Development of an LC–DAD–MS-Based Method for the Analysis of Hydroxyanthracene Derivatives in Food Supplements and Plant Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solvents and Materials
2.2. Preparation of Standard Solutions and Samples
2.3. Chromatographic Conditions
2.4. MS Parameters
2.5. Method Validation
3. Results
3.1. Selectivity, Linearity, LOQ, and LOD
3.2. Precision and Accuracy
3.3. Application
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gonfa, Y.H.; Beshah, F.; Tadesse, M.G.; Bachheti, A.; Bachheti, R.K. Phytochemical Investigation and Potential Pharmacologically Active Compounds of Rumex Nepalensis: An Appraisal. J. Basic Appl. Sci. 2021, 10, 18. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Safety of Hydroxyanthracene Derivatives for Use in Food. EFSA J. 2018, 16, e05090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandith, S.; Hussain, A.; Bhat, W.; Dhar, N.; Qazi, A.; Rana, S.; Razdan, S.; Wani, T.; Shah, M.; Bedi, Y.S.; et al. Evaluation of Anthraquinones from Himalayan Rhubarb (Rheum Emodi Wall. Ex Meissn.) as Antiproliferative Agents. S. Afr. J. Bot. 2014, 95, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Malik, E.M.; Müller, C.E. Anthraquinones As Pharmacological Tools and Drugs. Med. Res. Rev. 2016, 36, 705–748. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, N.; Bettiol, A.; Crescioli, G.; Maggini, V.; Gallo, E.; Sivelli, F.; Sofi, F.; Gensini, G.F.; Vannacci, A.; Firenzuoli, F. Association between Anthraquinone Laxatives and Colorectal Cancer: Protocol for a Systematic Review and Meta-Analysis. Syst. Rev. 2020, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Mascolo, N.; Capasso, R.; Capasso, F. Senna. A Safe and Effective Drug. PTR 1998, 12, 143–145. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of a Health Claim Related to Hydroxyanthracene Derivatives and Improvement of Bowel Function Pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2013, 11, 3412. [Google Scholar] [CrossRef] [Green Version]
- Chien, S.-C.; Wu, Y.-C.; Chen, Z.-W.; Yang, W.-C. Naturally Occurring Anthraquinones: Chemistry and Therapeutic Potential in Autoimmune Diabetes. Evid. Based Complement. Altern. Med. 2015, 2015, 357357. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Mei, N. Aloe vera: A Review of Toxicity and Adverse Clinical Effects. J. Environ. Sci. Health C 2016, 34, 77–96. [Google Scholar] [CrossRef]
- Liu, Y.; Mapa, M.; Sprando, R. Liver Toxicity of Anthraquinones: A Combined in Vitro Cytotoxicity and in Silico Reverse Dosimetry Evaluation. Food Chem. Toxicol. 2020, 140, 111313. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Dial, S.L.; Boudreau, M.D.; Xia, Q.; Fu, P.P.; Levy, D.D.; Moore, M.M.; Mei, N. In Vitro Investigation of the Mutagenic Potential of Aloe vera Extracts. Toxicol. Res. 2014, 3, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Siegers, C.-P.; Siemers, J.; Baretton, G. Sennosides and Aloin Do Not Promote Dimethylhydrazine-Induced Colorectal Tumors in Mice. PHA 1993, 47, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Nusko, G.; Schneider, B.; Müller, G.; Kusche, J.; Hahn, E.G. Retrospective Study on Laxative Use and Melanosis Coli as Risk Factors for Colorectal Neoplasma. PHA 1993, 47, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, A.; Müller, A.D. Constipation and Cathartics as Risk Factors of Colorectal Cancer: A Meta-Analysis. PHA 1993, 47, 224–233. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex 32006R1925 EN EUR-Lex Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006. Available online: https://eur-lex.europa.eu/eli/reg/2006/1925 (accessed on 15 November 2021).
- EUR-Lex 32021R0468 EN EUR-Lex Commission Regulation (EU) 2021/468 of 18 March 2021. Available online: https://eur-lex.europa.eu/eli/reg/2021/468/oj (accessed on 7 October 2021).
- Standing Committee on Plants, Animals, Food and Feed Section General Food Law. 5 October 2020. Exchange of Views and Possible Opinion of the Committee on a Draft Commission Regulation (EU) Amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as Regards Botanical Species Containing Hydroxyanthracene Derivatives.Sante.Ddg2.g.5(2020)7913268. Available online: https://www.aade.gr/sites/default/files/2021-06/reg-com_gfl_20201005_sum_0.pdf (accessed on 12 January 2022).
