Production of Atosiban’s Key Intermediate Pentapeptide: Synthetic Approaches to the Development of a Peptide Synthesis with Less Racemization and Simplifier Purification Process
Abstract
:1. Introduction
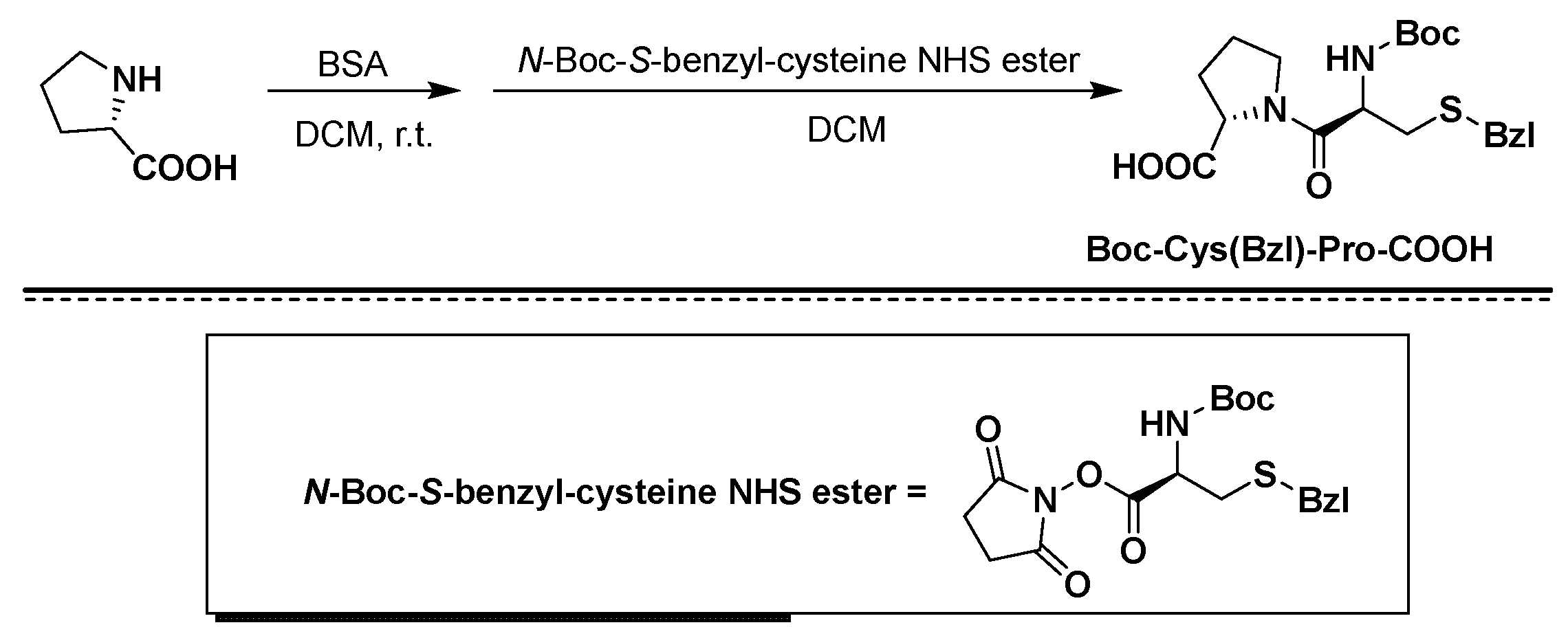
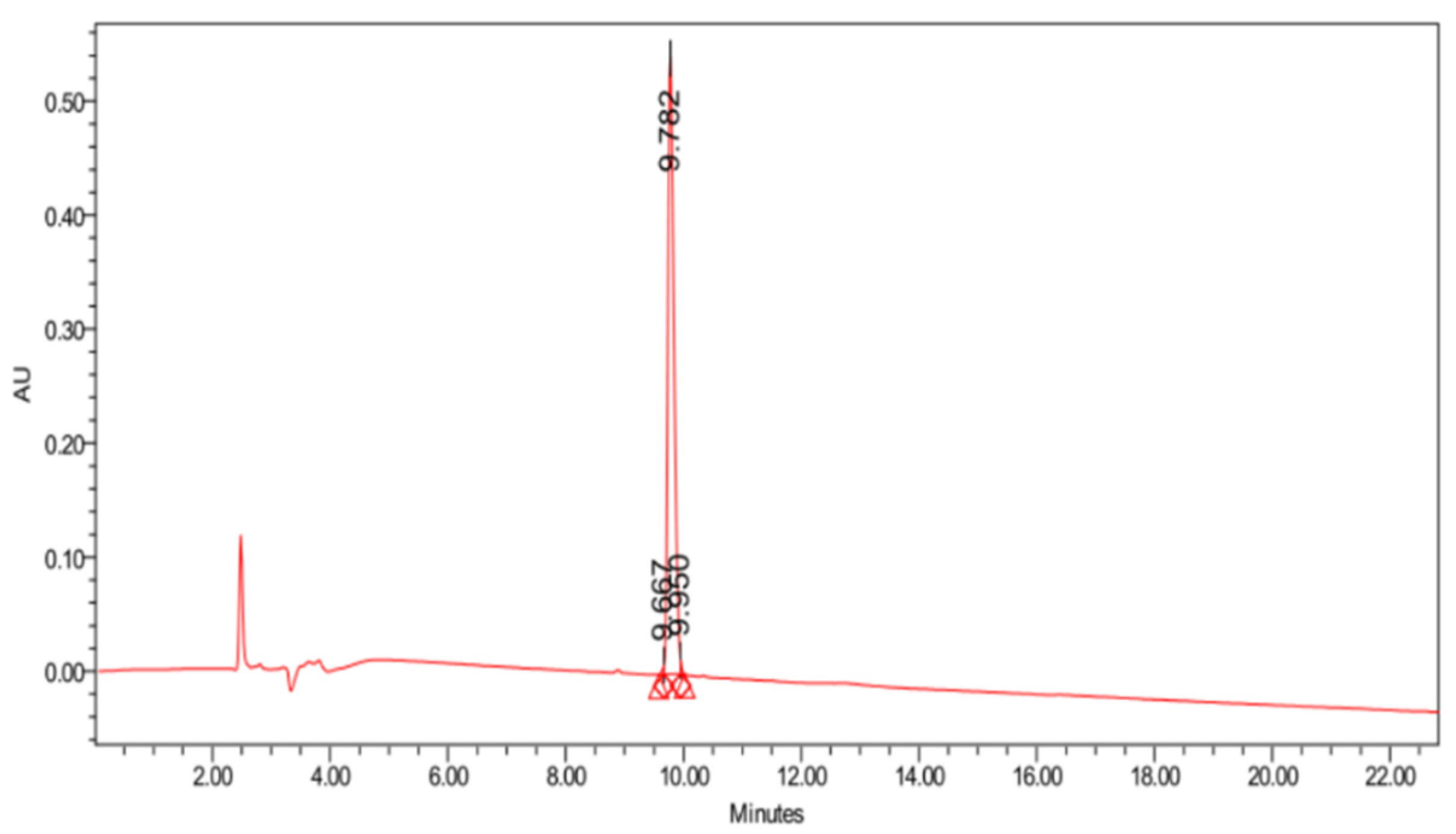
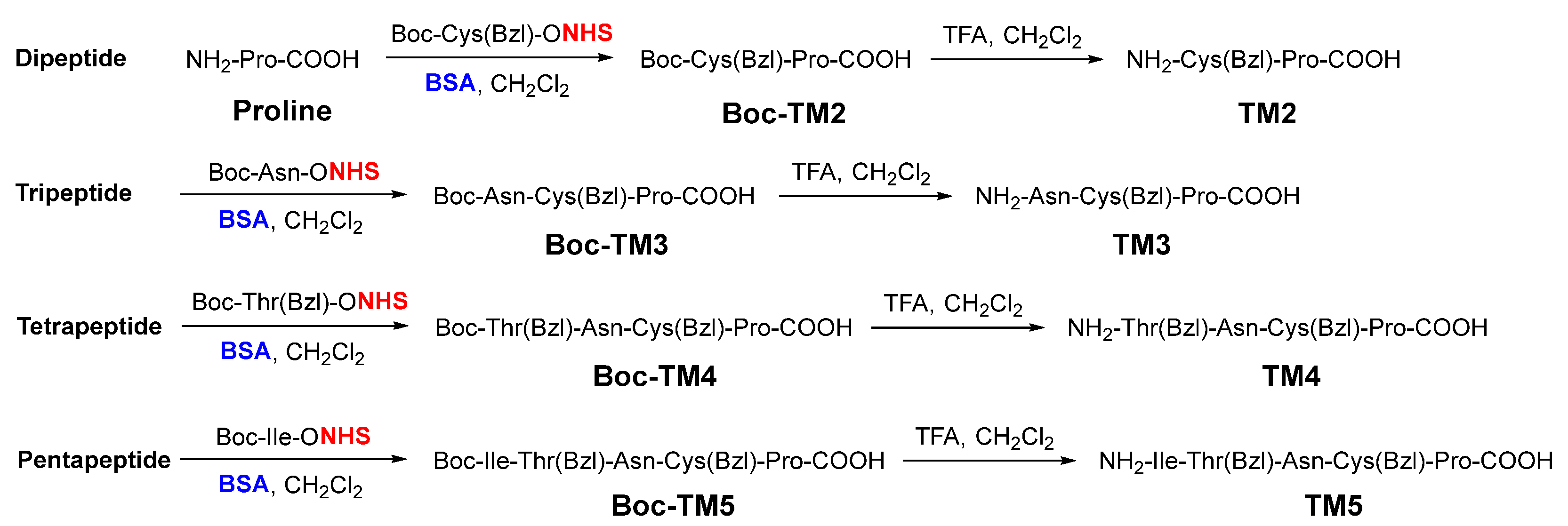
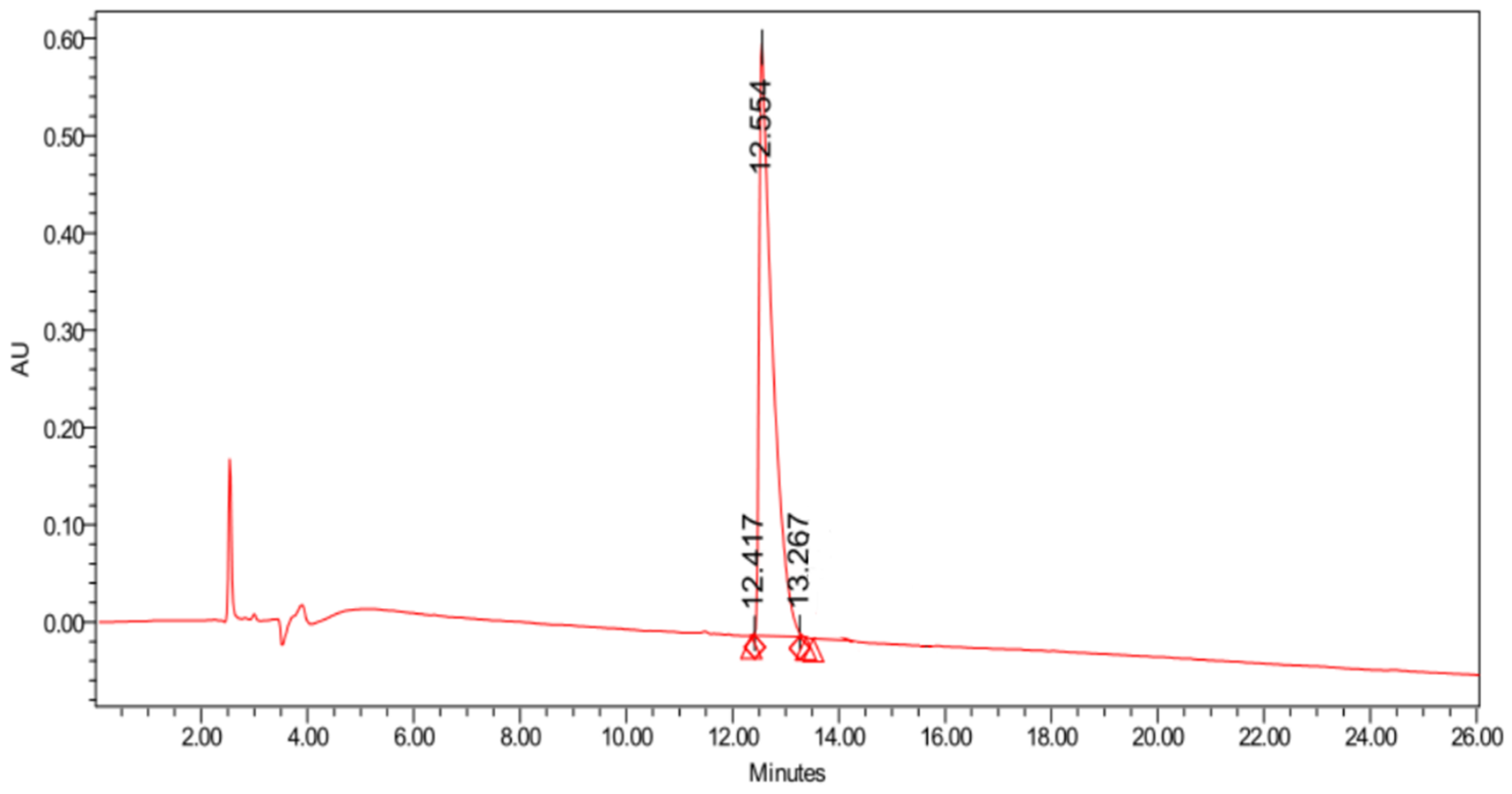
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wiśniewski, K.; Trojnar, J.; Riviere, P.; Haigh, R.; Yea, C.; Ashworth, D.; Melin, P.; Nilsson, A. The Synthesis of a New Class of Oxytocin Antagonists. Med. Chem. Lett. 1999, 9, 2801–2804. [Google Scholar] [CrossRef]
- Stymiest, J.L.; Mitchell, B.F.; Wong, S.; Vederas, J.C. Synthesis of Oxytocin Analogues with Replacement of Sulfur by Carbon Gives Potent Antagonists with Increased Stability. J. Org. Chem. 2005, 70, 7799–7809. [Google Scholar] [CrossRef] [PubMed]
