A Narrative Review of Sulfur Compounds in Whisk(e)y
Abstract
:1. Introduction
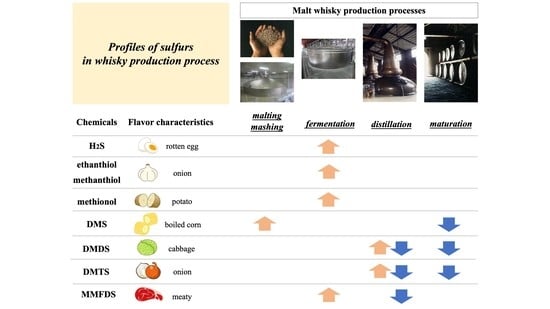
2. Formation and Removal of Sulfur Compounds in the Whisky Production Process
2.1. Formation of Dimethyl Sulfide in Malting
2.2. Formation of Sulfur Donors during Fermentation
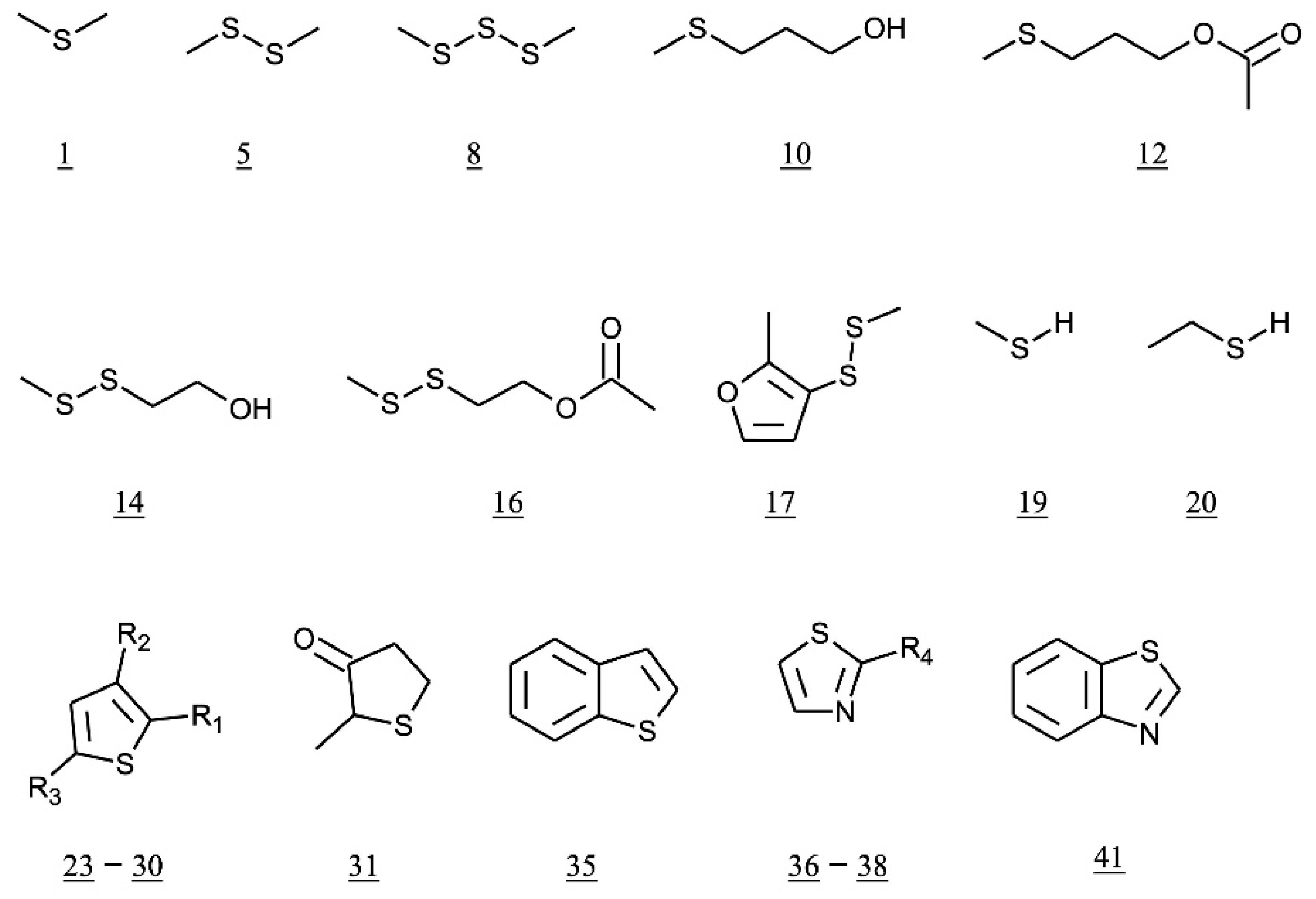
| No. | Class | Compound Name | Common Name | Odor Description (a) | References |
|---|---|---|---|---|---|
| 1 | sulfides | methylsulfanylmethane | dimethyl sulfide | sweet corn | Leppänen et al. [44] |
| 2 | ethylsulfanylethane | diethyl sulfide | garlic-like | Masuda and Nishmura [31] | |
| 3 | 1-propylsulfanylpropane | dipropyl sulfide | garlic, onion | Campillo et al. [32] | |
| 4 | methylsulfanylpropane | methyl propyl sulfide | green, leek | Campillo et al. [32] | |
| 5 | methyldisulfanylmethane | dimethyl disulfide | vegetable | Leppänen et al. [44] | |
| 6 | propyldisulfanylpropane | dipropyl disulfide | green onion | Campillo et al. [32] | |
| 7 | 2-methyl-1-(methyldisulfanyl)propane | iso-butyl methyl disulfide | nd | MacNamara [35] (b) | |
| 8 | methyltrisulfanylmethane | dimethyl trisulfide | onion, meaty | Leppänen et al. [44] | |
| 9 | 2-methylsulfanylethanol | 2-(methylthio) ethanol | meaty | Taniguchi et al. [33] | |
| 10 | 3-methylsulfanylpropan-1-ol | 3-(methylthio) propanol | boiled potato | Masuda and Nishimura [31] | |
| 11 | 3-methylsulfanylpropanal | 3-(methylthio) propanal | onion, meaty | Masuda and Nishimura [31] | |
| 12 | 3-methylsulfanylpropyl acetate | 3-(methylthio)propyl acetate | potato | Masuda and Nishimura [31] | |
| 13 | S-methyl ethanethioate | S-methyl thioacetate | cheese | Leppänen et al. [44] | |
| 14 | 2-(methyldisulfanyl)ethan-1-ol | 3,4-dithiapentyl alcohol | nd | Taniguchi et al. [33] | |
| 15 | 1-ethoxy-2-(methyldisulfanyl)ethane | 3,4-dithiapentyl ethyl ether | nd | Taniguchi et al. [33] | |
| 16 | 2-(methyldisulfanyl)ethyl acetate | 3,4-dithiapentyl acetate | nd | Taniguchi et al. [33] | |
| 17 | 2-methyl-3-(methyldisulfanyl)furan | methyl-(2-methyl-3-furyl) disulfide | meaty, sulfury | Cater-Tjimstra [34] | |
| 18 | thiols | sulfane | hydrogen sulfide | rotten egg | Ronkainen et al. [30] |
| 19 | methanethiol | rotten cabbage | Ronkainen et al. [30] | ||
| 20 | ethanethiol | leek | Ronkainen et al. [30] | ||
| 21 | mercapto esters | ethyl 3-methylsulfanyl propanoate | ethyl 3-(methylthio) propanoate | pineapple | Masuda and Nishimura [31] |
| 22 | ethyl 2-methylsulfanyl acetate | ethyl 2-(methylthio) acetate | green tropical | MacNamara [35] | |
| 23 | thiophenes | thiophene | garlic | Masuda and Nishimura [31] | |
| 24 | 2-methylthiophenone | meaty, cooked | Masuda and Nishimura [31] | ||
| 25 | 2,5-dimethylthiophene | nutty, green | Masuda and Nishimura [31] | ||
| 26 | thiophene-2-carbaldehyde | thiophene-2-carboxaldehyde | benzaldehyde-like | Masuda and Nishimura [31] | |
| 27 | thiophene-3-carbaldehyde | thiophene-3-carboxaldehyde | nd | Ochiai et al. [36] | |
| 28 | 3-methylthiophene-2-carbaldehyde | 3-methylthiophene-2-carboxaldehyde | nd | Ochiai et al. [36] | |
| 29 | 3-ethylthiophene-2-carbaldehyde | 3-ethylthiophene-2-carboxaldehyde | nd | Ochiai et al. [36] | |
| 30 | 5-methylthiophene-2-carbaldehyde | 5-methylthiophene-2-carboxaldehyde | benzaldehyde-like | Masuda and Nishimura [31] | |
| 31 | 2-methylthiolan-3-one | dihydro-2-methyl-3(2H)-thiophenone | sulfur, fruity | Masuda and Nishimura [31] | |
| 32 | 1-thiophen-2-ylethanone | 2-acetyl thiophene | nutty | MacNamara [35] | |
