Novel Pomegranate-Nanoparticles Ameliorate Cisplatin-Induced Nephrotoxicity and Improves Cisplatin Anti-Cancer Efficacy in Ehrlich Carcinoma Mice Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Pomegranate-Nanoparticles (PE-NPs)
2.2. Characterization of PE-NPs
2.3. Assessment of the Loading Ratio (LR) and Efficiency of Encapsulation (EE)
2.4. Animals and Treatment
2.5. ESC Induction
2.6. Samples Collection and Storage
2.7. Assessment of Kidney Function
2.8. Determination of Oxidative Stress Indicators
2.9. Determination of Indicators of Inflammation
2.10. Histopathological Investigation
2.11. Data Analysis
3. Results
3.1. Nutritional Composition of Pomegranate Fruit
3.2. Preparation and Physicochemical Characterization
3.3. Effect of PE-NPs on Oxidative Stress, Antioxidant, and Inflammatory Markers in Kidney Tissues
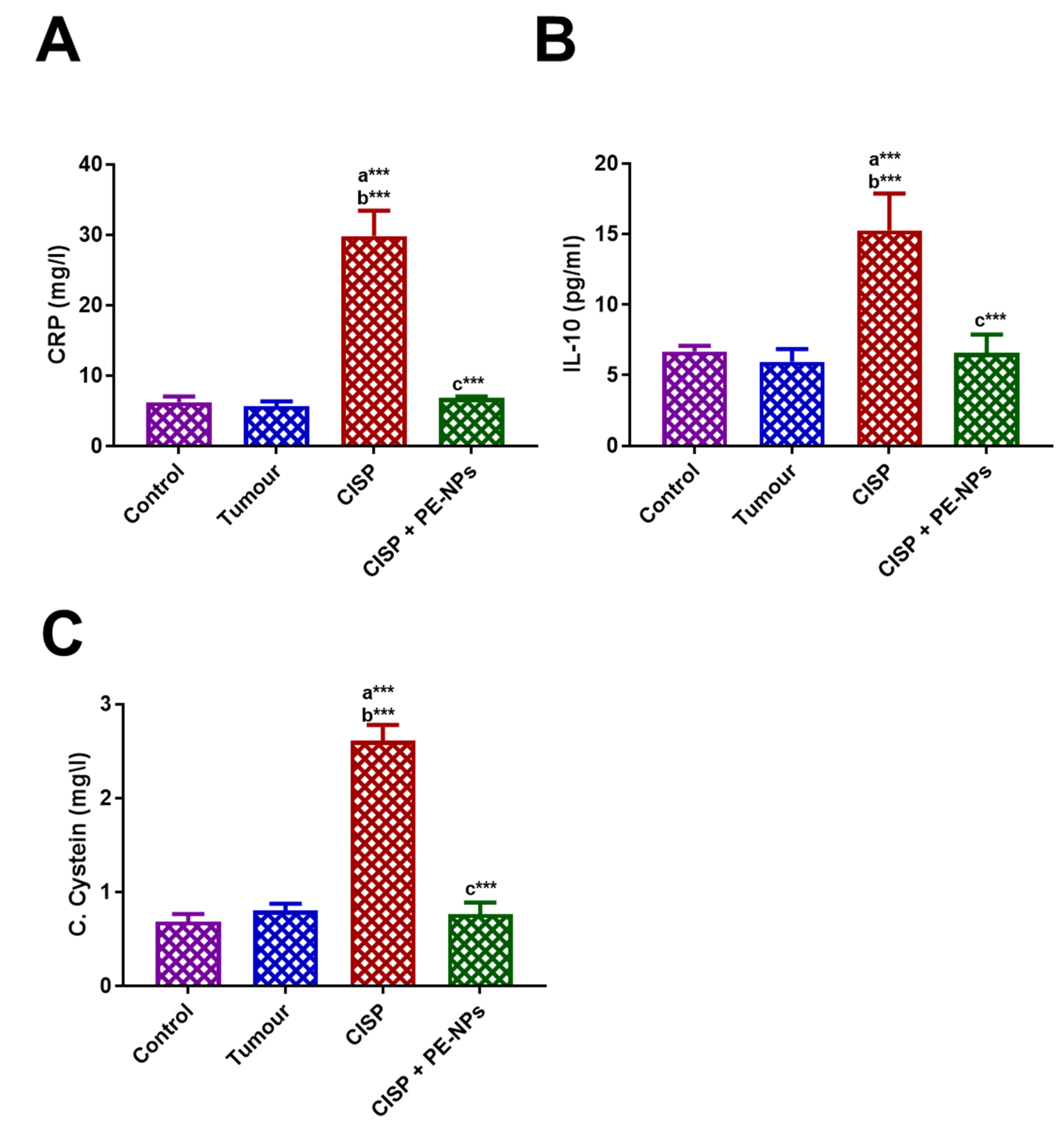
3.4. Effect of PE-NPs on Inflammatory Markers in Serum
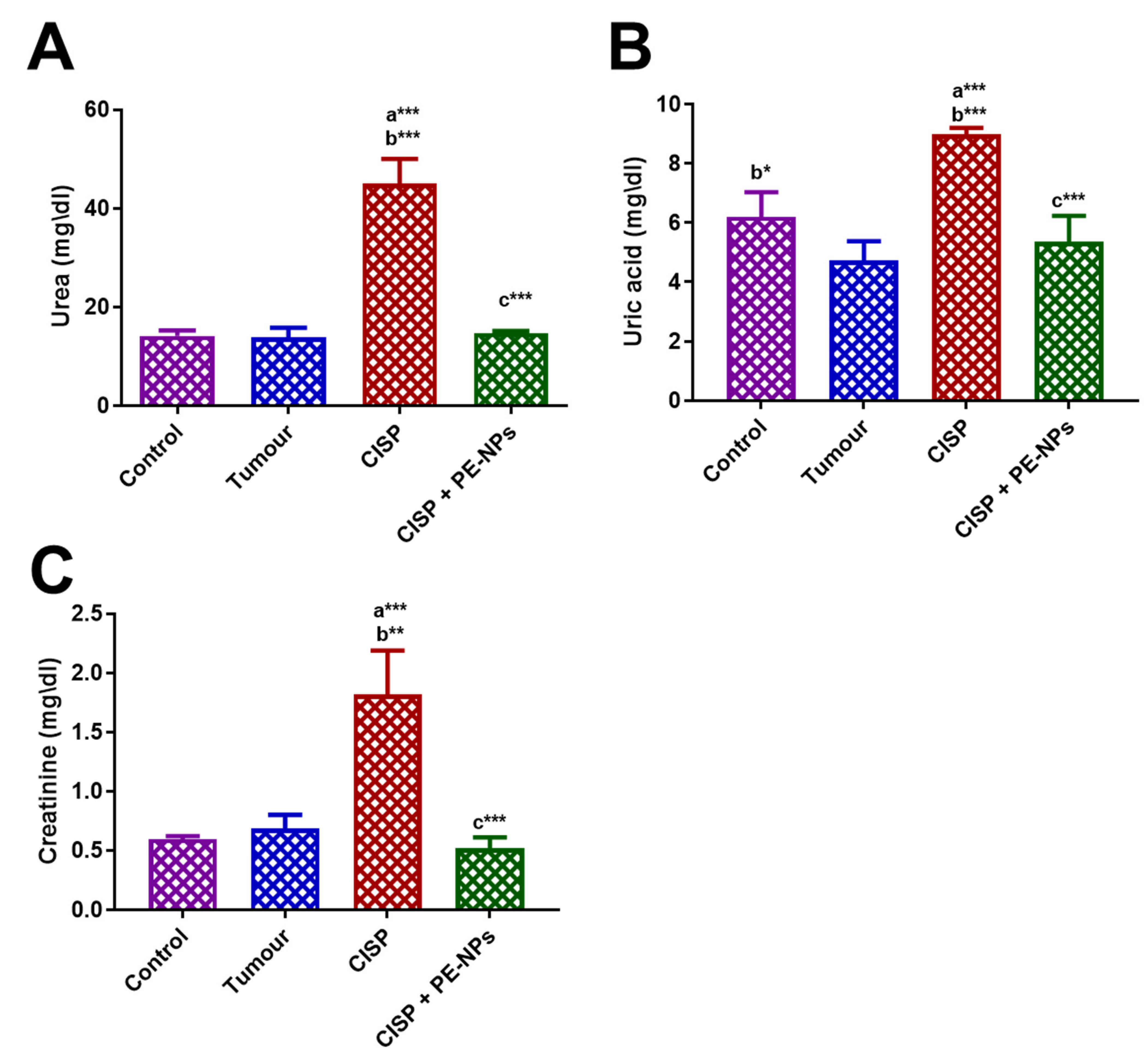
3.5. Effect of PE-NPs on Indicators of Kidney Function in Serum
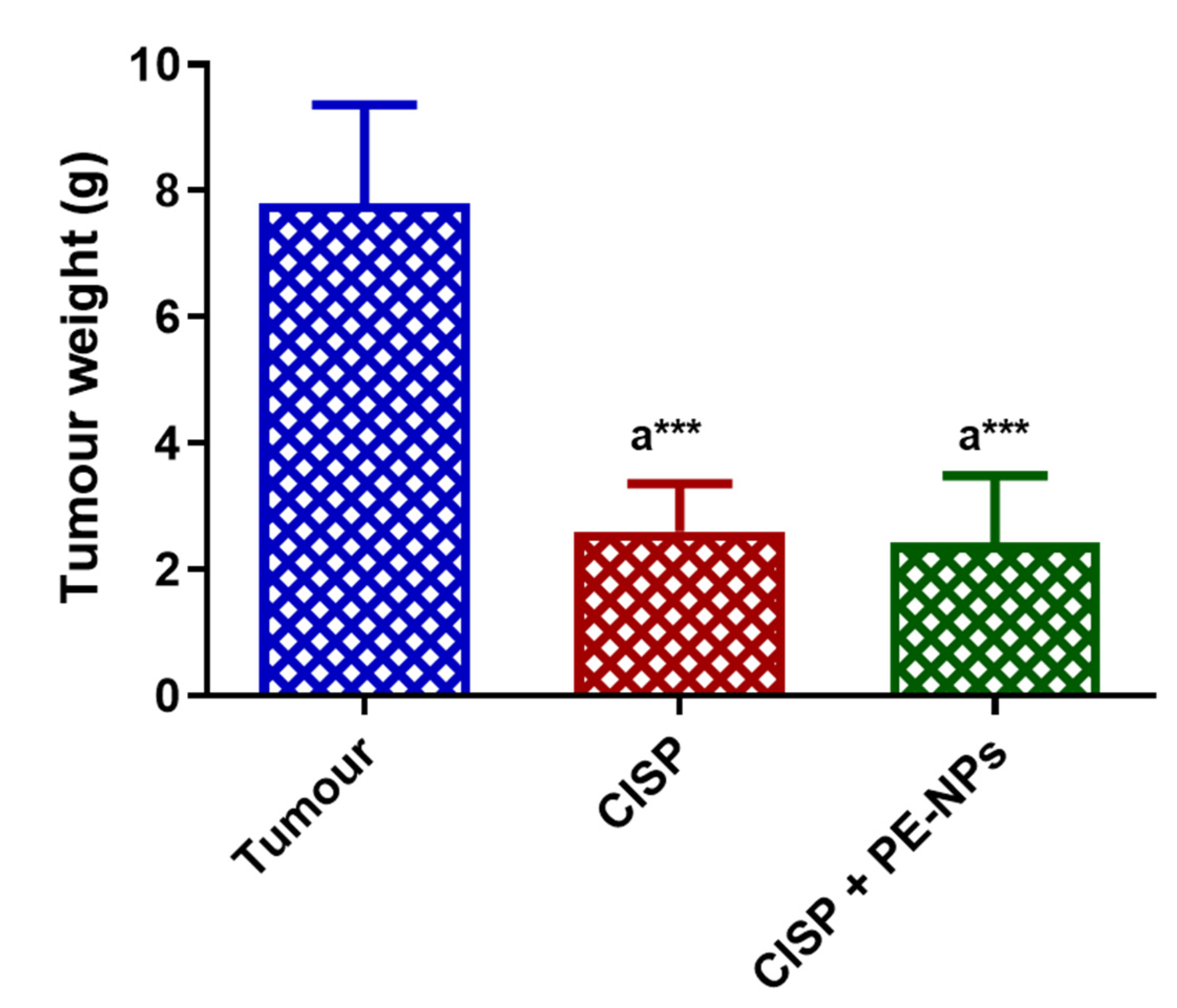
3.6. Effect PE-NPs on the Anticancer Activity of CISP in ESC Mice
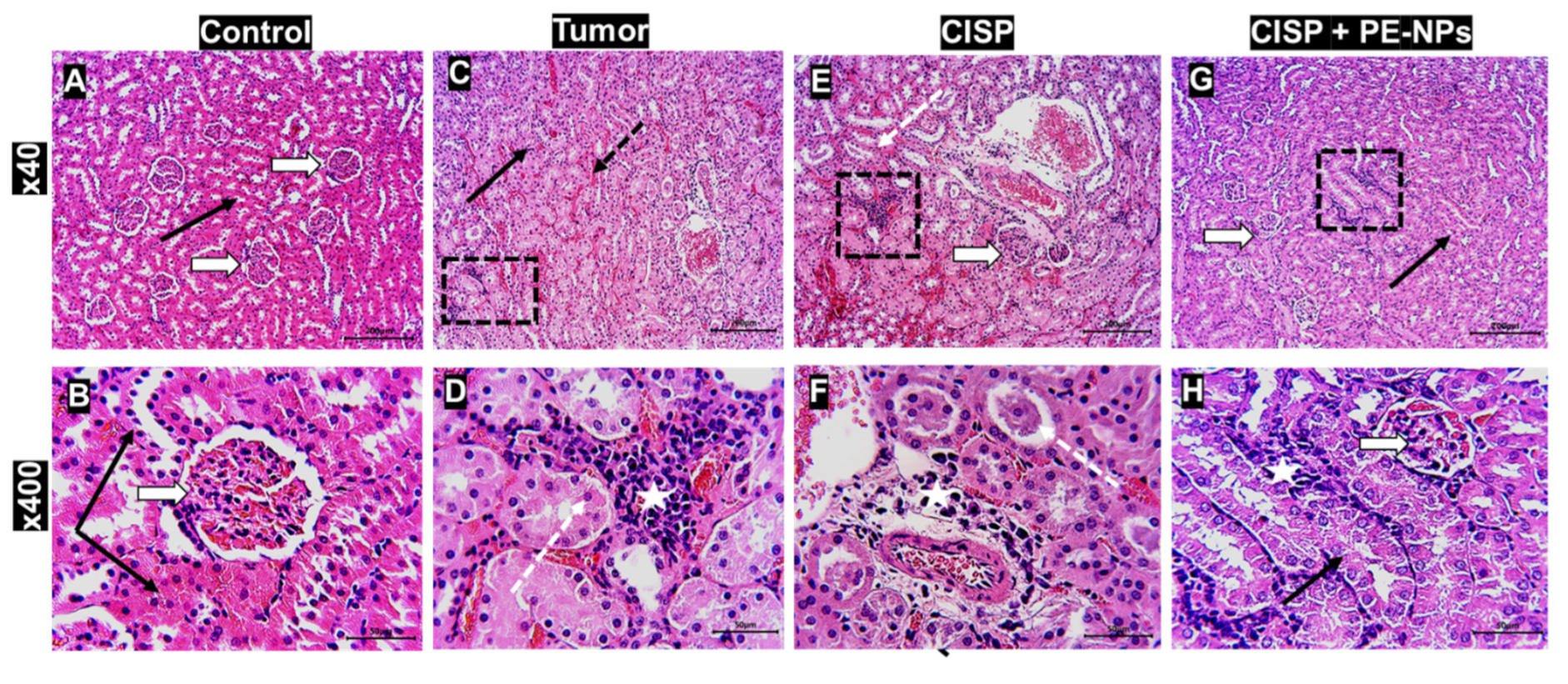
3.7. Effect of Combination of CISP with PE-NPs on Kidney Histopathology in ESC Mice
3.8. Evaluation of the Expression of Caspase-3 Immuno in Kidney Tissue Treated with the Combination of CISP and PE-NPs in ESC Mice
