Enhancing the Anticancer Activity of Sorafenib through Its Combination with a Nitric Oxide Photodelivering β-Cyclodextrin Polymer
Abstract
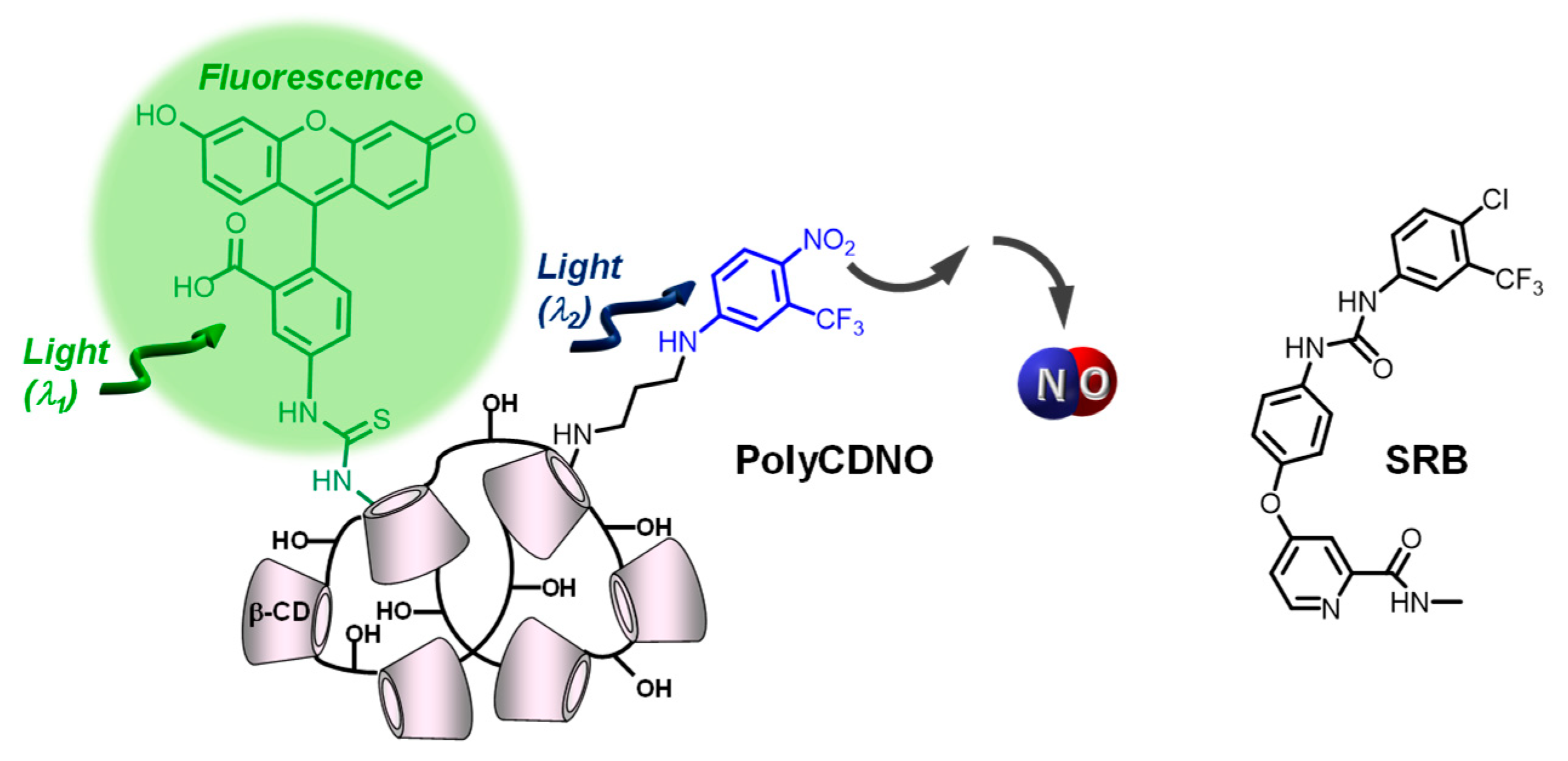
1. Introduction
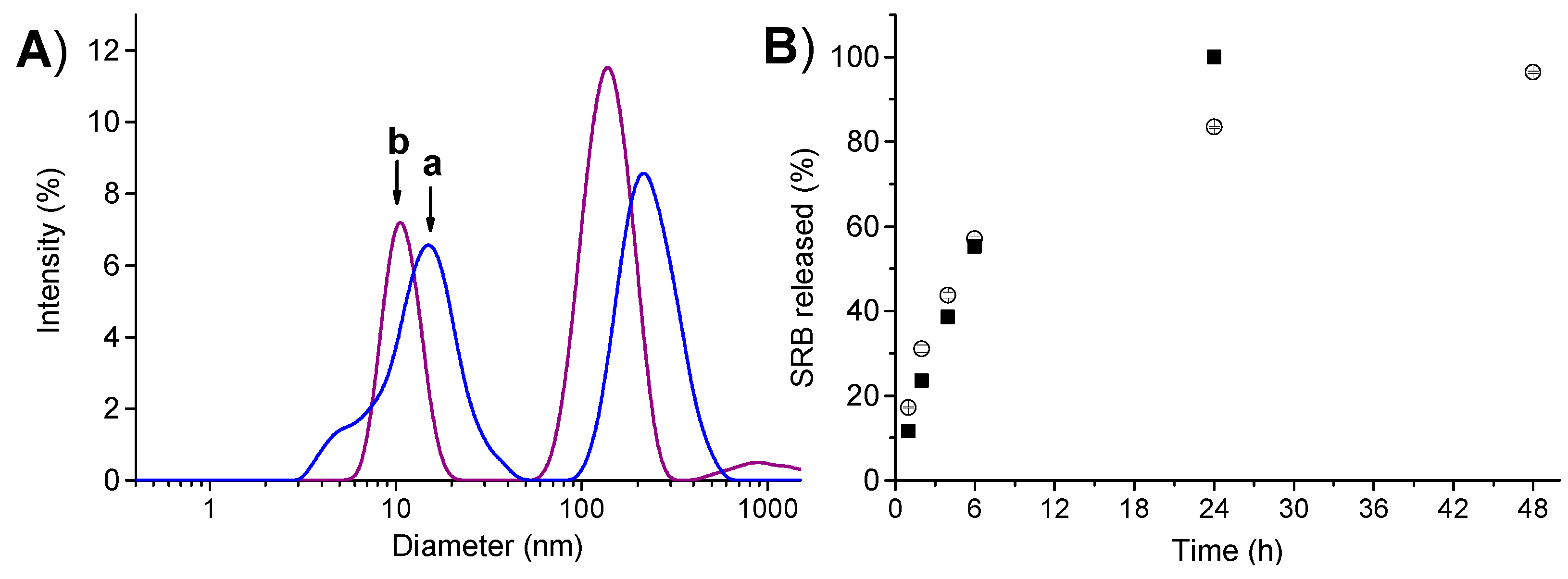
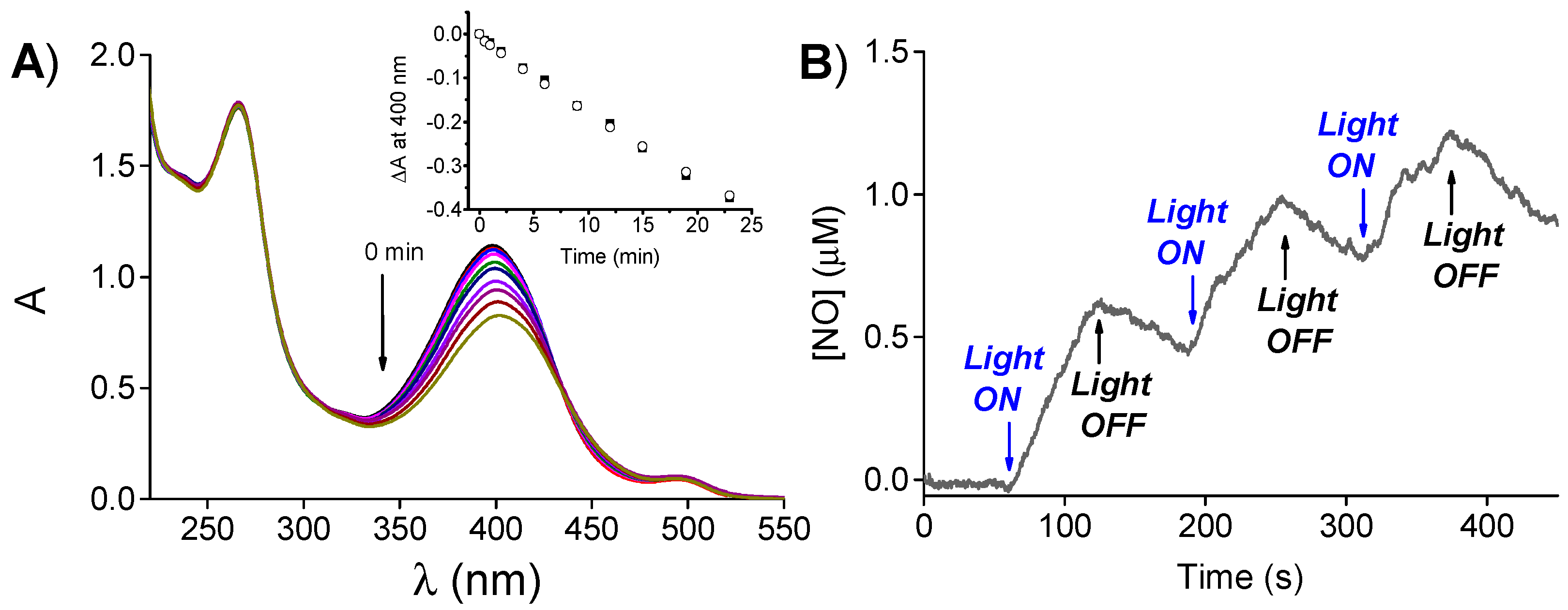
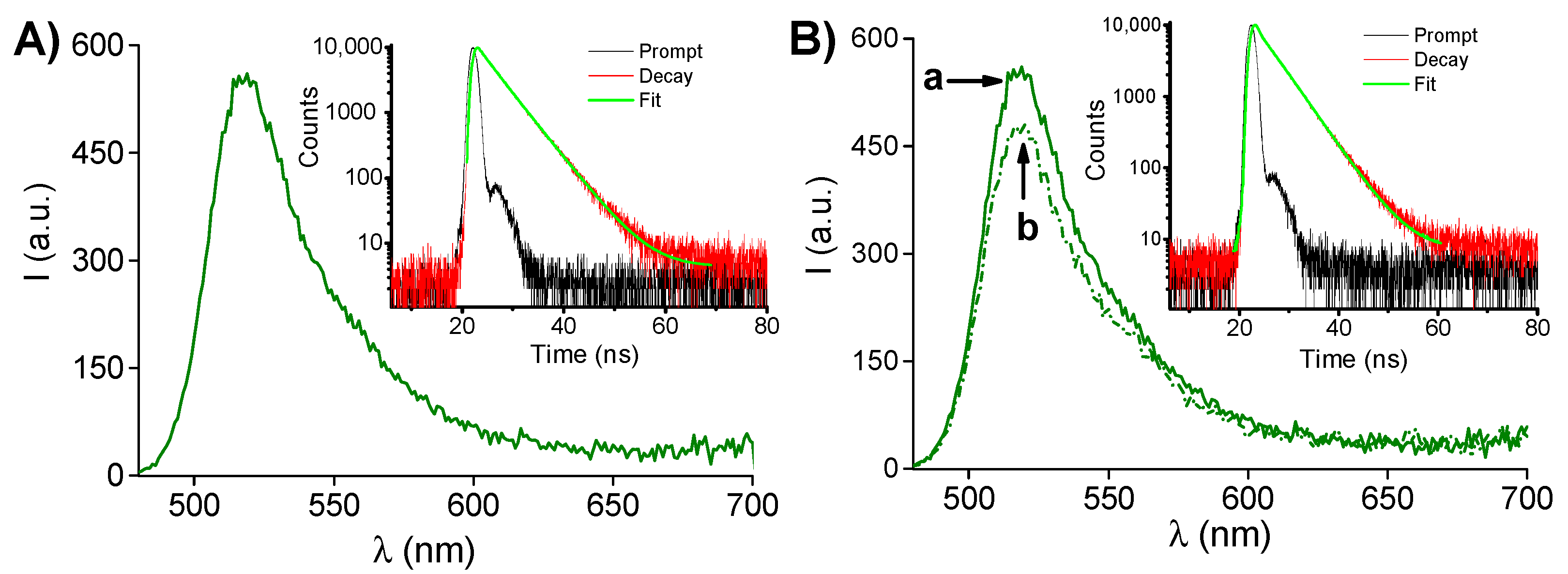
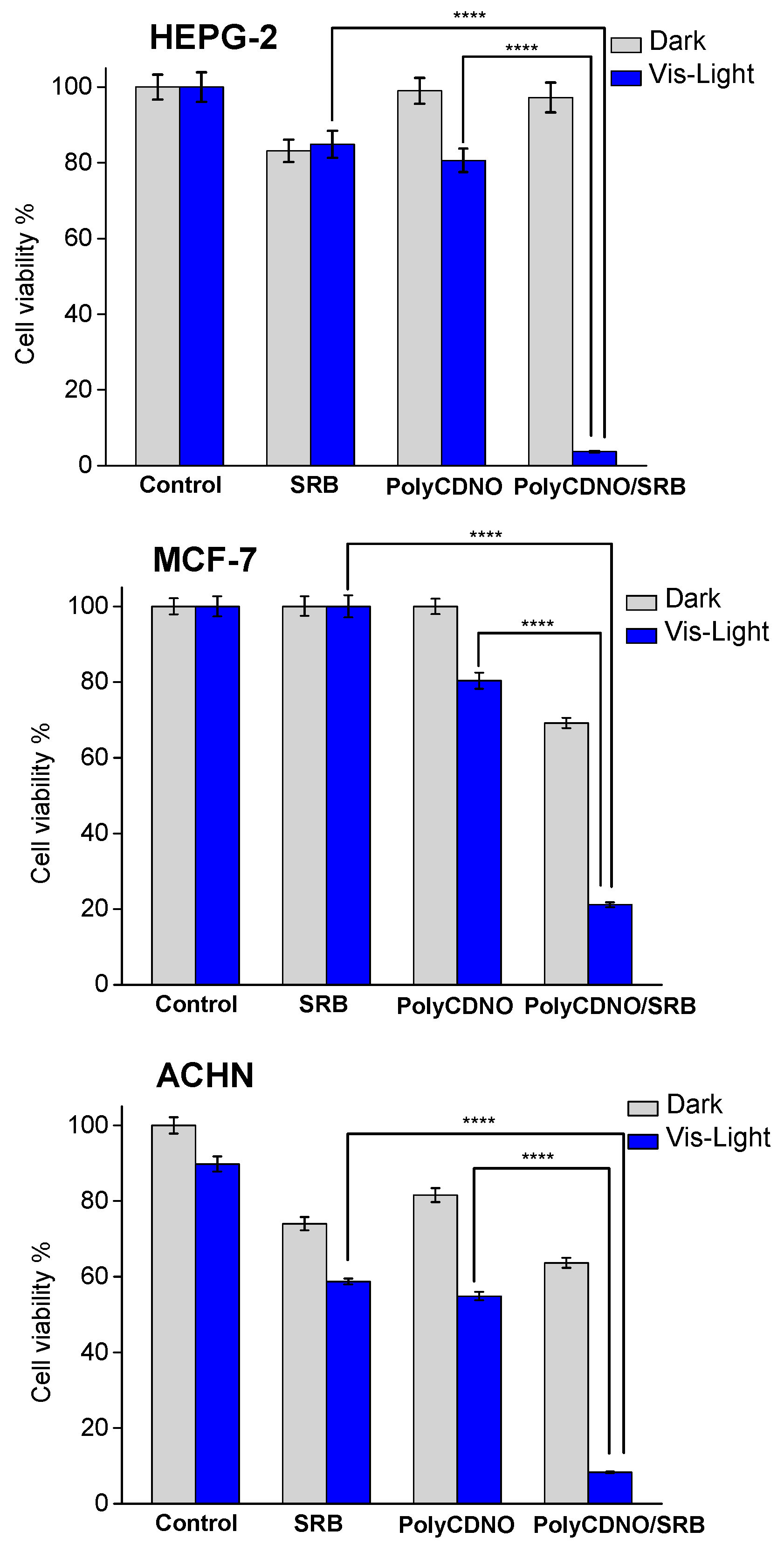
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. NO Photorelease and Fluorescence Quantum Yields
3.4. Preparation of the PolyCDNO/SRB Complex
3.5. Extent of Complexation of SRB in PolyCDNO/SRB and SRB Release
3.6. Biological Assays
3.6.1. Cell Lines
3.6.2. Fluorescence Microscopy
3.6.3. Viability Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kane, R.C.; Farrell, A.T.; Madabushi, R.; Booth, B.; Chattopadhyay, S.; Sridhara, R.; Justice, R.; Pazdur, R. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist 2009, 14, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Guevremont, C.; Jeldres, C.; Perrotte, P.; Karakiewicz, P.I. Sorafenib in the management of metastatic renal cell carcinoma. Curr. Oncol. 2009, 16, S27–S32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zafrakas, M.; Papasozomenou, P.; Emmanouilides, C. Sorafenib in breast cancer treatment: A systematic review and overview of clinical trials. World J. Clin. Oncol. 2016, 7, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Mangana, J.; Levesque, M.P.; Karpova, M.-B.; Dummer, R. Sorafenib in melanoma. Expert Opin. Investig. Drugs 2012, 21, 557–568. [Google Scholar]
