J. Mol. Sci. 2020, 21, 8246. [Google Scholar]
- Kim, J.-Y.; Jo, J.; Leem, J.; Park, K.-K. Kahweol ameliorates cisplatin-induced acute kidney injury through pleiotropic effects in mice. Biomedicines 2020, 8, 572. [Google Scholar] [CrossRef]
- Hu, W.; Gao, W.; Miao, J.; Xu, Z.; Sun, L. Alamandine, a derivative of angiotensin-(1-7), alleviates sepsis-associated renal inflammation and apoptosis by inhibiting the PI3K/Ak and MAPK pathways. Peptides 2021, 146, 170627. [Google Scholar]
- Sun, S.; Wang, J.; Wang, J.; Wang, F.; Yao, S.; Xia, H. Maresin 1 mitigates sepsis-associated acute kidney injury in mice via inhibition of the NF-κB/STAT3/MAPK pathways. Front. Pharmacol. 2019, 10, 1323. [Google Scholar] [PubMed] [Green Version]
- Kim, J.-Y.; Jo, J.; Leem, J.; Park, K.-K. Inhibition of p300 by garcinol protects against cisplatin-induced acute kidney injury through suppression of oxidative stress, inflammation, and tubular cell death in mice. Antioxidants 2020, 9, 1271. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Kawanaka, H.; Hori, M.; Kusamori, K.; Utsumi, D.; Tsukahara, T.; Amagase, K.; Horie, S.; Yamamoto, A.; Ozaki, H.; et al. Role of transient receptor potential melastatin 2 in surgical inflammation and dysmotility in a mouse model of postoperative ileus. Am. J. Physiol. Gastrointestinal. Liver Physiol. 2018, 315, G104–G116. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Jang, H.-J.; Leem, J.; Kim, G.-M. Protective effects of bee venom-derived phospholipase A2 against cholestatic liver disease in mice. Biomedicines 2021, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; Escrivá, C.; Dromant, M.; Borrás, C.; Viña, J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch. Biochem. Biophys. 2021, 709, 108941. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Leem, J.; Jeon, E.J. Protective effects of melatonin against aristolochic acid-induced nephropathy in mice. Biomolecules 2020, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Jee, S.-C.; Kim, M.; Kim, K.S.; Kim, H.-S.; Sung, J.-S. Protective effects of myricetin on benzo[a]pyrene-induced 8-hydroxy-2′-deoxyguanosine and BPDE-DNA adduct. Antioxidants 2020, 9, 446. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Choi, Y.; Leem, J.; Song, J.E. Heme oxygenase-1 induction by cobalt protoporphyrin ameliorates cholestatic liver disease in a xenobiotic-induced murine model. Int. J. Mol. Sci. 2021, 22, 8253. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Wu, M.; Liu, X.; Zhu, Y.; Zhu, J.; Xing, C. Humanized anti-TLR4 monoclonal antibody ameliorates lipopolysaccharide-related acute kidney injury by inhibiting TLR4/NF-κB signaling. Mol. Med. Rep. 2021, 24, 608. [Google Scholar] [CrossRef]
- Cai, B.; Wang, M.; Zhu, X.; Xu, J.; Zheng, W.; Zhang, Y.; Zheng, F.; Feng, Z.; Zhu, J. The fab fragment of a humanized anti-toll like receptor 4 (TLR4) monoclonal antibody reduces the lipopolysaccharide response via TLR4 in mouse macrophage. Int. J. Mol. Sci. 2015, 16, 25502–25515. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-Y.; Leem, J.; Hong, H.-L. Protective effects of SPA0355, a thiourea analogue, against lipopolysaccharide-induced acute kidney injury in mice. Antioxidants 2020, 9, 585. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Leem, J.; Park, K.-K. Antioxidative, antiapoptotic, and anti-inflammatory effects of apamin in a murine model of lipopolysaccharide-induced acute kidney injury. Molecules 2020, 25, 5717. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.R.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Microglia: Agents of the CNS pro-inflammatory response. Cells 2020, 9, 1717. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Wang, J.-X.; Parisini, E.; Dascher, C.C.; Nigrovic, P.A. Ly6 family proteins in neutrophil biology. J. Leukoc. Biol. 2013, 94, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Hwangbo, H.; Kim, M.Y.; Ji, S.Y.; Kim, S.Y.; Lee, H.; Kim, G.-Y.; Park, C.; Keum, Y.-S.; Hong, S.H.; Cheong, J.; et al. Auranofin attenuates non-alcoholic fatty liver disease by suppressing lipid accumulation and NLRP3 inflammasome-mediated hepatic inflammation in vivo and in vitro. Antioxidants 2020, 9, 1040. [Google Scholar] [CrossRef]