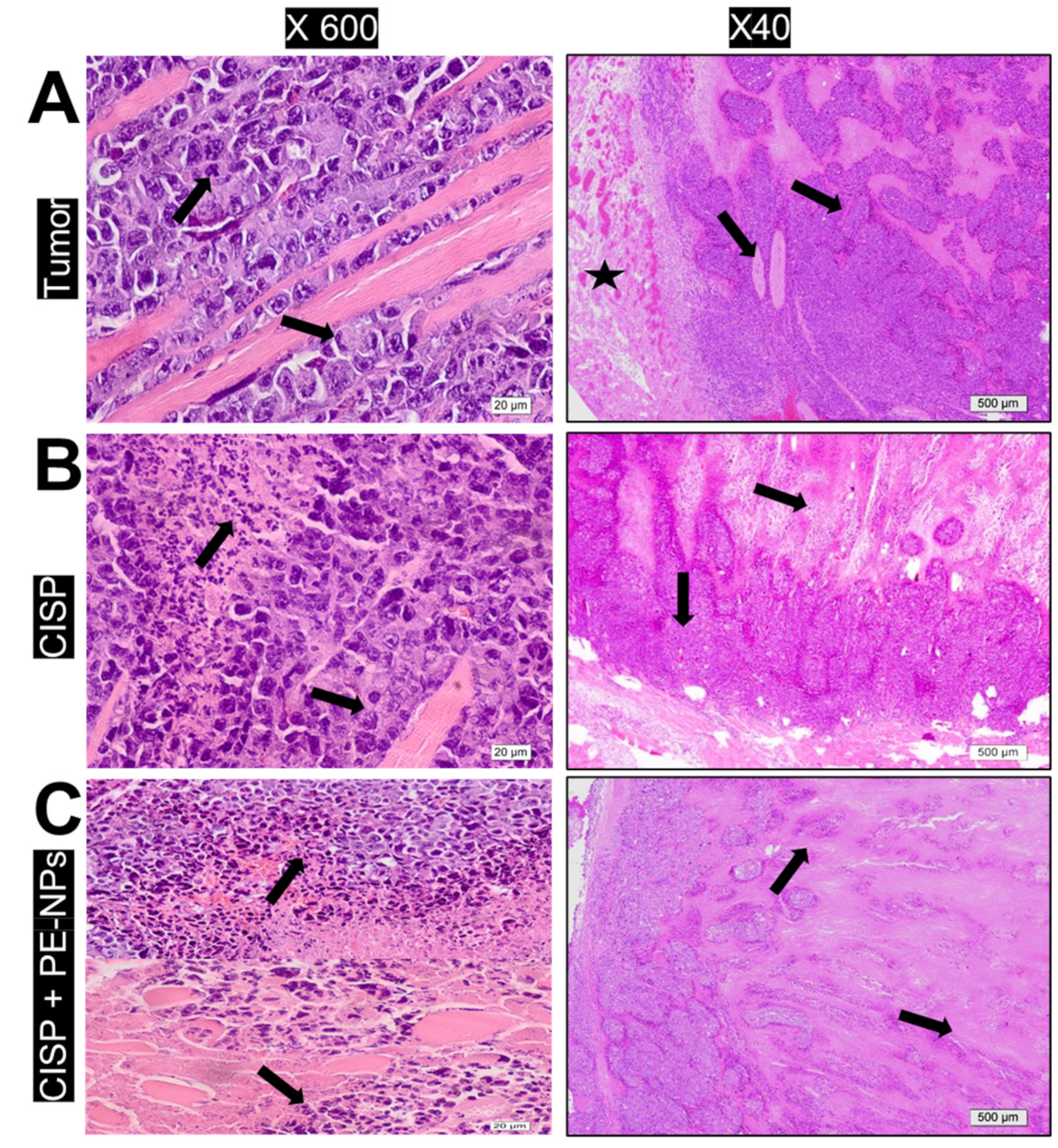
3.9. Effect of Combination of CISP with PE-NPs on Histopathological Features in ESC Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed]
- Desoize, B.; Madoulet, C. Particular aspects of platinum compounds used at present in cancer treatment. Crit. Rev. Oncol./Hematol. 2002, 42, 317–325. [Google Scholar] [CrossRef]
- Nematbakhsh, M.; Pezeshki, Z.; Eshraghi Jazi, F.; Mazaheri, B.; Moeini, M.; Safari, T.; Azarkish, F.; Moslemi, F.; Maleki, M.; Rezaei, A.; et al. Cisplatin-Induced Nephrotoxicity; Protective Supplements and Gender Differences. Asian Pac. J. Cancer Prev. 2017, 18, 295–314. [Google Scholar] [CrossRef]
- Tsang, R.Y.; Al-Fayea, T.; Au, H.-J. Cisplatin overdose. Drug Saf. 2009, 32, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef]
- Leibbrandt, M.E.; Wolfgang, G.H.; Metz, A.L.; Ozobia, A.A.; Haskins, J.R. Critical subcellular targets of cisplatin and related platinum analogs in rat renal proximal tubule cells. Kidney Int. 1995, 48, 761–770. [Google Scholar] [CrossRef][Green Version]
- Dobyan, D.C.; Levi, J.; Jacobs, C.; Kosek, J.; Weiner, M.W. Mechanism of cis-platinum nephrotoxicity: II. Morphologic observations. J. Pharmacol. Exp. Ther. 1980, 213, 551–556. [Google Scholar]
- Zhao, X.; Yuan, Z. Anthocyanins from Pomegranate (Punica granatum L.) and Their Role in Antioxidant Capacities in Vitro. Chem. Biodivers. 2021, 18, e2100399. [Google Scholar] [CrossRef]