- Müller, S.O.; Eckert, I.; Lutz, W.K.; Stopper, H. Genotoxicity of the Laxative Drug Components Emodin, Aloe-Emodin and Danthron in Mammalian Cells: Topoisomerase II Mediated? Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1996, 371, 165–173. [Google Scholar] [CrossRef]
- Metzger, W.; Reif, K. Determination of 1,8-Dihydroxyanthranoids in Senna. J. Chromatogr. A 1996, 740, 133–138. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Lin, B.; Zhou, Y.; Suo, W.; Wei, J.; Zhang, L.; Lin, J.; Xiao, F.; Zhao, L.; et al. Danthron Attenuates Experimental Atherosclerosis by Targeting Foam Cell Formation. Exp. Physiol. 2021, 106, 653–662. [Google Scholar] [CrossRef]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Huyiligeqi; Ni, J. Emodin: A Review of Its Pharmacology, Toxicity and Pharmacokinetics. PTR 2016, 30, 1207–1218. [Google Scholar] [CrossRef]
- Harlev, E.; Nevo, E.; Lansky, E.P.; Ofir, R.; Bishayee, A. Anticancer Potential of Aloes: Antioxidant, Antiproliferative, and Immunostimulatory Attributes. Planta Med. 2012, 78, 843–852. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Albayrak, S.; Antolak, H.; Kręgiel, D.; Pawlikowska, E.; Sharifi-Rad, M.; Uprety, Y.; Tsouh Fokou, P.V.; Yousef, Z.; Amiruddin Zakaria, Z.; et al. Aloe Genus Plants: From Farm to Food Applications and Phytopharmacotherapy. Int. J. Mol. Sci. 2018, 19, 2843. [Google Scholar] [CrossRef] [Green Version]
- Duval, J.; Pecher, V.; Poujol, M.; Lesellier, E. Research Advances for the Extraction, Analysis and Uses of Anthraquinones: A Review. Ind. Crops Prod. 2016, 94, 812–833. [Google Scholar] [CrossRef]
- Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Veterinary Medicine (CMV). Bioanalytical Method Validation Guidance for Industry; U.S. Departement of Health and Human Service: Washington, DC, USA, 2018. [Google Scholar]