- Watson, A.M.D.; Olukman, M.; Koulis, C.; Tu, Y.; Samijono, D.; Yeun, D.; Lee, C.; Behm, D.J.; Cooper, M.E.; Jandeleit-Dahm, K.A.M.; et al. Urotensin II receptor antagonism confers vasoprotective effects in diabetes associated atherosclerosis: Studies in humans and in a mouse model of diabetes. Diabetologia 2013, 56, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Ow, C.P.C.; Trask-Marino, A.; Betrie, A.H.; Evans, R.G.; May, C.N.; Lankadeva, Y.R. Targeting oxidative stress in septic acute kidney injury: From theory to practice. J. Clin. Med. 2021, 10, 3798. [Google Scholar] [CrossRef] [PubMed]
- Kockara, A.; Kayatas, M. Renal cell apoptosis and new treatment options in sepsis-induced acute kidney injury. Ren. Fail. 2013, 35, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Kut, K.; Bartosz, G.; Soszyński, M.; Sadowska-Bartosz, I. Antioxidant properties of hispidulin. Nat. Prod. Res. 2022, 1–4. [Google Scholar] [CrossRef]
- Wang, C.; Liu, T.; Tong, Y.; Cui, R.; Qu, K.; Liu, C.; Zhang, J. Ulinastatin protects against acetaminophen-induced liver injury by alleviating ferroptosis via the SIRT1/NRF2/HO-1 pathway. Am. J. Transl. Res. 2021, 13, 6031–6042. [Google Scholar]
- Venkatachalam, K.; Prabhu, S.D.; Reddy, V.S.; Boylston, W.H.; Valente, A.J.; Chandrasekar, B. Neutralization of interleukin-18 ameliorates ischemia/reperfusion-induced myocardial injury. J. Biol. Chem. 2009, 284, 7853–7865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almatroodi, S.A.; Almatroudi, A.; Alsahli, M.A.; Aljasir, M.A.; Syed, M.A.; Rahmani, A.H. Epigallocatechin-3-Gallate (EGCG), an active compound of green tea attenuates acute lung injury regulating macrophage polarization and Krüpple-like-factor 4 (KLF4) expression. Molecules 2020, 25, 2853. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-X.; Qiu, G.; Cheng, F.-R.; Pei, Z.; Yang, Z.; Deng, X.-H.; Zhu, J.-H.; Chen, L.; Chen, C.-C.; Lin, W.-F.; et al. Berberine protects secondary injury in mice with traumatic brain injury through anti-oxidative and anti-inflammatory modulation. Neurochem. Res. 2018, 43, 1814–1825. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Cha, D.R.; Kim, B.; An, E.J.; Lee, S.R.; Cha, J.J.; Kang, Y.S.; Ghee, J.Y.; Han, J.Y.; Bae, Y.S. LPS-induced acute kidney injury is mediated by Nox4-SH3YL1. Cell Rep. 2020, 33, 108245. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.J.; Ferrandiz, M.L.; Cejudo, M.; Terencio, M.C.; Gil, B.; Bustos, G.; Ubeda, A.; Gunasegaran, R.; Alcaraz, M.J. Influence of a series of natural flavonoids on free radical generating systems and oxidative stress. Xenobiotica 1994, 24, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.A.; Park, C.W. Catalytic Antioxidants in the Kidney. Antioxidants 2021, 10, 130. [Google Scholar] [CrossRef]
- Guerrero-Hue, M.; Rayego-Mateos, S.; Vázquez-Carballo, C.; Palomino-Antolín, A.; García-Caballero, C.; Opazo-Rios, L.; Morgado-Pascual, J.L.; Herencia, C.; Mas, S.; Ortiz, A.; et al. Protective role of Nrf2 in renal disease. Antioxidants 2021, 10, 39. [Google Scholar] [CrossRef]
- Feng, L.-X.; Zhao, F.; Liu, Q.; Peng, J.-C.; Duan, X.-J.; Yan, P.; Wu, X.; Wang, H.-S.; Deng, Y.-H.; Duan, S.-B. Role of Nrf2 in lipopolysaccharide-induced acute kidney injury: Protection by human umbilical cord blood mononuclear cells. Oxid. Med. Cell. Longev. 2020, 2020, 6123459. [Google Scholar] [CrossRef]
- Zhou, K.; Xie, M.; Yi, S.; Tang, Y.; Luo, H.; Xiao, Q.; Xiao, J.; Li, Y. Dimethyl fumarate ameliorates endotoxin-induced acute kidney injury against macrophage oxidative stress. Ren. Fail. 2021, 43, 1229–1239. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Y.; Wang, H.; Zhang, J.; Xie, L.; Huo, Y.; Ma, W.; Li, H.; Chen, X.; Shi, P. The role of miR-199b-3p in regulating Nrf2 pathway by dihydromyricetin to alleviate septic acute kidney injury. Free Radic. Res. 2021, 55, 842–852. [Google Scholar] [CrossRef]
