Rutin Inhibits Ox-LDL-Mediated Macrophage Inflammation and Foam Cell Formation by Inducing Autophagy and Modulating PI3K/ATK Signaling
Abstract
:1. Introduction
2. Results
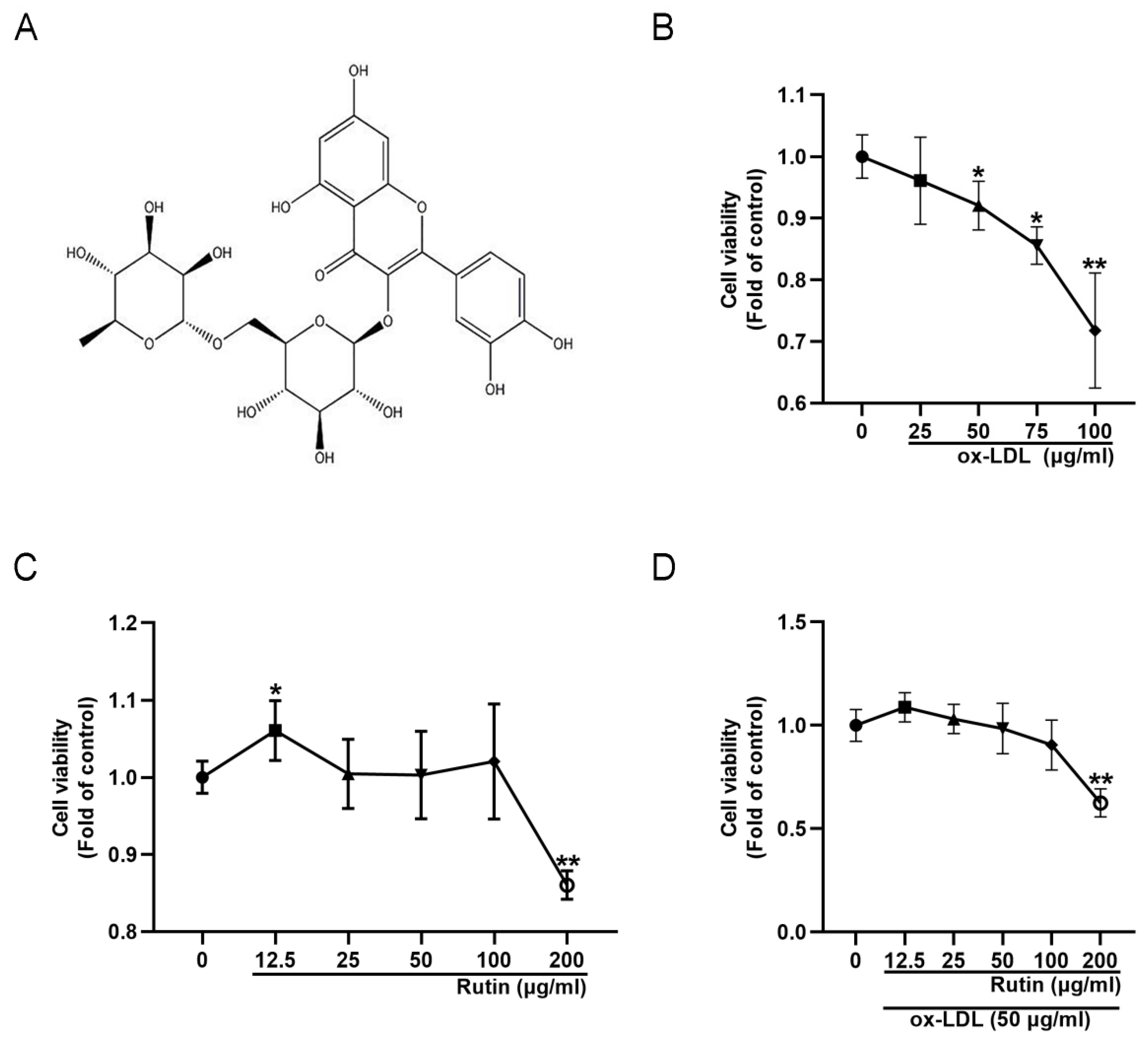
2.1. Rutin Can Improve the Viability of RAW264.7 Cells
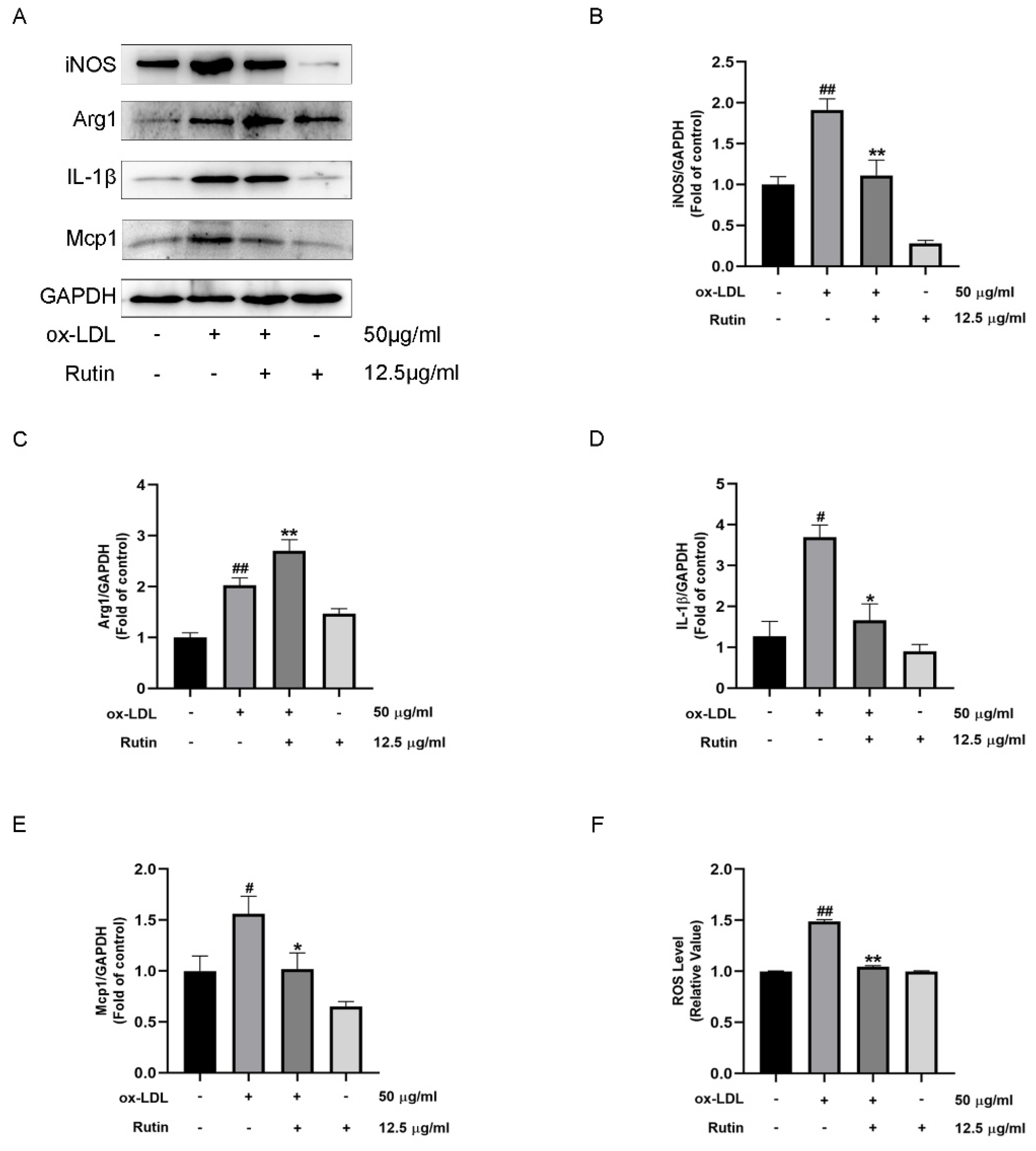
2.2. Rutin Can Inhibit Macrophage Inflammation
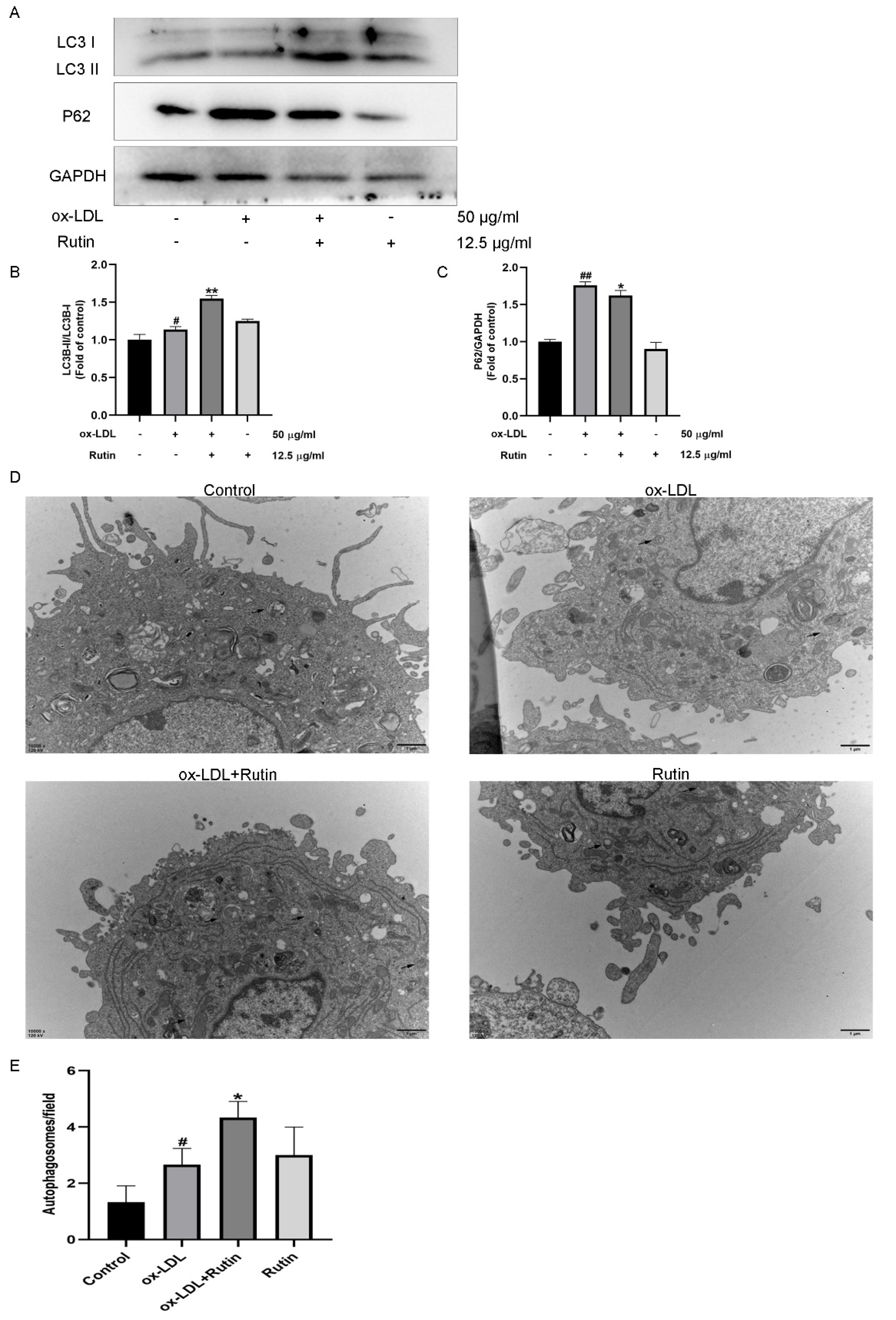
2.3. Rutin Can Promote Autophagy in Macrophages
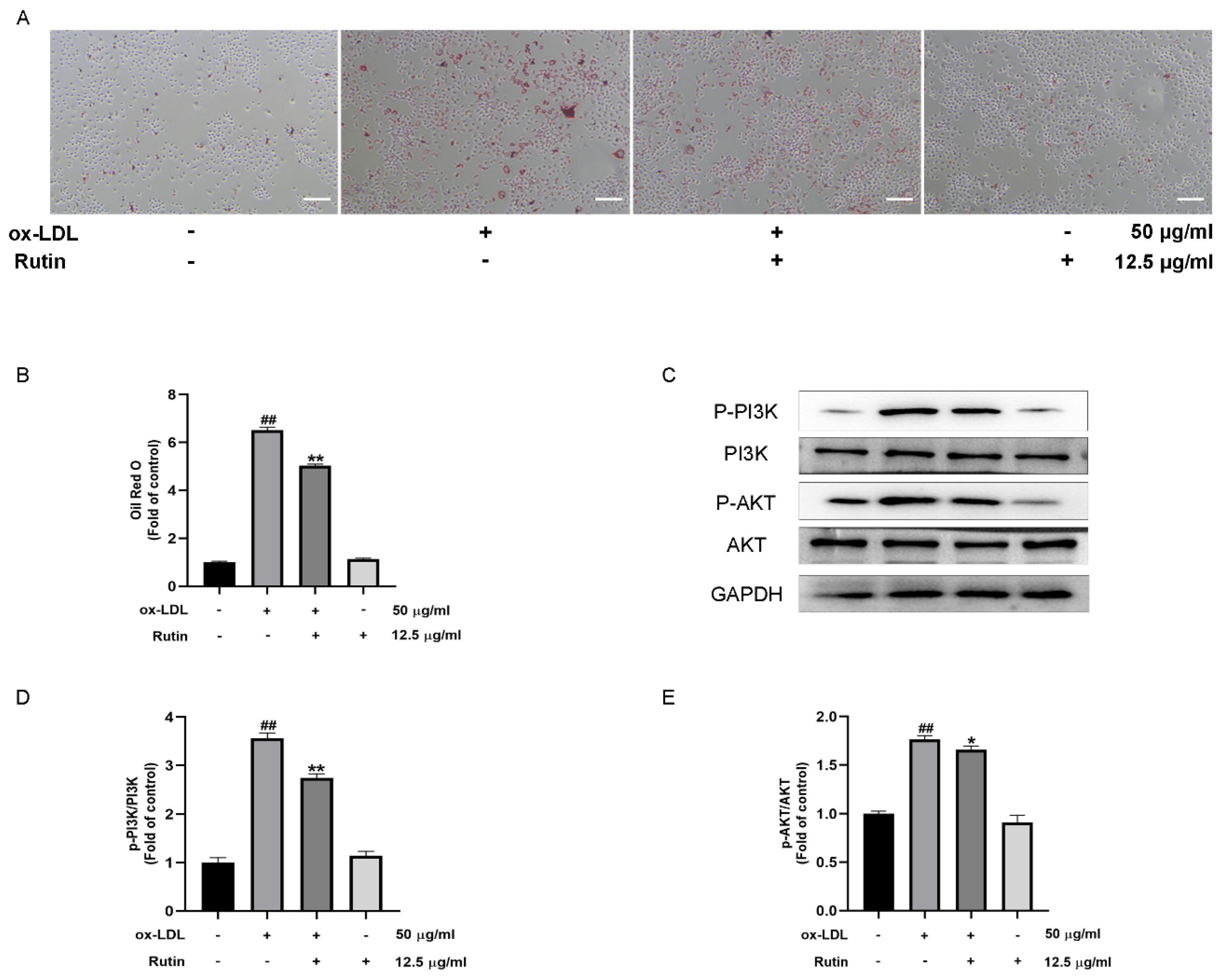
2.4. Rutin Can Inhibit the Production of Foam Cells Induced by ox-LDL
2.5. Rutin Can Inhibit the PI3K/AKT Signaling Pathway
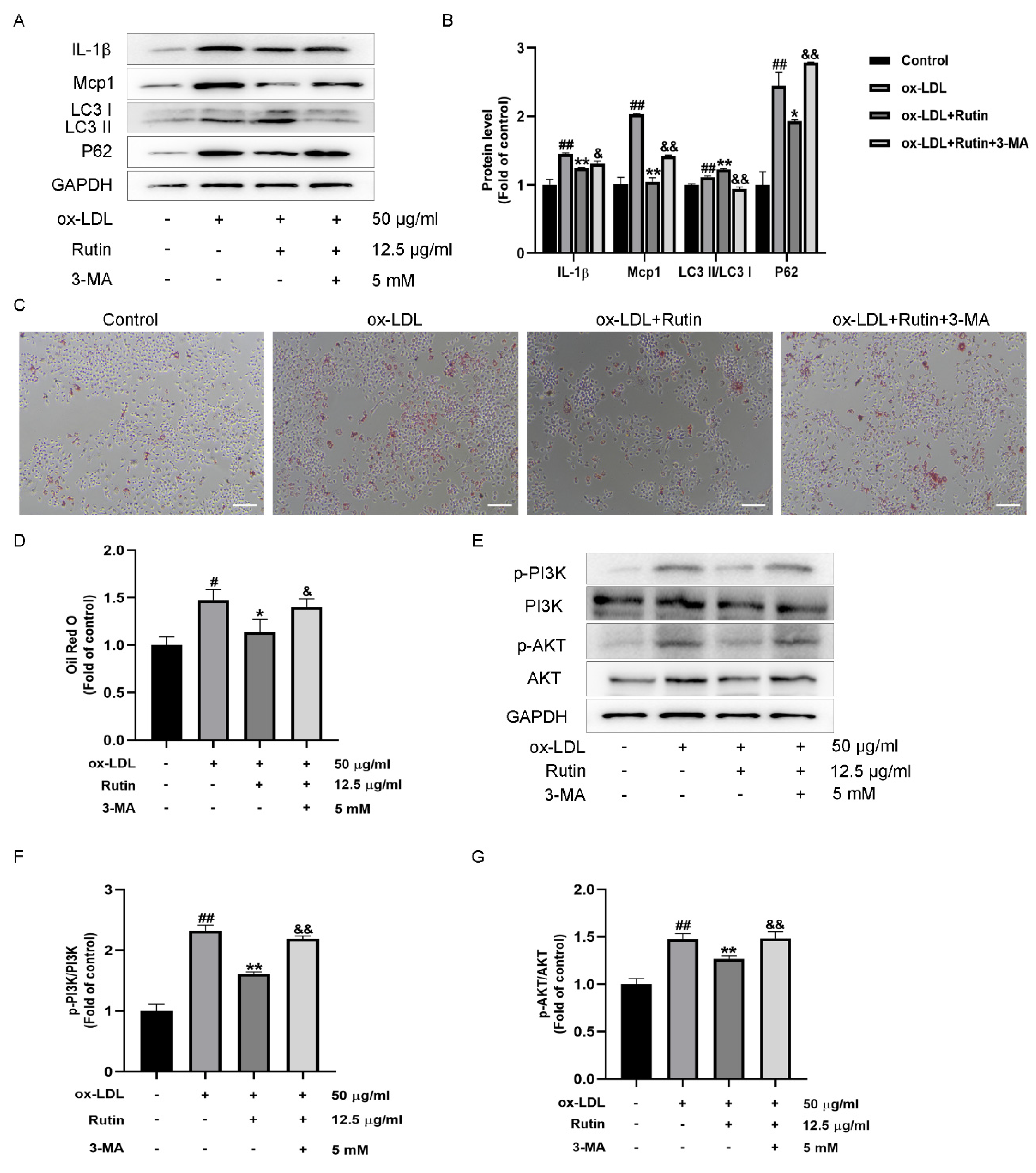
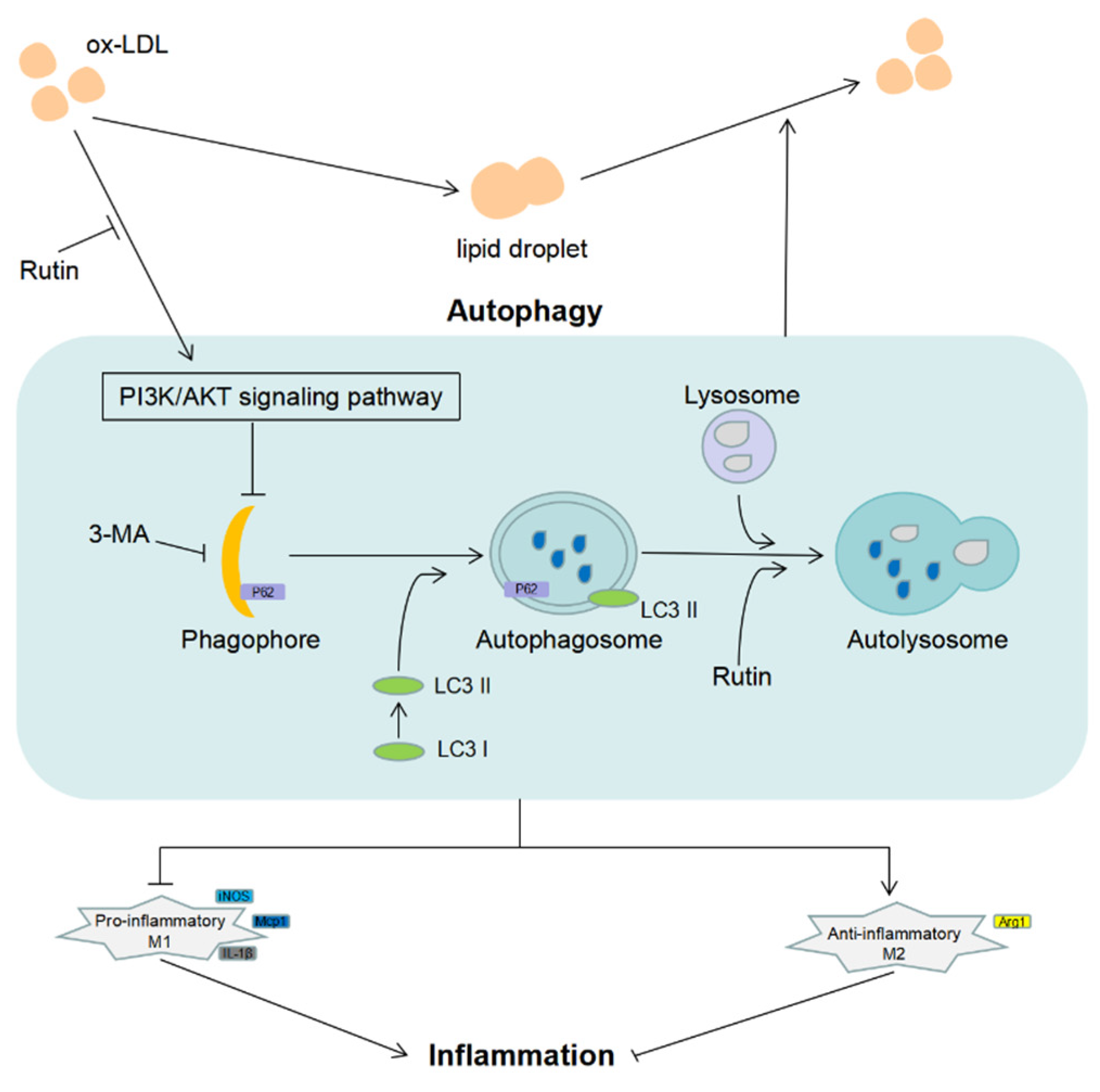
2.6. Rutin Can Reduce Macrophage Inflammation and Foam Cell Production via Modulating PI3K/ATK Signaling and Activating Autophagy
3. Discussion
4. Materials and Methods
4.1. Cells and Reagents
4.2. Cell Culture
4.3. Cell Counting Kit 8 Assays
4.4. Western Blot Analysis
4.5. Reactive Oxygen Species Assay of Cells