- Pitoia, F.; Jerkovich, F. Selective use of sorafenib in the treatment of thyroid cancer. Drug Des. Dev. Ther. 2016, 10, 1119–1131. [Google Scholar] [CrossRef]
- Kacan, T.; Nayir, E.; Altun, A.; Kilickap, S.; Babacan, N.A.; Ataseven, H.; Kaya, T. Antitumor activity of sorafenib on colorectal cancer. J. Oncol. Sci. 2016, 2, 53–57. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Z.-H.; Qu, X.-J. The adverse effects of sorafenib in patients with advanced cancers. Basic Clin. Pharmacol. Toxicol. 2015, 116, 216–221. [Google Scholar] [CrossRef]
- Almeida e Sousa, L.; Reutzel-Edens, S.M.; Stephenson, G.A.; Taylor, L.S. Assessment of the amorphous solubility of a group of diverse drugs using new experimental and theoretical approaches. Mol. Pharm. 2015, 12, 484–495. [Google Scholar] [CrossRef]
- Khan, M.A.; Raza, A.; Ovais, M.; Sohail, M.F.; Ali, S. Current state and prospects of nano-delivery systems for sorafenib. Int. J. Pol. Mater. Pol. Biomater. 2018, 67, 1105–1115. [Google Scholar] [CrossRef]
- Chen, F.; Fang, Y.; Chen, X.; Deng, R.; Zhang, Y.; Shao, J. Recent advances of sorafenib nanoformulations for cancer therapy: Smart nanosystem and combination therapy. Asian J. Pharm. Sci. 2021, 16, 318–336. [Google Scholar] [CrossRef]
- Craparo, E.F.; Sardo, C.; Serio, R.; Zizzo, M.G.; Bondì, M.L.; Giammona, G.; Cavallaro, G. Galactosylated polymeric carriers for liver targeting of sorafenib. Int. J. Pharm. 