| 33 | 1-thiophen-2-ylbutan-1-one | 2-butanoyl thiophene | meaty | MacNamara [35] | |
| 34 | 1-(5-methylthiophen-2-yl)ethanone | 2-acetyl-5-methyl thiophene | sweet, spicy | MacNamara [35] (b) | |
| 35 | 1-benzothiophene | rubbery | Masuda and Nishimura [31] | ||
| 36 | thiazoles | 1,3-thiazole | nutty, meaty | Masuda and Nishimura [31] | |
| 37 | 2-methyl-1,3-thiazole | vegetable | Ochiai et al. [36] | ||
| 38 | 1-(1,3-thiazol-2-yl)ethanone | 2-acetyl-1,3-thiazole | popcorn | MacNamara and Hoffmann [38] | |
| 39 | 5-ethenyl-4-methyl-1,3-thiazole | 4-methyl-5-vinyl-1,3-thiazole | nutty | Piggott [37] | |
| 40 | 1,3-benzothiazole | rubbery | Masuda and Nishimura [31] | ||
| 41 | 2-methyl-1,3-benzothiazole | rubbery, coffee | Ochiai et al. [36] | ||
| 42 | 3-ethyl-1,3-benzothiazole-2-thione | 3-ethyl-1,3-benzothiazolethione | nd | Ochiai et al. [36] | |
| 43 | 2-(furan-2-yl)-1,3-thiazole | 2-(2-furanyl)-thiazole | nd | MacNamara [35] |
2.3. Formation and Removal of Sulfur Volatiles during Distillation
2.4. Decrease in Alkyl Sulfides during Maturation
3. Chemical Analysis of Sulfur Compounds
| Sample Preparation | Separation | Detector | References |
|---|---|---|---|
| headspace | GC | FPD | Ronkaine et al. [30] |
| headspace | GC | FPD | Leppänen et al. [66] |
| liquid/liquid extraction | GC | MS | Masuda and Nishimura [31] |
| liquid/liquid extraction | MDGC | MS, ECD | Carter-Tijmstra [34] |
| not shown | MDGC | SCD, NTD, MS | MacNamara an Hoffmann [38] |
| vacuum distillation, preparative GC | MDGC | SCD, MS | MacNamara [35] |
| counter-current chromatography | GC | MS | Taniguchi et al. [33] |
| headspace SPME | GC | MS | Campillo et al. [32] |
| headspace Tenax | GC | SCD | Harrison et al. [14] |
| full evaporation dynamic headspace | MDGC | SCD, NCD, MS | Ochiai et al. [36] |
| headspace SPME | GC | MS | Dziekońska-Kubczak et al. [67] |
| solvent assisted flavor extraction | GC | MS | Kerley and Munafo [68] |
| headspace SPME | GC | MS | Daute et al. [13] |
4. Sensory Evaluation and Its Contribution to Quality
5. Control of Sulfur Compounds in Whisky
6. Conclusions and Looking Forward
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wright, S.; Pilkington, H. Whiskies of Canada and the United States. In Whisky and Other Spirits; Russell, I., Stewart, G.G., Kellershohn, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 87–104. [Google Scholar]
- Fukuyo, S.; Myojo, Y. Japanese whisky. In Whisky and Other Spirits; Russell, I., Stewart, G.G., Kellershohn, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 7–16. [Google Scholar]
- Quinn, D.; Nation, B. Irish whiskey. In Whisky and Other Spirits; Russell, I., Stewart, G.G., Kellershohn, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 125–136. [Google Scholar]
- Wright, S.A. Worldwide distilled spirits production. In The Alcohol Textbook, 6th ed.; Walker, G.M., Abbas, C., Ingledew, W.M., Pilgrim, C., Eds.; Lallemand Biofuels & Distilled Spirits: Duluth, MN, USA, 2017; pp. 23–39. ISBN 9789692930885. [Google Scholar]
- Pielech-Przybylska, K.; Balcerek, M. New trends in spirit beverages production. In Alcoholic Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 65–111. ISBN 9780128152690. [Google Scholar]
- Walker, G.M.; Lappe-Oliveras, P.; Moreno-Terrazas, C.R.; Kirchmayr, M.; Arellano-Plaza, M.; Gschaedler-Mathis, A.C. Yeasts associated with the production of distilled alcoholic beverages. In Yeasts in the Production of Wine; Romano, P., Ciani, M., Fleet, G.H., Eds.; Springer: New York, NY, USA, 2019; pp. 477–512. ISBN 9781493997824. [Google Scholar]