- Huang, L.; Huang, K.; Ning, H. Hispidulin prevents sevoflurane—Induced memory dysfunction in aged rats. Biomed. Pharmacother. 2018, 97, 412–422. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Lohakul, J.; Soontrapa, K.; Sampattavanich, S.; Akarasereenont, P.; Panich, U. Activation of Nrf2 reduces UVA-mediated MMP-1 upregulation via MAPK/AP-1 signaling cascades: The photoprotective effects of sulforaphane and hispidulin. J. Pharmacol. Exp. Ther. 2017, 360, 388–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Li, S.; Zhang, N.; Huang, W.; Li, X.; Wang, M.; Bai, D.; Han, B. Hispidulin alleviates high-glucose-induced podocyte injury by regulating protective autophagy. Biomed. Pharmacother. 2018, 104, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Huang, K.; Ning, H. Autophagy induction by hispidulin provides protection against sevoflurane-induced neuronal apoptosis in aged rats. Biomed. Pharmacother. 2018, 98, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Ashaq, A.; Maqbool, M.F.; Maryam, A.; Khan, M.; Shakir, H.A.; Irfan, M.; Qazi, J.I.; Li, Y.; Ma, T. Hispidulin: A novel natural compound with therapeutic potential against human cancers. Phytother. Res. 2021, 35, 771–789. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Huang, W.J.; Lee, H.J.; Chen, H.L.; Fan, P.C.; Ku, Y.L.; Chiou, L.C. Hispidulin, a constituent of Clerodendrum inerme that remitted motor tics, alleviated methamphetamine-induced hyperlocomotion without motor impairment in mice. J. Ethnopharmacol. 2015, 166, 18–22. [Google Scholar] [CrossRef]
- Lin, T.Y.; Lu, C.W.; Wang, S.J.; Huang, S.K. Protective effect of hispidulin on kainic acid-induced seizures and neurotoxicity in rats. Eur. J. Pharmacol. 2015, 755, 6–15. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Jo, J.; Kim, K.; An, H.-J.; Gwon, M.-G.; Gu, H.; Kim, H.-J.; Yang, A.Y.; Kim, S.-W.; Jeon, E.J.; et al. Pharmacological activation of Sirt1 ameliorates cisplatin-induced acute kidney injury by suppressing apoptosis, oxidative stress, and inflammation in mice. Antioxidants 2019, 8, 322. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Dai, Y.; Song, K.; Huang, Y.; Zhang, L.; Zhang, C.; Yan, Q.; Gao, H. Pre-emptive pharmacological inhibition of fatty acid-binding protein 4 attenuates kidney fibrosis by reprogramming tubular lipid metabolism. Cell Death Dis. 2021, 12, 572. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Leem, J.; Hong, H.L. Melittin ameliorates endotoxin-induced acute kidney injury by inhibiting inflammation, oxidative stress, and cell death in mice. Oxid. Med. Cell. Longev. 2021, 2021, 8843051. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Park, J.-H.; Jeon, E.J.; Leem, J.; Park, K.-K. Melatonin prevents transforming growth factor-β1-stimulated transdifferentiation of renal interstitial fibroblasts to myofibroblasts by suppressing reactive oxygen species-dependent mechanisms. Antioxidants 2020, 9, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Gene | Primer Sequence (5′→3′) | Accession No. |
|---|---|---|
| TNF-α | Forward: CACAGAAAGCATGATCCGCGACGT Reverse: CGGCAGAGAGGAGGTTGACTTTCT | NM_013693 |
| IL-6 | Forward: TAGTCCTTCCTACCCCAATTTCC Reverse: TTGGTCCTTAGCCACTCCTTC | NM_031168 |
| IL-1β | Forward: CGCAGCAGCACATCAACAAGAGC Reverse: TGTCCTCATCCTGGAAGGTCCACG | NM_008361 |
| TLR4 | Forward: CCTGACACCAGGAAGCTTGAA Reverse: TCTGATCCATGCATTGGTAGGT | NM_021297 |
| NOX4 | Forward: CCCAAGTTCCAAGCTCATTTCC Reverse: TGGTGACAGGTTTGTTGCTCCT | NM_015760 |
| GAPDH | Forward: ACTCCACTCACGGCAAATTC Reverse: TCTCCATGGTGGTGAAGACA | NM_001289726 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Leem, J. Hispidulin Ameliorates Endotoxin-Induced Acute Kidney Injury in Mice. Molecules 2022, 27, 2019. https://doi.org/10.3390/molecules27062019
Kim K, Leem J. Hispidulin Ameliorates Endotoxin-Induced Acute Kidney Injury in Mice. Molecules. 2022; 27(6):2019. https://doi.org/10.3390/molecules27062019
Chicago/Turabian StyleKim, Kiryeong, and Jaechan Leem. 2022. "Hispidulin Ameliorates Endotoxin-Induced Acute Kidney Injury in Mice" Molecules 27, no. 6: 2019. https://doi.org/10.3390/molecules27062019
APA StyleKim, K., & Leem, J. (2022). Hispidulin Ameliorates Endotoxin-Induced Acute Kidney Injury in Mice. Molecules, 27(6), 2019. https://doi.org/10.3390/molecules27062019