4.6. Oil Red O Staining
4.7. Transmission Electron Microscopy (TEM)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Atherosclerosis |
| CCK-8 | Cell Counting Kit 8 |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal Bovine Serum |
| OSO4 | Osmium (VIII) oxide |
| ox-LDL | Oxygenated Low-density Lipoprotein |
| ROS | Reactive Oxygen Species |
| TEM | Transmission Electron Microscopy |
| 3-MA | 3-Methyladenine |
References
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Pedro-Botet, J.; Climent, E.; Benaiges, D. Atherosclerosis and inflammation. New therapeutic approaches. Med. Clin. 2020, 155, 256–262. [Google Scholar] [CrossRef]
- Maguire, E.M.; Pearce, S.W.A.; Xiao, Q. Foam cell formation: A new target for fighting atherosclerosis and cardiovascular disease. Vascul. Pharmacol. 2019, 112, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Ren, P.; Zhang, Y.; Li, S.; Li, M.; Li, P.; Shang, J.; Liu, W.; Liu, H. Shen-Yuan-Dan Capsule Attenuates Atherosclerosis and Foam Cell Formation by Enhancing Autophagy and Inhibiting the PI3K/Akt/mTORC1 Signaling Pathway. Front. Pharmacol. 2019, 10, 603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qin, Y.; Wan, X.; Liu, H.; Lv, C.; Ruan, W.; He, L.; Lu, L.; Guo, X. Rosuvastatin exerts anti-atherosclerotic effects by improving macrophage-related foam cell formation and polarization conversion via mediating autophagic activities. J. Transl. Med. 2021, 19, 62. [Google Scholar] [CrossRef]
- Miao, J.; Zang, X.; Cui, X.; Zhang, J. Autophagy, Hyperlipidemia, and Atherosclerosis. Adv. Exp. Med. Biol. 2020, 1207, 237–264. [Google Scholar]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, L.; Ma, J.; Lu, L.; Wang, X.; Ren, J.; Yang, J. Rutin attenuates doxorubicin-induced cardiotoxicity via regulating autophagy and apoptosis. Biochim. Biophys. Acta Mol. Basis. Dis. 2017, 1863, 1904–1911. [Google Scholar] [CrossRef]