- Ługowoj, S.; Balcerek, M. Traditional and new raw materials for spirit beverage production. Folia Biol. Oecologica 2021, 17, 70–78. [Google Scholar] [CrossRef]
- Piggott, J.R. Whisky, whiskey and bourbon: Composition and analysis of whisky. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 514–518. ISBN 9780123849533. [Google Scholar] [CrossRef]
- Wanikawa, A. Flavors in malt whisky: A review. J. Am. Soc. Brew. Chem. 2020, 78, 260–278. [Google Scholar] [CrossRef]
- Palmer, G.H. Beverages: Distilled. In Encyclopedia of Food Grains, 2nd ed.; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Academic Press: Oxford, UK, 2016; pp. 193–205. ISBN 9780123947864. [Google Scholar]
- Steele, G.M.; Fotheringham, R.N.; Jack, F.R. Understanding flavour development in scotch whisky. In Distilled Spirits: Tradition and Innovation; Bryce, J.H., Stewart, G.G., Eds.; Nottingham University Press: Nottingham, UK, 2004; pp. 161–167. ISBN 9781897676394. [Google Scholar]
- Murry, D.M. Distillation practices for beverage alcohol and impact on flavour. In The Alcohol Textbook, 6th ed.; Walker, G.M., Abbas, C., Ingledew, W.M., Pilgrim, C., Eds.; Lallemand Biofuels & Distilled Spirits: Duluth, MN, USA, 2017; pp. 455–470. ISBN 9789692930885. [Google Scholar]
- Daute, M.; Jack, F.; Baxter, I.; Harrison, B.; Grigor, J.; Walker, G. Comparison of three approaches to assess the flavour characteristics of scotch whisky spirit. Appl. Sci. 2021, 11, 1410. [Google Scholar] [CrossRef]
- Harrison, B.; Fagnen, O.; Jack, F.; Brosnan, J. The impact of copper in different parts of malt whisky pot stills on new make spirit composition and aroma. J. Inst. Brew. 2011, 117, 106–112. [Google Scholar] [CrossRef]
- Russell, I.; Stewart, G.G. Distilling yeast and fermentation. In Whisky and Other Spirits; Russell, I., Stewart, G.G., Kellershohn, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 213–236. ISBN 9780128220764. [Google Scholar]
- Stewart, G.G. The production of secondary metabolites with flavour potential during brewing and distilling wort fermentations. Fermentation 2017, 3, 63. [Google Scholar] [CrossRef] [Green Version]
- Walker, G.M.; Hill, A.E. Saccharomyces cerevisiae in the production of whisk(e)y. Beverages 2016, 2, 38. [Google Scholar] [CrossRef] [Green Version]
- Masuda, M.; Komura, H. Whisky flavour (3). J. Brew. Soc. JPN 1993, 88, 201–207. [Google Scholar] [CrossRef]
- Masuda, M.; Nishimura, K.-I.-C. Occurrence and formation of damascenone, trans-2, 6, 6-Trimethyl- 1-Crotonyl-Cyclohexa-1, 3-Dien in alcohol beverages. J. Food Sci. 1980, 45, 396–397. [Google Scholar] [CrossRef]
- Strickland, M. Cask chemistry. In Cask Management for Distillers; White Mule Press: Hayward, UK, 2020; pp. 45–51. ISBN 9781732235496. [Google Scholar]
- Piggott, J.R. Whisky. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Sanromán, M.Á., Du, G., Soccol, C.R., Dussap, C.-G., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 435–450. ISBN 9780444636669. [Google Scholar]
- Nishimura, K.; Matsuyama, R. Maturation and maturation chemistry. In The Science and Technology of Whiskies; Piggott, J.R., Sharp, R., Duncan, R.E.B., Eds.; Longman Scientific & Technical: Harlow, UK, 1989; pp. 235–263. ISBN 9780582044289. [Google Scholar]
- Buxton, I.; Hughes, P.S. Wood chemistry and maturation of whisky. In The Science and Commerce of Whisky; Buxton, I., Hughes, P.S., Eds.; Royal Society of Chemistry: London, UK, 2020; pp. 143–180. ISBN 9781788017107. [Google Scholar]