- Kinder, V.A.; Thornton, S.; Ashford, M.L.J.; Melin, P.; Smith, S.K. The effect of the oxytocin antagonists F314 and F792 on the in vitvo contractility of human myometrium. Br. J. Obstet. Gynaecol. 1996, 103, 373–375. [Google Scholar] [CrossRef]
- Schmitz, T.; Cabrol, D. Tocolysis. Atosiban, an ocytcin-receptor antagonist. Obstet. Biol. Reprod. 2001, 30, 238–245. [Google Scholar]
- Andersson, L.H.H.; Sköldbäck, J.A. Synthesis of Cyclic Peptides. European Patent EP0710243, 29 June 1993. [Google Scholar]
- Dong, H.; Guo, D.; Wen, Y. Preparation Method of Atosiban. CN110759972A, 7 February 2020. [Google Scholar]
- Peng, Y.; Chang, M.; Wang, R.; Xue, H. Method of Preparing Atosiban Acetate by Convergent Condensation. CN104447960A, 25 March 2015. [Google Scholar]
- Yang, D.H.; Lu, Y.; Zhou, L.; Shen, K. Solid-Phase Cyclization Synthesis Method of Atosiban. CN103980350A, 13 August 2014. [Google Scholar]
- Johansson, C.; Blomberg, L.; Hlebowicz, E.; Nicklasson, H.; Nilsson, B.; Andersson, L. Industrial production of an oxytocin antagonist: Synthetic approaches to the development of a multi-kilogram scale solution synthesis. Pept.-Eur. Symp. 1995, 13, 34–35. [Google Scholar]