- Lavoro, A.; Falzone, L.; Gattuso, G.; Salemi, R.; Cultrera, G.; Leone, G.M.; Scandurra, G.; Candido, S.; Libra, M. Pomegranate: A promising avenue against the most common chronic diseases and their associated risk factors (Review). Int. J. Funct. Nutr. 2021, 2, 6. [Google Scholar] [CrossRef]
- Vidal, A.; Fallarero, A.; Peña, B.R.; Medina, M.E.; Gra, B.; Rivera, F.; Gutierrez, Y.; Vuorela, P.M. Studies on the toxicity of Punica granatum L. (Punicaceae) whole fruit extracts . J. Ethnopharmacol. 2003, 89, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Ameh, M.P.; Mohammed, M.; Ofemile, Y.P.; Mohammed, M.G.; Gabriel, A.; Isaac, A.O. Detoxifying Action of Aqueous Extracts of Mucuna pruriens Seed and Mimosa pudica Root against Venoms of Naja nigricollis and Bitis arietans. Recent Pat. Biotechnol. 2020, 14, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Jurenka, J.S. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. J. Clin. Ther. 2008, 13, 128–144. [Google Scholar]
- Zhu, H.; Rockhold, R.W.; Baker, R.C.; Kramer, R.E.; Ho, I.K. Effects of single or repeated dermal exposure to methyl parathion on behavior and blood cholinesterase activity in rats. J. Biomed. Sci. 2001, 8, 467–474. [Google Scholar] [CrossRef]