- Flamini, R.; Panighel, A.; De Marchi, F. Mass spectrometry in the study of wood compounds released in the barrel-aged wine and spirits. Mass Spectrom. Rev. 2021, e21754. [Google Scholar] [CrossRef]
- Morishima, K.; Nakamura, N.; Matsui, K.; Tanaka, Y.; Masunaga, H.; Mori, S.; Iwashita, T.; Li, X.; Shibayama, M. Formation of clusters in whiskies during the maturation process. J. Food Sci. 2019, 84, 59–64. [Google Scholar] [CrossRef]
- Shortreen, W.; Richard, P.; Swan, J.S.; Burtles, S. The flavour terminology of scotch whisky. Brew. Guard. 1979, 108, 57, 59, 61–62. [Google Scholar]
- Lee, K.-Y.M.; Paterson, A.; Piggott, J.R.; Richardson, G.D. Origins of flavour in whiskies and a revised flavour wheel: A review. J. Inst. Brew. 2001, 107, 287–313. [Google Scholar] [CrossRef] [Green Version]
- Jack, F.R. Whiskies: Composition, sensory properties and sensory analysis. In Alcoholic Beverages; Piggott, J., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 379–392. ISBN 9780857090515. [Google Scholar]
- Miller, G.H. Whisky Science: A Condensed Distillation; Springer: Cham, Switzerland, 2019; ISBN 9783030137328. [Google Scholar]
- Ronkainen, P.; Denslow, J.; Leppänen, O. The gas chromatographic analysis of some volatile sulfur compounds. J. Chromatogr. Sci. 1973, 11, 384–390. [Google Scholar] [CrossRef]
- Masuda, M.; Nishimura, K.-I.-C. Changes in volatile sulfur compounds of whisky during aging. J. Food Sci. 1982, 47, 101–105. [Google Scholar] [CrossRef]
- Campillo, N.; Peñalver, R.; López-García, I.; Hernández-Córdoba, M. Headspace solid-phase microextraction for the determination of volatile organic sulphur and selenium compounds in beers, wines and spirits using gas chromatography and atomic emission detection. J. Chromatogr. A 2009, 1216, 6735–6740. [Google Scholar] [CrossRef]
- Taniguchi, T.; Miyajima, N.; Komura, H. An application of centrifugal counter-current chromatography on flavor chemistry- separation of aroma substances in whisky new distillates-. In Developments in Food Science; Charalambous, G., Ed.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 37, pp. 1767–1778. [Google Scholar]
- Carter-Tijmstra, J. Whiskey Flavour Analysis. In Proceedings of the 3rd Aviemore Conference on Malting, Brewing and Distilling, Aviemore, UK, 20–24 May 1990; Institute of Brewing: London, UK, 1990; pp. 468–471. [Google Scholar]
- MacNamara, M. Investigation of medium volatil sulfur compounds in whiskey. In Élaboration et Connaissance Des Spiritueux; Cantagrel, R., Ed.; Lavoiseir-Tec & Doc: Paris, France, 1993; pp. 385–391. [Google Scholar]
- Ochiai, N.; Sasamoto, K.; MacNamara, K. Characterization of sulfur compounds in whisky by full evaporation dynamic headspace and selectable one-dimensional/two-dimensional retention time locked gas chromatography-mass spectrometry with simultaneous element-specific detection. J. Chromatogr. A 2012, 1270, 296–304. [Google Scholar] [CrossRef]
- Piggott, J.R.; Paterson, A.; Conner, J.M.; Haack, G. Heterocyclic Nitrogen Compounds in Whisky. In Development of Food Science, Food Flavour, Ingrediens and Composition; Charalambous, G., Ed.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 521–532. [Google Scholar]
- MacNamara, K.; Hoffmann, A. Simultaneous Nitrogen, Sulfur, and Mass Spectrometric Analysis after Multi-Column Switching of Complex Whiskey Flavour Extracts. Available online: https://gerstel.com/de/pdf/p-gc-an-1993-02-ar.pdf (accessed on 19 December 2019).