2014, 466, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Cervello, M.; Pitarresi, G.; Bavuso Volpe, A.; Porsio, B.; Balasus, D.; Emma, M.R.; Azzolina, A.; Puleio, R.; Loria, G.R.; Puleo, S.; et al. Nanoparticles of a polyaspartamide-based brush copolymer for modified release of sorafenib: In vitro and in vivo evaluation. J. Control. Release 2017, 266, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Sukkar, F.; Shafaa, M.; El-Nagdy, M.; Darwish, W. Polymeric nanocarriers for effective synergistic action of sorafenib tosylate and gold-sensitized gamma radiation against HepG2 cells. Int. J. Nanomed. 2021, 16, 8309–8321. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ye, L.; Liu, J.; Lian, D.; Li, X. Sorafenib-loaded nanoparticles based on biodegradable dendritic polymers for enhanced therapy of hepatocellular carcinoma. Int. J. Nanomed. 2020, 15, 1469–1480. [Google Scholar] [CrossRef]
- Lin, T.T.; Gao, D.Y.; Liu, Y.C.; Sung, Y.C.; Wan, D.; Liu, J.Y.; Chiang, T.; Wang, L.; Chen, Y. Development and characterization of sorafenib-loaded PLGA nanoparticles for the systemic treatment of liver fibrosis. J. Control. Release 2016, 221, 62–70. [Google Scholar] [CrossRef]
- Tang, W.; Chen, Z.; Zhang, W.; Cheng, Y.; Zhang, B.; Wu, F.; Wang, Q.; Wang, S.; Rong, D.; Reiter, F.P.; et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: Theoretical basis and therapeutic aspects. Signal Transduct. Target Ther. 2020, 5, 87. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancer 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Riganti, C.; Miraglia, E.; Viarisio, D.; Costamagna, C.; Pescarmona, G.; Ghigo, D.; Bosia, A. Nitric oxide reverts the resistance to doxorubicin in human colon cancer cells by inhibiting the drug efflux. Cancer Res. 2005, 65, 516–525. [Google Scholar]
- Ignarro, L.J. Nitric Oxide: Biology and Pathobiology, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Wink, D.A.; Mitchell, J.R. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 1998, 25, 434–456. [Google Scholar] [CrossRef]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Diers, A.R.; Hogg, N. Cancer cell metabolism and the modulating effects of nitric oxide. Free Radic. Biol. Med. 2015, 79, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Fu, J.; Zhang, Y. Nitric oxide-based cancer therapy. J. Med. Chem. 2017, 60, 7617–7635. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Erusalimsky, J.D. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat. Rev. Mol. Cell Biol. 2002, 3, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Vodovotz, Y.; Laval, J.; Laval, M. The multifaceted roles of nitric oxide in cancer. Carcinogenesis 1998, 19, 711–721. [Google Scholar] [CrossRef]
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric oxide donors: Chemical activities and biological applications. Chem. Rev. 2002, 102, 1091–1134. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; Schoenfisch, M.H. Nitric oxide release: Part II. Therapeutic applications. Chem. Soc. Rev. 2012, 41, 3742–3752. [Google Scholar] [CrossRef]