- Lamidi, I.Y.; Mikail, H.G.; Adamu, S.; Akefe, I.O.; Tijjani, M.B.; Salihu, S.I.; Olatunji, A.O.; Hassan, A.; Daniel, N.; Adegoke, V.A. Flavonoid fractions of diosmin and hesperidin mitigate lead acetate-induced biochemical, oxidative stress, and histopathological alterations in Wistar rats. Toxicol. Res. 2021, 37, 473–484. [Google Scholar] [CrossRef]
- Akefe, I.O.; Ayo, J.O.; Sinkalu, V.O. Assortment of kaempferol and zinc gluconate improves noise-induced biochemical imbalance and deficits in body weight gain. Exp. Results 2021, 2, e37. [Google Scholar] [CrossRef]
- Akefe, I.; Yusuf, I.; Adegoke, V. C-glycosyl flavonoid orientin alleviates learning and memory impairment by radiofrequency electromagnetic radiation in mice via improving antioxidant defence mechanism. Asian Pac. J. Trop. Biomed. 2019, 9, 518–523. [Google Scholar] [CrossRef]
- Newman, R.A.; Lansky, E.P.; Block, M.L. Pomegranate: The Most Medicinal Fruit; Basic Health Publications, Inc.: Laguna Beach, CA, USA, 2007. [Google Scholar]
- Al-Olayan, E.M.; El-Khadragy, M.F.; Metwally, D.M.; Abdel Moneim, A.E. Protective effects of pomegranate (Punica granatum) juice on testes against carbon tetrachloride intoxication in rats. BMC Complementary Altern. Med. 2014, 14, 164. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J. Pomegranate and its many functional components as related to human health: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef]