- Stewart, G.G.; Ryder, D.S. Sulfur metabolism during brewing. Tech. Q. Master Brew. Assoc. Am. 2019, 56, 39–46. [Google Scholar] [CrossRef]
- Parker, J.K.; Elmore, S.; Methven, L. Introduction to aroma copounds in foods. In Flavour Development, Analysis and Perception in Food and Beverages; Parker, J.K., Elmore, J.S., Methven, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–30. ISBN 9781782421115. [Google Scholar]
- Watts, S.H.; Fotheringham, R.N.; Brosnan, J.M.; Meaden, P.G.; Komura, H. The Contribution of MMFDS to the flavour of scotch whisky. In Distilled Spirits: Tradition and Innovation; Bryce, J.K., Stewart, G.G., Eds.; Nottingham University Press: Nottingham, UK, 2004; pp. 181–185. [Google Scholar]
- Bamforth, C.W. Dimethyl sulfide—Significance, origins, and control. J. Am. Soc. Brew. Chem. 2014, 72, 165–168. [Google Scholar] [CrossRef] [Green Version]
- Bathgate, G.N. The influence of malt and wort processing on spirit character: The lost styles of scotch malt whisky. J. Inst. Brew. 2019, 125, 200–213. [Google Scholar] [CrossRef]
- Leppänen, O.; Denslow, J.; Ronkainen, P. Determination of thiolacetates and some other volatile sulfur compounds in alcoholic beverages. J. Agric. Food Chem. 1980, 28, 359–362. [Google Scholar] [CrossRef]
- Schreier, P.; Drawert, F.; Junker, A.; Barton, H.; Leupold, G. Biosynthesis of aroma compounds by microorganisms II. Formation of sulphur containing flavour substances from methionine by Saccharomyces cerevisiae. Z. Lebensm. Unters. Forsch. 1976, 162, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Etschmann, M.M.W.; Kötter, P.; Hauf, J.; Bluemke, W.; Entian, K.-D.; Schrader, J. Production of the aroma chemicals 3-(methylthio)-1-Propanol and 3-(methylthio)-Propylacetate with yeasts. Appl. Microbiol. Biotechnol. 2008, 80, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Deed, R.C.; Hou, R.; Kinzurik, M.I.; Gardner, R.C.; Fedrizzi, B. The role of yeast ARO8, ARO9 and ARO10 genes in the biosynthesis of 3-(methylthio)-1-Propanol from L-Methionine during fermentation in synthetic grape medium. FEMS Yeast Res. 2019, 19, foy109. [Google Scholar] [CrossRef] [PubMed]
- Kinzurik, M.I.; Herbst-Johnstone, M.; Gardner, R.C.; Fedrizzi, B. Hydrogen sulfide production during yeast fermentation causes the accumulation of ethanethiol, s-ethyl thioacetate and diethyl disulfide. Food Chem. 2016, 209, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Kinzurik, M.I.; Deed, R.C.; Herbst-Johnstone, M.; Slaghenaufi, D.; Guzzon, R.; Gardner, R.C.; Larcher, R.; Fedrizzi, B. Addition of volatile sulfur compounds to yeast at the early stages of fermentation reveals distinct biological and chemical pathways for aroma formation. Food Microbiol. 2020, 89, 103435. [Google Scholar] [CrossRef]
- Noba, S.; Yako, N.; Kobayashi, M.; Masuda, S.; Watanabe, T. Search for compounds contributing to onion-like off-flavor in beer and investigation of the cause of the flavor. J. Biosci. Bioeng. 2017, 124, 419–424. [Google Scholar] [CrossRef]
- Noba, S.; Kikuchi, K.; Kato, T.; Kusunoki, K.; Toyota, S.; Kobayashi, M.; Uemura, K.; Nishiyama, M. Elucidation of the formation mechanism of 2-mercapto-3-Methyl-1-Butanol in beer. Part II: Identification of the key enzymes in yeast. J. Am. Soc. Brew. Chem. 2021, 79, 82–89. [Google Scholar] [CrossRef]
- Daute, M.; Jack, F.; Harrison, B.; Walker, G. Experimental whisky fermentations: Influence of wort pretreatments. Foods 2021, 10, 2755. [Google Scholar] [CrossRef]
- Waymark, C.; Hill, A.E. The influence of yeast strain on whisky new make spirit aroma. Fermentation 2021, 7, 311. [Google Scholar] [CrossRef]
- Yomo, H.; Noguchi, Y.; Yonezawa, T. Effect on new-make spirit character due to the performance of brewer’s yeast—(I)physiological changes of yeast during propagation and brewing. In Distilled Spirits: Production, Technology and Innovation; Bryce, J.H., Piggott, J.R., Stewart, G.G., Eds.; Nottingham University Press: Nottigham, UK, 2008; pp. 109–116. [Google Scholar]
- Copper Stills vs. Stainless Steel Stills. Available online: https://www.clawhammersupply.com/blogs/moonshine-still-blog/54804996-copper-stills-vs-stainless-steel-stills (accessed on 3 February 2022).