- Wang, P.G.; Zhou, W.; Wamer, W.G.; Krynitsky, A.J.; Rader, J.I. Simultaneous Determination of Aloin A and Aloe Emodin in Products Containing Aloe vera by Ultra-Performance Liquid Chromatography with Tandem Mass Spectrometry. Anal. Methods 2012, 4, 3612–3619. [Google Scholar] [CrossRef]
- Koyama, J.; Takeuchi, A.; Morita, I.; Nishino, Y.; Shimizu, M.; Inoue, M.; Kobayashi, N. Characterization of Emodin Metabolites in Raji Cells by LC–APCI-MS/MS. Bioorg. Med. Chem. 2009, 17, 7493–7499. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.J.; Li, C.H.; Hu, Y.J.; Chen, H.; Yang, F.Q. Comparative Assessment of In Vitro Thrombolytic and Fibrinolysis Activity of Four Aloe Species and Analysis of Their Phenolic Compounds by LC–MS. S. Afr. J. Bot. 2018, 119, 325–334. [Google Scholar] [CrossRef]
- Food and Drug Administration. HHS Status of Certain Additional Over-the-Counter Drug Category II and III Active Ingredients. Final Rule. Fed. Regist. 2002, 67, 31125–31127. [Google Scholar]
- Sibhat, G.; Kahsay, G.; Van Schepdael, A.; Adams, E. Fast and Easily Applicable LC-UV Method for Analysis of Bioactive Anthrones from Aloe Leaf Latex. J. Pharm. Biomed. Anal. 2021, 195, 113834. [Google Scholar] [CrossRef]
- Kline, D.; Ritruthai, V.; Babajanian, S.; Gao, Q.; Ingle, P.; Chang, P.; Swanson, G. Quantitative Analysis of Aloins and Aloin-Emodin in Aloe vera Raw Materials and Finished Products Using High-Performance Liquid Chromatography: Single-Laboratory Validation, First Action 2016.09. J. AOAC Int. 2017, 100, 661–670. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, W.; Li, J.; He, J.; Zhang, P.; Zheng, F.; Zhang, B.; Gao, X.; Chang, Y. Influence of Processing on Pharmacokinetic of Typical Constituents in Radix Polygoni Multiflori after Oral Administration by LC–ESI–MS/MS. J. Ethnopharmacol. 2013, 148, 246–253. [Google Scholar] [CrossRef]