- Sheehan, J.C.; Preston, J.; Cruickshank, P.A. A Rapid Synthesis of Oligopeptide Derivatives without Isolation of Intermediates. J. Am. Chem. Soc. 1965, 87, 2492–2493. [Google Scholar] [CrossRef] [PubMed]
- Carpino, L.A.; Ismail, M.; Truran, G.A.; Mansour, E.M.E.; Iguchi, S.; Ionescu, D.; El-Faham, A.; Riemer, C.; Warrass, R. The 1,1-Dioxobenzo[b]thiophene-2-ylmethyloxycarbonyl (Bsmoc) Amino-Protecting Group. J. Org. Chem. 1999, 64, 4324–4338. [Google Scholar] [CrossRef]
- Carpino, L.A.; Ghassemi, S.; Ionescu, D.; Ismail, M.; Sadat-Aalaee, D.; Truran, G.A.; Mansour, E.M.E.; Siwruk, G.A.; Eynon, J.S.; Morgan, B. Rapid, Continuous Solution-Phase Peptide Synthesis: Application to Peptides of Pharmaceutical Interest. Org. Process Res. Dev. 2003, 7, 28–37. [Google Scholar] [CrossRef]
- Meneses, C.; Nicoll, S.L. Trembleau, Multigram-Scale Synthesis of Short Peptides via a Simplified Repetitive Solution-Phase Procedure. J. Org. Chem. 2010, 75, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Feng, W.H. N,O-bis(trimethylsilyl)acetamide/N-hydroxysuccinimide ester (BSA/NHS) as coupling agents for dipeptide synthesis. Chin. Chem. Lett. 2016, 27, 357–360. [Google Scholar] [CrossRef]
- Andersson, L.; Blomberg, L.; Flegel, M.; Lepsa, L.; Nilsson, B.; Verlander, M. Large-scale synthesis of peptides. Biopolymers 2000, 55, 227–250. [Google Scholar] [CrossRef]
- Montalbetti, C.A.G.N.; Falque, V. Amide bond formation and peptide coupling. Tetrahedron 2005, 61, 10827–10852. [Google Scholar] [CrossRef]
- Mikolajczyk, M.; Kielbasinski, P. Recent Developments in The Carbodiimide Chemistry. Tetrahedron 1981, 37, 233–284. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Zheng, Y.; Liu, J.; Li, X.; Feng, W. Production of Atosiban’s Key Intermediate Pentapeptide: Synthetic Approaches to the Development of a Peptide Synthesis with Less Racemization and Simplifier Purification Process. Molecules 2022, 27, 1920. https://doi.org/10.3390/molecules27061920
Ma C, Zheng Y, Liu J, Li X, Feng W. Production of Atosiban’s Key Intermediate Pentapeptide: Synthetic Approaches to the Development of a Peptide Synthesis with Less Racemization and Simplifier Purification Process. Molecules. 2022; 27(6):1920. https://doi.org/10.3390/molecules27061920
Chicago/Turabian StyleMa, Chunying, Yixuan Zheng, Jinwei Liu, Xiaobao Li, and Wenhua Feng. 2022. "Production of Atosiban’s Key Intermediate Pentapeptide: Synthetic Approaches to the Development of a Peptide Synthesis with Less Racemization and Simplifier Purification Process" Molecules 27, no. 6: 1920. https://doi.org/10.3390/molecules27061920
APA StyleMa, C., Zheng, Y., Liu, J., Li, X., & Feng, W. (2022). Production of Atosiban’s Key Intermediate Pentapeptide: Synthetic Approaches to the Development of a Peptide Synthesis with Less Racemization and Simplifier Purification Process. Molecules, 27(6), 1920. https://doi.org/10.3390/molecules27061920