- Hopfer, H.; Gilleland, G.; Ebeler, S.E.; Nelson, J. Elemental Profiles of whisk(e)y allow differentiation by type and region. Beverages 2017, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Webster, A.; Raffkin, G.; Walker, G.; Murry, D.; Russell, A. A development of organo-sulphur compounds in malt whisky during spirit distillation from two distilleries using different condensers. In Proceedings of the 7th Worldwide Distilled Spirts Conference, Virtual Conference, Edinburgh, UK, 6–8 September 2021. [Google Scholar]
- Nedjma, M.; Hoffmann, N. Hydrogen sulfide reactivity with thiols in the presence of copper (II) in hydroalcoholic solutions or cognac brandies: Formation of symmetrical and unsymmetrical dialkyl trisulfides. J. Agric. Food Chem. 1996, 44, 3935–3938. [Google Scholar] [CrossRef]
- Furusawa, T. The Formation and Reactions of Sulphur Compounds during Distillation. Ph.D. Thesis, Heriot-Watt University, Edinburgh, UK, 1996. [Google Scholar]
- Jack, F.R.; Brosnan, J.M.; Campbell, K.A.; Fagnen, O.; Fotheringham, R.N.; Goodall, I.C. Sensory implications of modified distillation practice in scotch malt whisky production. In Distilled Spirits: Production, Technology and Innovation; Bryce, J.H., Piggott, J.R., Stewart, G.G., Eds.; Nottingham University Press: Nottingham, UK, 2008; pp. 205–211. [Google Scholar]
- Leppänen, O.; Ronkainen, P.; Denslow, J.; Laakso, R.; Lindeman, A.; Nykanen, I. Polysulphides and thiophenes in whisky. In Flavour of Distilled Beverages: Origin and Development; Piggott, J.R., Ed.; E. Horwood Ltd.: Chichester, UK, 1983; pp. 206–214. [Google Scholar]
- Zavahir, J.S.; Nolvachai, Y.; Marriott, P.J. Molecular spectroscopy—Information rich detection for gas chromatography. Trends Analyt. Chem. 2018, 99, 47–65. [Google Scholar] [CrossRef]
- Marriott, P.J.; Chin, S.-T.; Nolvachai, Y. Techniques and application in comprehensive multidimensional gas chromatography—Mass spectrometry. J. Chromatogr. A 2021, 1636, 461788. [Google Scholar] [CrossRef] [PubMed]
- Dimandja, J.-M.D. Chapter 1—Introduction and historical background: The “inside” story of comprehensive two-dimensional gas chromatography. In Separation Science and Technology; Snow, N.H., Ed.; Academic Press: London, UK, 2020; Volume 12, pp. 1–40. [Google Scholar]
- Lebanov, L.; Tedone, L.; Kaykhaii, M.; Linford, M.R.; Paull, B. Multidimensional gas chromatography in essential oil analysis. Part 2: Application to characterisation and identification. Chromatographia 2019, 82, 399–414. [Google Scholar] [CrossRef]
- Leppänen, O.; Denslow, J.; Ronkainen, P. A gas chromatographic method for the accurate determination of low concentrations of volatile sulphur compounds in alcoholic beverages. J. Inst. Brew. 1979, 85, 350–353. [Google Scholar] [CrossRef]
- Dziekońska-Kubczak, U.; Pielech-Przybylska, K.; Patelski, P.; Balcerek, M. Development of the method for determination of Volatile Sulfur Compounds (VSCs) in fruit brandy with the use of HS–SPME/GC–MS. Molecules 2020, 25, 1232. [Google Scholar] [CrossRef] [Green Version]
- Kerley, T.; Munafo, J.P. Changes in tennessee whiskey odorants by the lincoln county process. J. Agric. Food Chem. 2020, 68, 9759–9767. [Google Scholar] [CrossRef]
- Ronkainen, P.; Leppänen, O.; Harju, K. The re-use of stillage water in the mashing of grain as a means of energy conservation. J. Inst. Brew. 1978, 84, 115–117. [Google Scholar] [CrossRef]
- Jack, F. Development of guidelines for the preparation and handling of sensory samples in the scotch whisky industry. J. Inst. Brew. 2003, 109, 114–119. [Google Scholar] [CrossRef]