- Elsohly, M.A.; Gul, W.; Avula, B.; Khan, I.A. Determination of the Anthraquinones Aloe-Emodin and Aloin-A by Liquid Chromatography with Mass Spectrometric and Diode Array Detection. J. AOAC Int. 2007, 90, 28–42. [Google Scholar] [CrossRef] [Green Version]
- Mandrioli, R.; Mercolini, L.; Ferranti, A.; Fanali, S.; Raggi, M. Determination of Aloe Emodin in Aloe vera Extracts and Commercial Formulations by HPLC with Tandem UV Absorption and Fluorescence Detection. Food Chem. 2011, 126, 387–393. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, J.; Gao, X.; Amponsem, E.; Kang, L.; Hu, L.; Zhang, B.; Chang, Y. Simultaneous Determination of Stilbenes, Phenolic Acids, Flavonoids and Anthraquinones in Radix Polygoni Multiflori by LC–MS/MS. J. Pharm. Biomed. Anal. 2012, 62, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Chen, Z.; Shang, B.; Chen, Q. Identification and Quantification of Four Anthraquinones in Rhubarb and Its Preparations by Gas Chromatography-Mass Spectrometry. J. Chromatogr. Sci. 2018, 56, 195–201. [Google Scholar] [CrossRef] [PubMed]
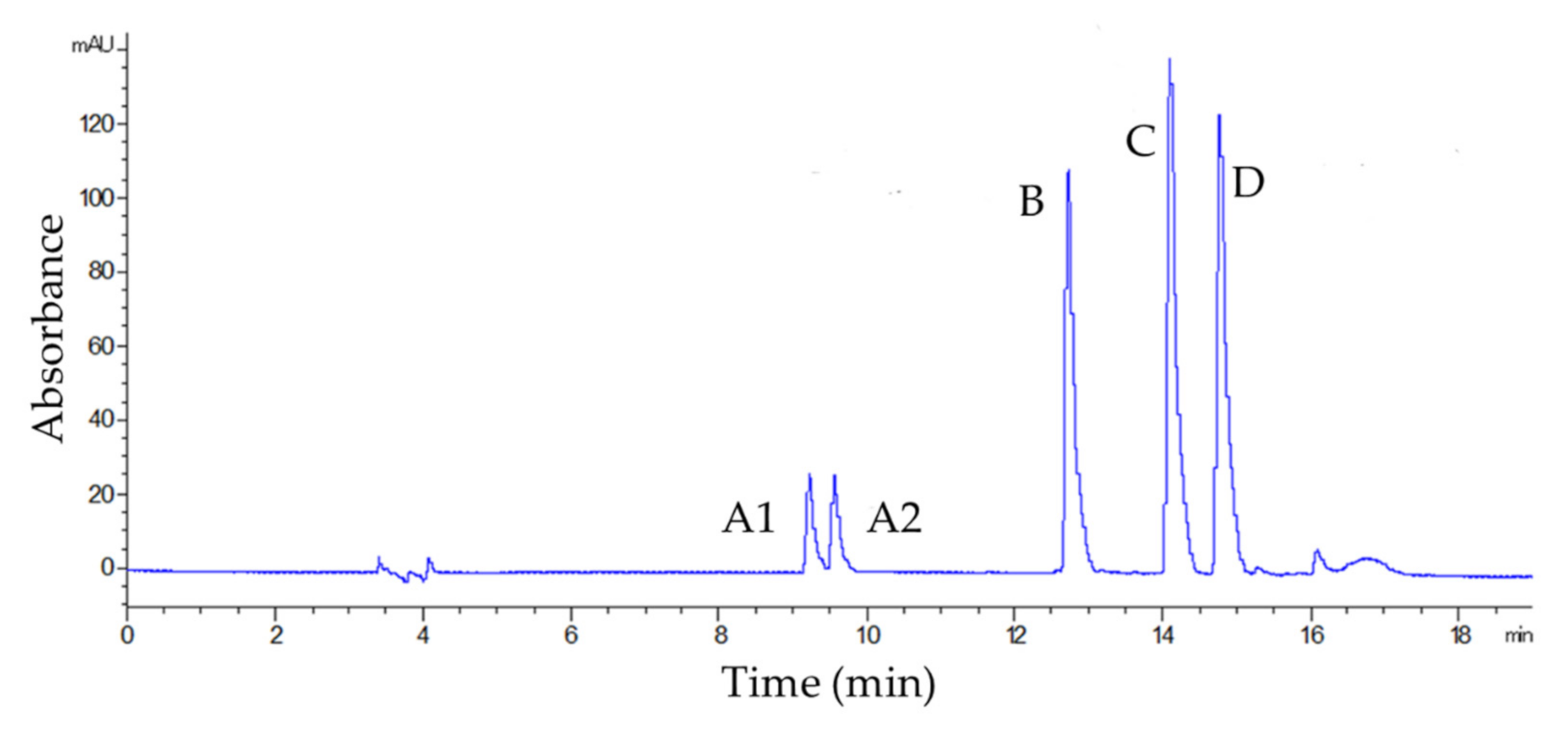
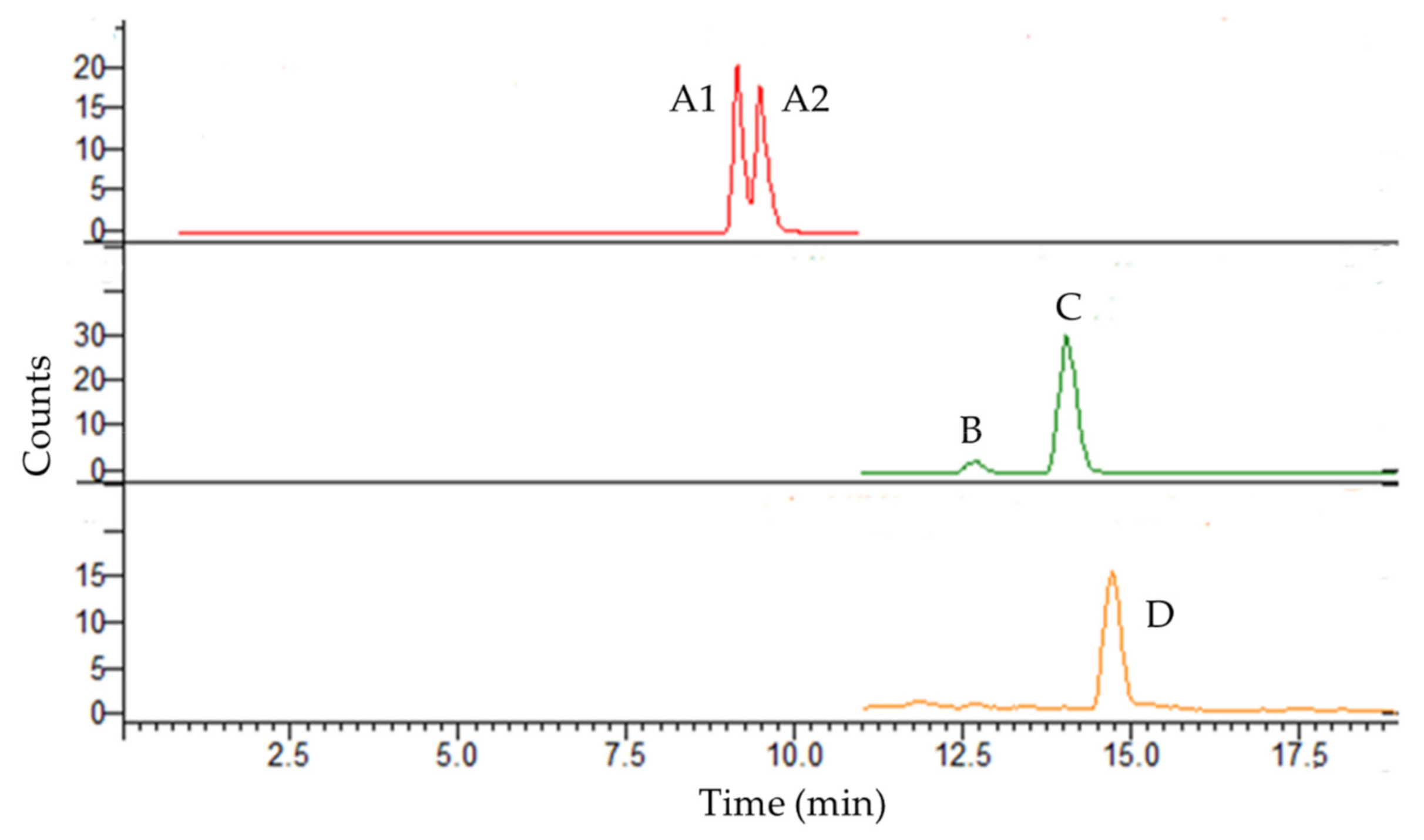
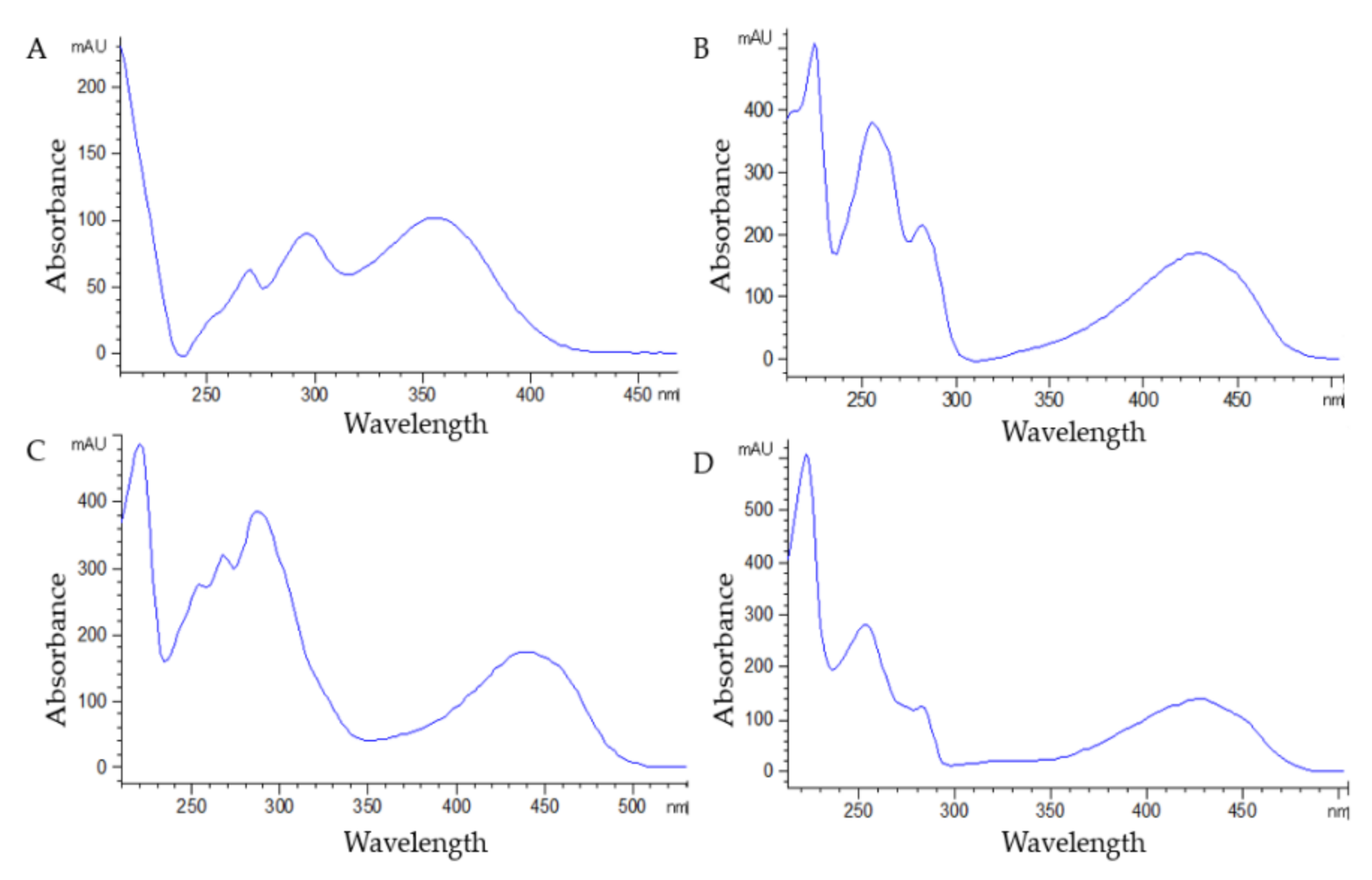
| Analyte | λ (nm) | Equation | r2 | Linearity (mg L−1) | LOQ (mg L−1) | LOD (mg L−1) | |
|---|---|---|---|---|---|---|---|
| Aloin B | 350 | y = 23.133 x − 0.0953 | 0.9993 | 0.099–19.8 | 0.059 | 0.020 | |
| Aloin A | 350 | y = 16.029 x + 2.0841 | 0.9866 | 0.10–20.4 | 0.060 | 0.020 | |
| Aloe emodin | 425 | y = 33.222 x + 0.6329 | 0.9999 | 0.105–21.0 | 0.052 | 0.017 | |
| Emodin | 425 | y = 30.072 x − 1.4086 | 0.9996 | 0.105–21.0 | 0.052 | 0.017 | |
| Danthron | 425 | y = 29.628 x − 0.4598 | 0.9989 | 0.11–21.2 | 0.082 | 0.027 | |
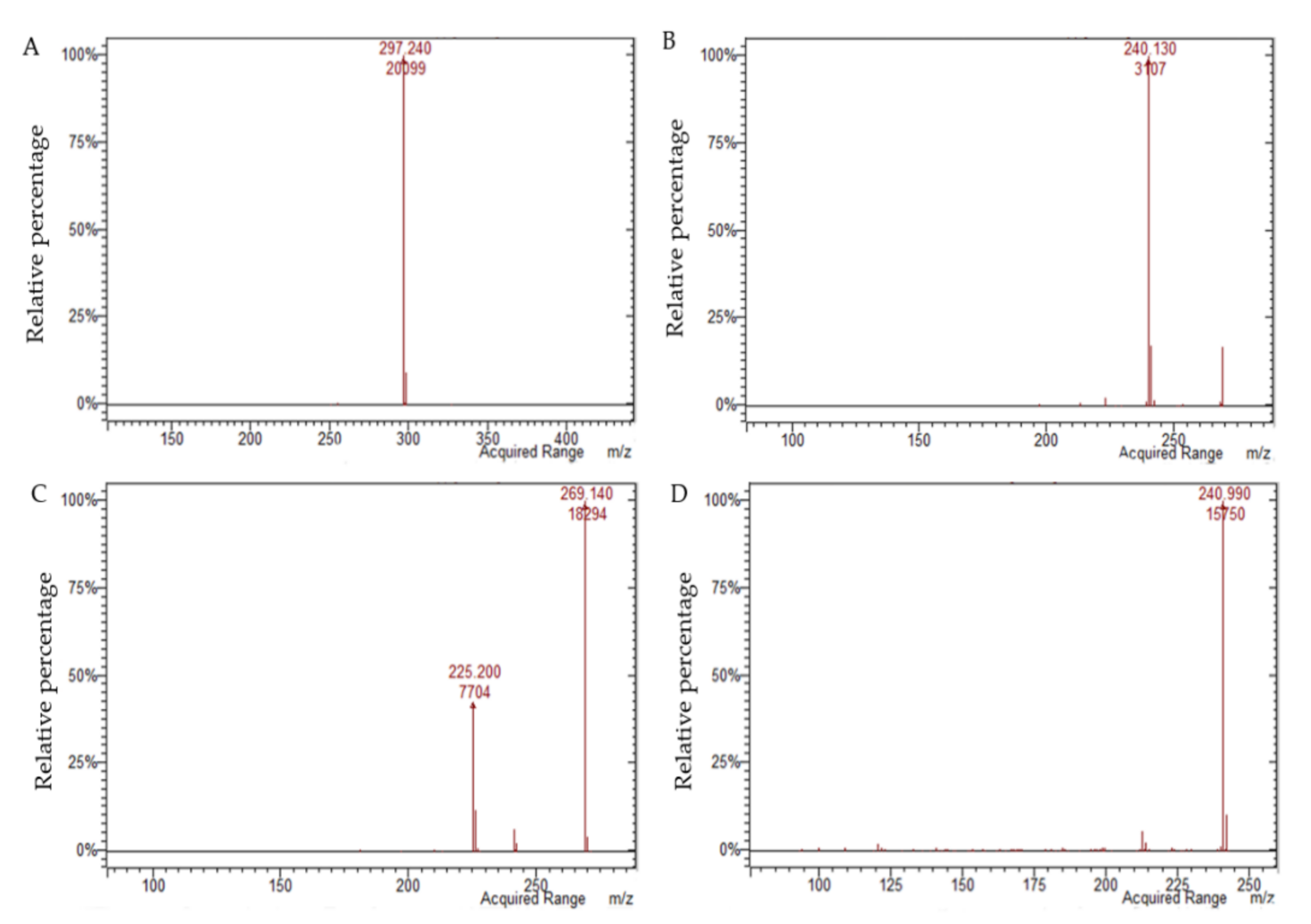
| Analyte | [M − H]− | [M + H]+ | Equation | r2 | Linearity (mg L−1) | LOQ (mg L−1) | LOD (mg L−1) |