- Li, Y.; Qin, R.; Yan, H.; Wang, F.; Huang, S.; Zhang, Y.; Zhong, M.; Zhang, W.; Wang, Z. Inhibition of vascular smooth muscle cells premature senescence with rutin attenuates and stabilizes diabetic atherosclerosis. J. Nutr. Biochem. 2018, 51, 91–98. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, X.; Liu, X.; Du, H.; Sun, C.; Shao, X.; Tian, J.; Gu, X.; Wang, H.; Tian, J.; et al. Adipose-Derived Exosomes Exert Proatherogenic Effects by Regulating Macrophage Foam Cell Formation and Polarization. J. Am. Heart Assoc. 2018, 7, e007442. [Google Scholar] [CrossRef]
- Geng, J.; Yang, C.; Wang, B.; Zhang, X.; Hu, T.; Gu, Y.; Li, J. Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomed. Pharmacother. 2018, 97, 941–947. [Google Scholar] [CrossRef]
- Lu, S.; Luo, Y.; Sun, G.; Sun, X. Ginsenoside Compound K Attenuates Ox-LDL-Mediated Macrophage Inflammation and Foam Cell Formation via Autophagy Induction and Modulating NF-kappaB, p38, and JNK MAPK Signaling. Front. Pharmacol. 2020, 11, 567238. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Ma, J.; Zhang, Z.; Sui, W.; Zhai, C.; Xu, D.; Wang, Z.; Lu, H.; Zhang, M.; Zhang, C.; et al. Deficient Chaperone-Mediated Autophagy Promotes Inflammation and Atherosclerosis. Circ. Res. 2021, 129, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Jia, Q.; Yan, L.; Chen, C.; Xing, S.; Shen, D. Quercetin Suppresses the Progression of Atherosclerosis by Regulating MST1-Mediated Autophagy in ox-LDL-Induced RAW264.7 Macrophage Foam Cells. Int. J. Mol. Sci. 2019, 20, 6093. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.; Sun, S.; Cai, H.; Zou, X.; Wang, S.; Hao, X.; Wan, X.; Tian, J.; Li, Z.; He, Z.; et al. IRGM/Irgm1 facilitates macrophage apoptosis through ROS generation and MAPK signal transduction: Irgm1 (+/−) mice display increases atherosclerotic plaque stability. Theranostics 2021, 11, 9358–9375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, R.; Zhang, L. Triclosan stimulates human vascular endothelial cell injury via repression of the PI3K/Akt/mTOR axis. Chemosphere 2020, 241, 125077. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.H.; Tang, C.; Xue, M.; Li, X.Y.; Chang, Y.P.; Cheng, Y.; Li, T.; Yu, X.C.; Sun, B.; et al. Calcium Dobesilate Restores Autophagy by Inhibiting the VEGF/PI3K/AKT/mTOR Signaling Pathway. Front. Pharmacol. 2019, 10, 886. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Qin, H.; Zhang, H.; Feng, X.; Yang, L.; Hou, D.X.; Chen, J. Fisetin inhibits inflammation and induces autophagy by mediating PI3K/AKT/mTOR signaling in LPS-induced RAW264.7 cells. Food Nutr. Res. 2021, 65, 6355. [Google Scholar]
- Zhuo, X.; Wu, Y.; Yang, Y.; Gao, L.; Qiao, X.; Chen, T. Knockdown of LSD1 meliorates Ox-LDL-stimulated NLRP3 activation and inflammation by promoting autophagy via SESN2-mesiated PI3K/Akt/mTOR signaling pathway. Life Sci. 2019, 233, 116696. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Varghese, J.F.; Patel, R.; Singh, M.; Yadav, U.C.S. Fisetin Prevents Oxidized Low-density Lipoprotein-Induced Macrophage Foam Cell Formation. J. Cardiovasc. Pharmacol. 2021, 78, e729–e737. [Google Scholar] [CrossRef]