- Akefe, I.O.; Ayo, J.O.; Sinkalu, V.O. Kaempferol and zinc gluconate mitigate neurobehavioral deficits and oxidative stress induced by noise exposure in Wistar rats. PLoS ONE 2020, 15, e0236251. [Google Scholar] [CrossRef] [PubMed]
- El-Shitany, N.A.E.-A.; Abbas, A.T.; Ali, S.S.; Eid, B.; Harakeh, S.; Neamatallah, T.; Al-Abd, A.; Mousa, S. Nanoparticles Ellagic Acid Protects Against Cisplatin-induced Hepatotoxicity in Rats Without Inhibiting its Cytotoxic Activity. Int. J. Pharmacol. 2019, 15, 465–477. [Google Scholar] [CrossRef]
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055. [Google Scholar]
- Feczkó, T.; Tóth, J.; Gyenis, J. Comparison of the preparation of PLGA–BSA nano-and microparticles by PVA, poloxamer and PVP. Colloids Surf. A Physicochem. Eng. Asp. 2008, 319, 188–195. [Google Scholar] [CrossRef]
- Alam, S.; Khan, Z.I.; Mustafa, G.; Kumar, M.; Islam, F.; Bhatnagar, A.; Ahmad, F.J. Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: A pharmacoscintigraphic study. Int. J. Nanomed. 2012, 7, 5705. [Google Scholar] [CrossRef]
- Almuhayawi, M.S.; Ramadan, W.S.; Harakeh, S.; Al Jaouni, S.K.; Bharali, D.J.; Mousa, S.A.; Almuhayawi, S.M. The potential role of pomegranate and its nano-formulations on cerebral neurons in aluminum chloride induced Alzheimer rat model. Saudi J. Biol. Sci. 2020, 27, 1710–1716. [Google Scholar] [CrossRef]