| Aloin B | 417.1 | na | y = 210.97 x2 + 14,693 x | 0.9998 | 0.099–9.9 | 0.079 | 0.026 |
| Aloin A | 417.1 | na | y = 406.57 x2 + 13,190 x | 0.9993 | 0.10–10.2 | 0.080 | 0.026 |
| Aloe emodin | 269.1 | na | y = 77.238 x2 + 2332.7 x | 0.9989 | 0.105–10.5 | 0.087 | 0.029 |
| Emodin | 269.1 | na | y = −7018.8 x2 + 135,531 x | 0.9948 | 0.105–10.5 | 0.042 | 0.014 |
| Danthron | na | 241.1 | y = −555.63 x2 + 16,579 x | 0.9979 | 0.11–10.6 | 0.094 | 0.031 |
| Analyte | Nominal Concentration (μg g−1) | μg g−1 ± SD (CV), LC–DAD | Deviation (%), LC–DAD | μg g−1 ± SD (CV), LC–MS | Deviation (%), LC–MS | |
|---|---|---|---|---|---|---|
| Intraday precision (n = 3) | Aloin B | 4.95 | 4.94 ± 0.08 (1.73) | 99.8 | 5.10 ± 0.09 (1.78) | 103.1 |
| 0.99 | 0.94 ± 0.02 (2.51) | 94.9 | 1.05 ± 0.11 (10.58) | 105.7 | ||
| 0.49 | 0.43 ± 0.01 (1.9) | 87.75 | 0.49 ± 0.06 (12.15) | 98.4 | ||
| Aloin A | 5.10 | 4.92 ± 0.11 (1.97) | 96.5 | 4.98 ± 0.07 (1.44) | 97.7 | |
| 1.02 | 0.98 ± 0.04 (4.45) | 96.1 | 1.01 ± 0.12 (11.99) | 99.4 | ||
| 0.51 | 0.48 ± 0.005 (1.12) | 94.1 | 0.48 ± 0.04 (8.98) | 93.5 | ||
| Aloe emodin | 5.25 | 5.34 ± 0.12 (2.18) | 101.7 | 5.07 ± 0.44 (8.61) | 96.6 | |
| 1.05 | 1.01 ± 0.02 (2.43) | 96.2 | 1.05 ± 0.06 (5.58) | 100.1 | ||
| 0.52 | 0.53 ± 0.01 (1.98) | 101.9 | 0.49 ± 0.05 (9.49) | 93.7 | ||
| Emodin | 5.25 | 5.26 ± 0.09 (1.83) | 100.2 | 5.22 ± 0.08 (1.57) | 99.1 | |
| 1.05 | 1.02 ± 0.02 (1.83) | 97.1 | 1.04 ± 0.09 (8.62) | 99.2 | ||
| 0.52 | 0.51 ± 0.02 (3.95) | 98.1 | 0.60 ± 0.03 (5.43) | 108.2 | ||
| Danthron | 5.30 | 5.45 ± 0.16 (2.89) | 102.8 | 5.35 ± 0.33 (6.2) | 100.9 | |
| 1.06 | 0.98 ± 0.03 (3.39) | 92.4 | 0.99 ± 0.07 (6.75) | 93.1 | ||
| 0.53 | 0.52 ± 0.01 (1.55) | 98.1 | 0.49 ± 0.04 (8.44) | 92.8 | ||
| Interday precision (n = 3) | Aloin B | 4.95 | 4.96 ± 0.06 (1.29) | 99.8 | 5.08 ± 0.06 (1.21) | 102.7 |