- Lin, P.; Ji, H.H.; Li, Y.J.; Guo, S.D. Macrophage Plasticity and Atherosclerosis Therapy. Front. Mol. Biosci. 2021, 8, 679797. [Google Scholar] [CrossRef]
- Fei, J.; Sun, Y.; Duan, Y.; Xia, J.; Yu, S.; Ouyang, P.; Wang, T.; Zhang, G. Low concentration of rutin treatment might alleviate the cardiotoxicity effect of pirarubicin on cardiomyocytes via activation of PI3K/AKT/mTOR signaling pathway. Biosci. Rep. 2019, 39, BSR20190546. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Zhang, B.; Li, B.; Cao, X.; Chen, X.; Xue, Q. PTBP3 promotes migration of non-small cell lung cancer through regulating E-cadherin in EMT signaling pathway. Cancer Cell Int. 2020, 20, 172. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Ji, Y.; Yi, C.; Wang, X.; Liu, C.; Wang, C.; Lu, X.; Xu, X.; Wang, X. Rutin Inhibits Ox-LDL-Mediated Macrophage Inflammation and Foam Cell Formation by Inducing Autophagy and Modulating PI3K/ATK Signaling. Molecules 2022, 27, 4201. https://doi.org/10.3390/molecules27134201
Li B, Ji Y, Yi C, Wang X, Liu C, Wang C, Lu X, Xu X, Wang X. Rutin Inhibits Ox-LDL-Mediated Macrophage Inflammation and Foam Cell Formation by Inducing Autophagy and Modulating PI3K/ATK Signaling. Molecules. 2022; 27(13):4201. https://doi.org/10.3390/molecules27134201
Chicago/Turabian StyleLi, Ben, Yumeng Ji, Chenlong Yi, Xufeng Wang, Chaoyang Liu, Chufan Wang, Xiaohu Lu, Xiaohan Xu, and Xiaowei Wang. 2022. "Rutin Inhibits Ox-LDL-Mediated Macrophage Inflammation and Foam Cell Formation by Inducing Autophagy and Modulating PI3K/ATK Signaling" Molecules 27, no. 13: 4201. https://doi.org/10.3390/molecules27134201
APA StyleLi, B., Ji, Y., Yi, C., Wang, X., Liu, C., Wang, C., Lu, X., Xu, X., & Wang, X. (2022). Rutin Inhibits Ox-LDL-Mediated Macrophage Inflammation and Foam Cell Formation by Inducing Autophagy and Modulating PI3K/ATK Signaling. Molecules, 27(13), 4201. https://doi.org/10.3390/molecules27134201