- AlDubayan, S.H.; Pyle, L.C.; Gamulin, M.; Kulis, T.; Moore, N.D.; Taylor-Weiner, A.; Hamid, A.A.; Reardon, B.; Wubbenhorst, B.; Godse, R.; et al. Association of Inherited Pathogenic Variants in Checkpoint Kinase 2 (CHEK2) With Susceptibility to Testicular Germ Cell Tumors. JAMA Oncol. 2019, 5, 514–522. [Google Scholar] [CrossRef]
- Elkhawaga, O.-A.; Gebril, S.; Salah, N. Evaluation of anti-tumor activity of metformin against Ehrlich ascites carcinoma in Swiss albino mice. Egypt. J. Basic Appl. Sci. 2019, 6, 116–123. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: New York, NY, USA, 2018. [Google Scholar]
- Jilanchi, S.; Nematbakhsh, M.; Mazaheri, S.; Talebi, A.; Zolfaghari, B.; Pezeshki, Z.; Eshraghi-Jazi, F.; Moeini, M. Pomegranate Flower Extract does not Prevent Cisplatin-Induced Nephrotoxicity in Female Rats. Int. J. Prev. Med. 2014, 5, 1621–1625. [Google Scholar]
- Dehghani, H.; Hashemi, M.; Entezari, M.; Mohsenifar, A. The comparison of anticancer activity of thymoquinone and nanothymoquinone on human breast adenocarcinoma. Iran. J. Pharm. Res. IJPR 2015, 14, 539. [Google Scholar]
- Kuhlmann, M.; Burkhardt, G.; Köhler, H. Insights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical application. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 1997, 12, 2478–2480. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.; Catao, C.; Martins, N.; Curti, C.; Bianchi, M.; Santos, A. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch. Toxicol. 