- Lahne, J.; Collins, T.S.; Heymann, H. replication improves sorting-task results analyzed by distatis in a consumer study of american bourbon and rye whiskeys. J. Food Sci. 2016, 81, S1263–S1271. [Google Scholar] [CrossRef]
- Lahne, J.; Abdi, H.; Collins, T.; Heymann, H. Bourbon and rye whiskeys are legally distinct but are not discriminated by sensory descriptive analysis. J. Food Sci. 2019, 84, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.J.; Ochoa, A.; Kerth, C.R.; Miller, R.K.; Murray, S.C. Assessing the impact of corn variety and texas terroir on flavor and alcohol yield in new-make bourbon whiskey. PLoS ONE 2019, 14, e0220787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyraleou, M.; Herb, D.; O’Reilly, G.; Conway, N.; Bryan, T.; Kilcawley, K.N. the impact of terroir on the flavour of single malt whisk(e)y new make spirit. Foods 2021, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Jack, F.R.; Fotheringham, R.N. Others new-make malt spirit quality: A statistical approach. In Distilled Spirits: Tradition and Innovation; Bryce, J.H., Stewart, G.G., Eds.; Nottingham University Press: Nottingham, UK, 2004; pp. 169–172. ISBN 9781897676394. [Google Scholar]
- Lee, K.-Y.M.; Paterson, A.; Piggott, J.R.; Richardson, G.D. Perception of whisky flavour reference compounds by scottish distillers. J. Inst. Brew. 2000, 106, 203–208. [Google Scholar] [CrossRef]
- Daubly, H.; Wardlaw, A. Assessment of charcoals for removal of organic compound. In Distilled Spirits: Local Roots, Global Reach, Delivering Distilling Expertise to the World; Jack, F., Dabrowska, D., Davies, S., Garden, M., Maskell, D., Murry, D., Eds.; Nottingham University Press: Nottingham, UK, 2018; pp. 135–136. [Google Scholar]
- Htet, K.M.M.; Hengniran, P. The Utilization of High Quality Bamboo Charcoal as Adsorbent for Enhancing Distilled Whisky by Filtration Process. Ph.D. Thesis, Kasetsart University, Bangkok, Thailand, 2021. [Google Scholar]
- Cai, L.; Rice, S.; Koziel, J.A.; Jenks, W.S.; van Leeuwen, J.H. Further purification of food-grade alcohol to make a congener-free product. J. Inst. Brew. 2016, 122, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Balcerek, M.; Pielech-Przybylska, K.; Patelski, P.; Dziekońska-Kubczak, U.; Jusel, T. Treatment with activated carbon and other adsorbents as an effective method for the removal of volatile compounds in agricultural distillates. Food Addit. Contam. Part A 2017, 34, 714–727. [Google Scholar] [CrossRef]
- Magee, S. Spirit filtration with activated carbon: Flavour and aroma. In Proceedings of the 7th Worldwide Distilled Spirits Conference, Virtual Conference, Edinburgh, UK, 6–8 September 2021. [Google Scholar]
- Sugimoto, T.; Murata, M.; Kagami, N.; Kawashima, Y.; Asahi, A.; Hosoi, K. Selective removal of sulfide compounds from new make spirit using silver-supported zeolite filtration. In Distilled Spirits: Local Roots, Global Reach, Delivering Distilling Expertise to the World; Jack, F., Dabrowska, D., Davies, S., Garden, M., Maskell, D., Murry, D., Eds.; Nottingham University Press: Nottingham, UK, 2018; pp. 123–128. [Google Scholar]
- Controlling Sulfurs. Available online: https://issuu.com/americancraftspirits/docs/craftspirit_nov2021?fr=sNDQ4NjQ0NjE1NTc (accessed on 1 February 2022).
- American Craft Spirits Association Control of Sulfur Compounds in Malt Whisk(e)y. Available online: https://americancraftspirits.org/product/webinar-control-of-sulfur-compounds-in-malt-whiskey/ (accessed on 22 July 2021).