- Sortino, S. Light-controlled nitric oxide delivering molecular assemblies. Chem. Soc. Rev. 2010, 39, 2903–2913. [Google Scholar] [CrossRef]
- Fraix, A.; Parisi, C.; Seggio, M.; Sortino, S. Nitric oxide photoreleasers with fluorescent reporting. Chem. Eur. J. 2021, 27, 12714–12725. [Google Scholar] [CrossRef]
- Ford, P.C. Photochemical delivery of nitric oxide. Nitric Oxide 2013, 34, 56–64. [Google Scholar] [CrossRef]
- Fry, N.L.; Mascharak, P.K. Photoactive ruthenium nitrosyls as NO donors: How to sensitize them toward visible light. Acc. Chem. Res. 2011, 44, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Ieda, N.; Oka, Y.; Yoshihara, T.; Tobita, S.; Sasamori, T.; Kawiguchi, M.; Nakagawa, H. Structure-efficiency relationship of photoinduced electron transfer-triggered nitric oxide releasers. Sci. Rep. 2019, 9, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Sodano, F.; Cavanagh, R.J.; Pearce, A.K.; Lazzarato, L.; Rolando, B.; Fraix, A.; Abelha, T.F.; Vasey, C.E.; Alexander, C.; Taresco, V.; et al. Enhancing doxorubicin anticancer activity with a novel polymeric platform photoreleasing nitric oxide. Biomater. Sci. 2020, 8, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Fraix, A.; Conte, C.; Gazzano, E.; Riganti, C.; Quaglia, F.; Sortino, S. Overcoming doxorubicin resistance with lipid-polymer hybrid nanoparticles photoreleasing nitric oxide. Mol. Pharm. 2020, 17, 2135–2144. [Google Scholar] [CrossRef]