| 0.99 | 0.93 ± 0.05 (5.87) | 93.9 | 0.92 ± 0.07 (8.05) | 92.5 | ||
| 0.49 | 0.44 ± 0.02 (3.95) | 88.9 | 0.47 ± 0.03 (7.44) | 94.9 | ||
| Aloin A | 5.10 | 4.92 ± 0.11 (1.97) | 96.5 | 4.95 ± 0.10 (2.06) | 97.2 | |
| 1.02 | 0.98 ± 0.04 (4.45) | 96.1 | 1.11 ± 0.02 (1.59) | 109.0 | ||
| 0.51 | 0.46 ± 0.005 (1.15) | 90.2 | 0.54 ± 0.04 (8.18) | 105.6 | ||
| Aloe emodin | 5.25 | 5.53 ± 0.34 (5.96) | 105.3 | 5.30 ± 0.10 (1.94) | 101.0 | |
| 1.05 | 1.00 ± 0.02 (2.43) | 95.2 | 1.01 ± 0.11 (11.19) | 96.4 | ||
| 0.52 | 0.52 ± 0.01 (2.08) | 99.0 | 0.51 ± 0.02 (4.62) | 96.8 | ||
| Emodin | 5.25 | 5.48 ± 0.21 (3.82) | 104.2 | 5.05 ± 0.31 (6.18) | 96.0 | |
| 1.05 | 1.06 ± 0.03 (2.82) | 100.9 | 1.04 ± 0.08 (7.78) | 98.6 | ||
| 0.52 | 0.56 ± 0.01 (1.55) | 105.7 | 0.59 ± 0.06 (9.47) | 111.5 | ||
| Danthron | 5.30 | 5.49 ± 0.11 (1.96) | 103.6 | 5.52 ± 0.08 (1.5) | 104.2 | |
| 1.06 | 0.96 ± 0.06 (5.94) | 90.6 | 0.94 ± 0.003 (0.3) | 88.8 | ||
| 0.53 | 0.51 ± 0.02 (3.89) | 96.2 | 0.51 ± 0.06 (12.72) | 95.8 |
| Sample | HAD | Spiking Concentration (μg g−1) | μg g−1 ± SD, LC–DAD | Recovery (%), LC–DAD | μg g−1 ± SD, LC–MS | Recovery (%), LC–MS |
|---|---|---|---|---|---|---|
| Echinacea root + HADs (n = 3) | Aloin B | 4.95 | 4.28 ± 0.10 | 86.5 | 5.03 ± 0.11 | 101.6 |
| Aloin A | 5.10 | 4.72 ± 0.08 | 92.5 | 5.55 ± 0.16 | 108.8 | |
| Aloe emodin | 5.25 | 5.69 ± 0.12 | 108.4 | 5.92 ± 0.23 | 112.8 | |
| Emodin | 5.26 | 4.66 ± 0.13 | 88.6 | 5.09 ± 0.12 | 96.8 | |
| Danthron | 5.30 | 4.80 ± 0.14 | 90.6 | 4.55 ± 0.15 | 85.8 | |
| Echinacea root + HADs (n = 3) | Aloin B | 0.99 | 0.91 ± 0.04 | 91.9 | 1.01 ± 0.08 | 102.0 |
| Aloin A | 1.02 | 0.97 ± 0.03 | 95.1 | 1.21 ± 0.15 | 118.6 | |
| Aloe emodin | 1.05 | 1.18 ± 0.06 | 112.4 | 1.14 ± 0.12 | 108.6 | |
| Emodin | 1.05 | 0.90 ± 0.04 | 85.7 | 0.86 ± 0.12 | 81.9 | |