- Murayama, H.; Yamamoto, Y.; Tone, M.; Hasegawa, T.; Kimura, M.; Ishida, T.; Isogai, A.; Fujii, T.; Okumura, M.; Tokunaga, M. Selective adsorption of 1,3-Dimethyltrisulfane (DMTS) responsible for aged odour in japanese sake using supported gold nanoparticles. Sci. Rep. 2018, 8, 16064. [Google Scholar] [CrossRef] [Green Version]
- Solar, S.; Castro, R.; Guerrero, E.D. New accelerating techniques applied to the ageing of oenological products. Food Rev. Int. 2021, 1–21. [Google Scholar] [CrossRef]
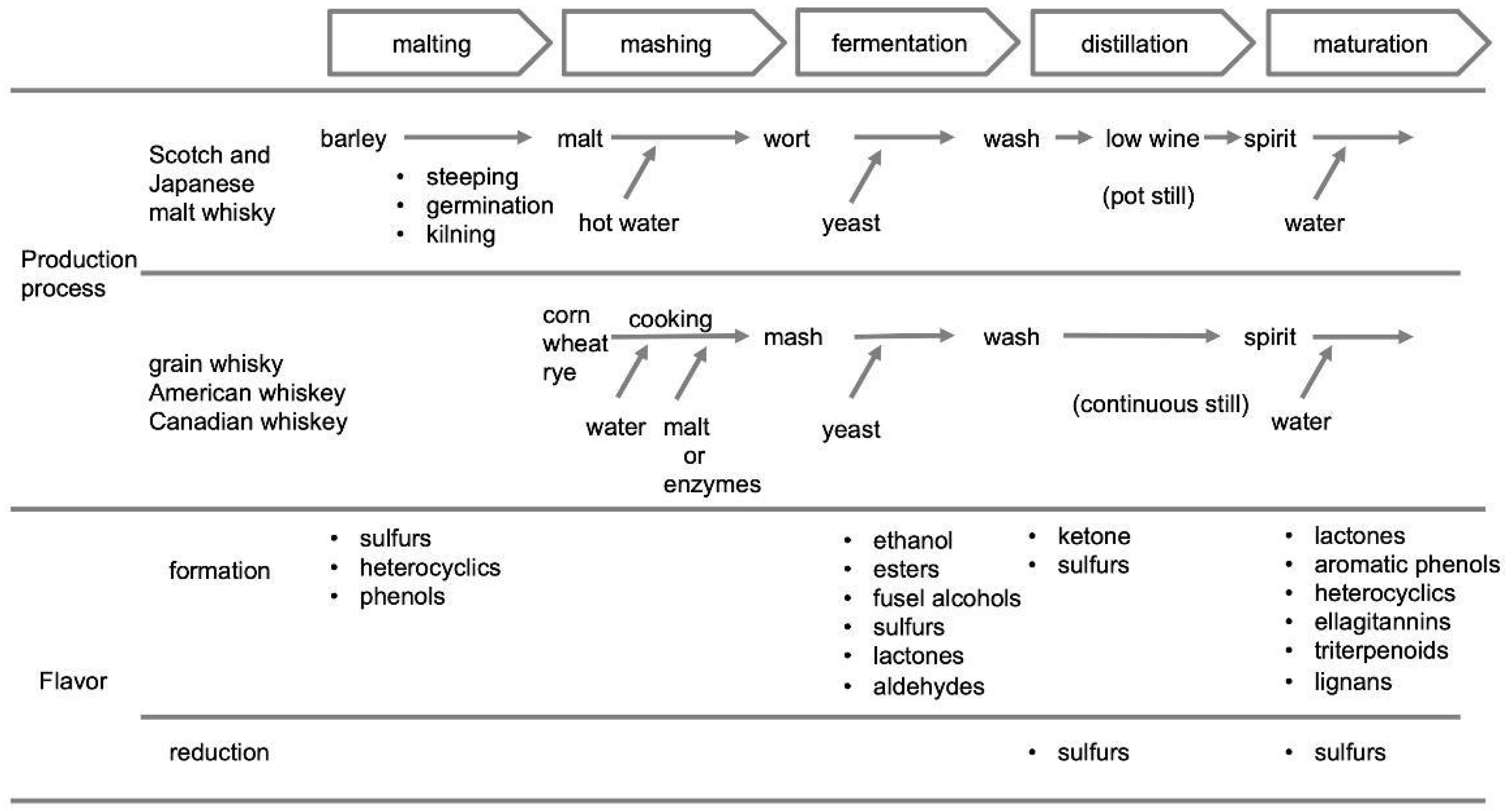
| Scotch and Japanese Whisky | Irish Whiskey | American Whiskey | Canadian Whiskey | |||
|---|---|---|---|---|---|---|
| Malt Whisky | Grain Whisky | Pot Still Whiskey | Grain Whisky | |||
| materials | malted barley | corn, wheat, malted barley | malted barley, barley | corn, wheat, barley | corn, wheat, rye, malted barley | corn, wheat, rye, malted barley, malted rye |
| distillation | batch | continuous | batch | continuous | continuous | continuous |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanikawa, A.; Sugimoto, T. A Narrative Review of Sulfur Compounds in Whisk(e)y. Molecules 2022, 27, 1672. https://doi.org/10.3390/molecules27051672
Wanikawa A, Sugimoto T. A Narrative Review of Sulfur Compounds in Whisk(e)y. Molecules. 2022; 27(5):1672. https://doi.org/10.3390/molecules27051672
Chicago/Turabian StyleWanikawa, Akira, and Toshikazu Sugimoto. 2022. "A Narrative Review of Sulfur Compounds in Whisk(e)y" Molecules 27, no. 5: 1672. https://doi.org/10.3390/molecules27051672
APA StyleWanikawa, A., & Sugimoto, T. (2022). A Narrative Review of Sulfur Compounds in Whisk(e)y. Molecules, 27(5), 1672. https://doi.org/10.3390/molecules27051672