2007, 81, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ramesh, G.; Norbury, C.; Reeves, W. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-α produced by renal parenchymal cells. Kidney Int. 2007, 72, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Saifi, M.A.; Sangomla, S.; Khurana, A.; Godugu, C. Protective effect of nanoceria on cisplatin-induced nephrotoxicity by amelioration of oxidative stress and pro-inflammatory mechanisms. Biol. Trace Elem. Res. 2019, 189, 145–156. [Google Scholar] [CrossRef]
- Ramesh, G.; Reeves, W.B. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Investig. 2002, 110, 835–842. [Google Scholar] [CrossRef]
- Minisy, F.M.; Shawki, H.H.; El Omri, A.; Massoud, A.A.; Omara, E.A.; Metwally, F.G.; Badawy, M.A.; Hassan, N.A.; Hassan, N.S.; Oishi, H. Pomegranate Seeds Extract Possesses a Protective Effect against Tramadol-Induced Testicular Toxicity in Experimental Rats. BioMed Res. Int. 2020, 2020, 2732958. [Google Scholar] [CrossRef]
- Gabriel, A.; Mohammed, M.; Magaji, M.G.; Ofemile, Y.P.; Matthew, A.P.; Akefe, I.O. In Vitro and In Vivo Neutralizing Activity of Uvaria chamae Leaves Fractions on the Venom of Naja nigricollis in Albino Rat and Bovine Blood. Recent Pat. Biotechnol. 2020, 14, 295–311. [Google Scholar] [CrossRef]
- Isaac, O.; Joseph, O.; Victor, O. Mitigative effects of antioxidants in Noise Stress. J. Clin. Nutr. Diet. 2017, 3, 21. [Google Scholar]