- Chegaev, K.; Fraix, A.; Gazzano, E.; Abd-Ellatef, G.E.F.; Blangetti, M.; Rolando, B.; Conoci, S.; Riganti, C.; Fruttero, R.; Gasco, A.; et al. Light-regulated NO release as a novel strategy to overcome doxorubicin multidrug resistance. ACS Med. Chem. Lett. 2017, 8, 361–365. [Google Scholar] [CrossRef]
- Fraix, A.; Parisi, C.; Failla, M.; Chegaev, K.; Spyrakis, F.; Lazzarato, L.; Fruttero, R.; Gasco, A.; Sortino, S. NO release regulated by doxorubicin as green light-harvesting antenna. Chem. Commun. 2020, 56, 6332–6335. [Google Scholar] [CrossRef]
- Ghionea, S.; Mabrouka, N.; Paula, C.; Bettaieba, A.; Plenchettea, S. Protein kinase inhibitor-based cancer therapies: Considering the potential of nitric oxide (NO) to improve cancer treatment. Biochem. Pharmacol. 2020, 176, 113855–113865. [Google Scholar] [CrossRef]
- Malanga, M.; Seggio, M.; Kirejev, V.; Fraix, A.; Di Bari, I.; Fenyvesi, E.; Ericson, M.B.; Sortino, S. A phototherapeutic fluorescent β-cyclodextrin branched polymer delivering nitric oxide. Biomater. Sci. 2019, 7, 2272–2276. [Google Scholar] [CrossRef]
- Giglio, V.; Viale, M.; Bertone, V.; Maric, I.; Vaccarone, R.; Vecchio, G. Cyclodextrin polymers as nanocarriers for sorafenib. Investig. New Drugs 2018, 36, 370–379. [Google Scholar] [CrossRef]
- Fraix, A.; Kirejev, V.; Malanga, M.; Fenyvesi, E.; Beni, S.; Ericson, M.B.; Sortino, S. A three-color fluorescent supramolecular nanoassembly of phototherapautics activatable by two-photon excitation with near infrared light. Chem. Eur. J. 2019, 23, 7091–7095. [Google Scholar] [CrossRef]