| Danthron | 1.06 | 0.97 ± 0.02 | 91.5 | 0.90 ± 0.11 | 84.9 | |
| Echinacea root + HADs (n = 3) | Aloin B | 0.51 | 0.42 ± 0.01 | 82.3 | 0.51 ± 0.05 | 100.0 |
| Aloin A | 0.49 | 0.43 ± 0.02 | 87.7 | 0.58 ± 0.06 | 118.4 | |
| Aloe emodin | 0.52 | 0.57 ± 0.03 | 91.2 | 0.57 ± 0.07 | 109.6 | |
| Emodin | 0.53 | 0.44 ± 0.03 | 83.0 | 0.45 ± 0.03 | 84.9 | |
| Danthron | 0.53 | 0.47 ± 0.02 | 88.7 | 0.47 ± 0.07 | 88.7 | |
| Simple syrup + HADs (n = 3) | Aloin B | 4.95 | 4.47 ± 0.11 | 90.3 | 4.76 ± 0.15 | 96.2 |
| Aloin A | 5.10 | 4.70 ± 0.12 | 92.1 | 5.42 ± 0.12 | 106.3 | |
| Aloe emodin | 5.25 | 5.69 ± 0.16 | 108.4 | 5.83 ± 0.15 | 111.0 | |
| Emodin | 5.26 | 5.56 ± 0.09 | 105.7 | 4.87 ± 0.12 | 92.6 | |
| Danthron | 5.30 | 5.45 ± 0.13 | 102.8 | 5.32± 0.12 | 100.4 | |
| Simple syrup + HADs (n = 3) | Aloin B | 0.99 | 0.92 ± 0.07 | 92.9 | 0.93 ± 0.11 | 93.9 |
| Aloin A | 1.02 | 0.92 ± 0.05 | 89.3 | 1.15 ± 0.07 | 112.7 | |
| Aloe emodin | 1.05 | 1.10 ± 0.04 | 104.8 | 0.89 ± 0.09 | 84.8 | |
| Emodin | 1.05 | 1.14 ± 0.06 | 108.6 | 0.97 ± 0.03 | 92.4 | |
| Danthron | 1.06 | 1.07 ± 0.03 | 100.9 | 0.93 ± 0.06 | 87.7 | |
| Simple syrup + HADs (n = 3) | Aloin B | 0.51 | 0.44 ± 0.04 | 86.3 | 0.45 ± 0.05 | 88.2 |
| Aloin A | 0.49 | 0.42 ± 0.02 | 85.7 | 0.49 ± 0.04 | 100.0 | |
| Aloe emodin | 0.52 | 0.44 ± 0.04 | 84.6 | 0.46 ± 0.03 | 88.5 | |
| Emodin | 0.53 | 0.63 ± 0.05 | 118.9 | 0.48 ± 0.06 | 90.6 | |
| Danthron | 0.53 | 0.51 ± 0.01 | 96.2 | 0.49 ± 0.06 | 92.4 |
| Sample | Aloin B | Aloin A | AloeEmodin | Emodin | Danthron | |
|---|---|---|---|---|---|---|
| Hydroalcoholic liquid single-dose bottles | nd | nd | 2.08 ± 0.09 | 1.04 ± 0.03 | nd | |
| Hydroalcoholic liquidoral spray | nd | nd | 10.39 ± 1.66 | 0.60 ± 0.025 | nd | |
| Aloe vera juice A | 0.26 ± 0.02 | 0.81 ± 0.10 | nd | nd | nd | |
| Aloe vera juice B | 8.19 ± 2.04 | 11.21 ± 2.82 | nd | nd | nd | |