- Swaminathan, J.; Garcia-Amros, A.; Fraix, A.; Kandoth, N.; Sortino, S.; Raymo, F.M. Photoresponsive polymer nanocarriers with a multifunctional cargo. Chem. Soc. Rev. 2014, 43, 4167–4178. [Google Scholar] [CrossRef] [PubMed]
- Kandoth, N.; Kirejev, V.; Monti, S.; Gref, R.; Ericson, M.B.; Sortino, S. Two-photon-fluorescence imaging and bimodal phototherapy of epidermal cancer cells with biocompatible self-assembled polymer nanoparticles. Biomacromolecules 2014, 15, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Kirejev, V.; Kandoth, N.; Gref, R.; Ericson, M.B.; Sortino, S. A polymer-based nanodevice for the photoregulated release of NO with two-photon fluorescence reporting in skin carcinoma cells. J. Mater. Chem. B 2014, 2, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Fraix, A.; Kandoth, N.; Manet, I.; Cardile, V.; Graziano, A.C.E.; Gref, R.; Sortino, S. An engineered nanoplatform for bimodal anticancer phototherapy with dual-color fluorescence detection of sensitizers. Chem. Commun. 2013, 49, 4459–4461. [Google Scholar] [CrossRef] [PubMed]
- Coriat, R.; Nicco, C.; Chereau, C.; Mir, O.; Alexandre, J.; Ropert, S.; Weill, B.; Chaussade, S.; Goldwasser, F.; Batteux, F. Sorafenib-induced hepatocellular carcinoma cell death depends on reactive oxygen species production in vitro and in vivo. Mol. Cancer Ther. 2012, 11, 2284–2293. [Google Scholar] [CrossRef]
- Goldstein, S.; Czapski, G. The role of the reactions of NO with superoxide and oxygen in biological systems: A kinetic approach. Free Radic. Biol. Med. 1995, 19, 505–510. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laneri, F.; Graziano, A.C.E.; Seggio, M.; Fraix, A.; Malanga, M.; Béni, S.; Longobardi, G.; Conte, C.; Quaglia, F.; Sortino, S. Enhancing the Anticancer Activity of Sorafenib through Its Combination with a Nitric Oxide Photodelivering β-Cyclodextrin Polymer. Molecules 2022, 27, 1918. https://doi.org/10.3390/molecules27061918
Laneri F, Graziano ACE, Seggio M, Fraix A, Malanga M, Béni S, Longobardi G, Conte C, Quaglia F, Sortino S. Enhancing the Anticancer Activity of Sorafenib through Its Combination with a Nitric Oxide Photodelivering β-Cyclodextrin Polymer. Molecules. 2022; 27(6):1918. https://doi.org/10.3390/molecules27061918
Chicago/Turabian StyleLaneri, Francesca, Adriana C. E. Graziano, Mimimorena Seggio, Aurore Fraix, Milo Malanga, Szabolcs Béni, Giuseppe Longobardi, Claudia Conte, Fabiana Quaglia, and Salvatore Sortino. 2022. "Enhancing the Anticancer Activity of Sorafenib through Its Combination with a Nitric Oxide Photodelivering β-Cyclodextrin Polymer" Molecules 27, no. 6: 1918. https://doi.org/10.3390/molecules27061918
APA StyleLaneri, F., Graziano, A. C. E., Seggio, M., Fraix, A., Malanga, M., Béni, S., Longobardi, G., Conte, C., Quaglia, F., & Sortino, S. (2022). Enhancing the Anticancer Activity of Sorafenib through Its Combination with a Nitric Oxide Photodelivering β-Cyclodextrin Polymer. Molecules, 27(6), 1918. https://doi.org/10.3390/molecules27061918