| Aloe vera juice C | 0.04 ± 0.01 | 0.06 ± 0.01 | nd | nd | nd | |
| Tablets | 27.08 ± 3.23 | 32.98 ± 2.79 | 63.96 ± 0.41 | 9.88 ± 0.01 | nd | |
| Capsules | nd | nd | nd | nd | nd | |
| Herbal tea A | nd | nd | 9.78 ± 0.88 | 0.31 ± 0.04 | nd | |
| Herbal tea B | nd | nd | 10.36 ± 0.92 | 0.13 ± 0.04 | nd | |
| Dry extract A | nd | nd | 3.23 ± 0.19 | 1.24 ± 0.08 | nd | |
| Dry extract B | nd | nd | nd | 1.13 ± 0.11 | nd | |
| Dry extract C | nd | nd | 1.85 ± 0.17 | 0.62 ± 0.21 | nd | |
| Dry extract D | nd | nd | 65.49 ± 4.26 | 64.57 ± 7.76 | nd | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loschi, F.; Faggian, M.; Sut, S.; Ferrarese, I.; Maccari, E.; Peron, G.; Dall’Acqua, S. Development of an LC–DAD–MS-Based Method for the Analysis of Hydroxyanthracene Derivatives in Food Supplements and Plant Materials. Molecules 2022, 27, 1932. https://doi.org/10.3390/molecules27061932
Loschi F, Faggian M, Sut S, Ferrarese I, Maccari E, Peron G, Dall’Acqua S. Development of an LC–DAD–MS-Based Method for the Analysis of Hydroxyanthracene Derivatives in Food Supplements and Plant Materials. Molecules. 2022; 27(6):1932. https://doi.org/10.3390/molecules27061932
Chicago/Turabian StyleLoschi, Francesca, Marta Faggian, Stefania Sut, Irene Ferrarese, Erica Maccari, Gregorio Peron, and Stefano Dall’Acqua. 2022. "Development of an LC–DAD–MS-Based Method for the Analysis of Hydroxyanthracene Derivatives in Food Supplements and Plant Materials" Molecules 27, no. 6: 1932. https://doi.org/10.3390/molecules27061932
APA StyleLoschi, F., Faggian, M., Sut, S., Ferrarese, I., Maccari, E., Peron, G., & Dall’Acqua, S. (2022). Development of an LC–DAD–MS-Based Method for the Analysis of Hydroxyanthracene Derivatives in Food Supplements and Plant Materials. Molecules, 27(6), 1932. https://doi.org/10.3390/molecules27061932