- Benzer, F.; Kandemir, F.; Yildirim, N.; Ozan, S. Effect of pomegranate seed extract on free radical damage and antioxidant activity under cisplatin-induced oxidative stress conditions in rabbit testes. Asian J. Chem. 2011, 23, 3231. [Google Scholar]
- Nasser, M.; Damaj, Z.; Hijazi, A.; Merah, O.; Al-Khatib, B.; Hijazi, N.; Trabolsi, C.; Damaj, R.; Nasser, M. Pomegranate Juice Extract Decreases Cisplatin Toxicity on Peripheral Blood Mononuclear Cells. Medicines 2020, 7, 66. [Google Scholar] [CrossRef]



| Groups (n = 7 Each) | Treatment |
|---|---|
| Group I (control) | PBS (0.2 mL)/mouse |
| Group II (negative control) | Tumour (2.6 × 106 cells in PBS) |
| Group III | Tumour + CISP (3.5 mg/kg) |
| Group IV | Tumour + CISP + PE-NPs (1.47 mg/kg) |
| Nutrients | Value Per 100 g | Units |
|---|---|---|
| Potassium | 236 | mg |
| Sodium | 3 | mg |
| Ascorbic acid, total | 10.2 | mg |
| Choline, total | 7.6 | mg |
| Calcium | 10 | mg |
| Iron | 0.3 | mg |
| Magnesium | 12 | mg |
| Phosphorus | 36 | mg |
| Ash | 0.53 | g |
| Carbohydrates | 18.7 | g |
| Fiber | 4 | g |
| Sugars, total | 13.67 | g |
| Water | 77.93 | g |
| Energy | 83 | Kcal |
| Protein | 1.67 | g |
| Total lipid (fat) | 1.17 | g |
| GSH (ng/mL) | SOD (u/mL) | CAT (Mu/L) | MDA (nmol/mL) | TNF-α (pg/mL) | IL10 (pg/mL) | NFKβ (ng/mL) | |
|---|---|---|---|---|---|---|---|
| Control | 12.86 ± 0.51 | 158 ± 2.68 | 111.2 ± 4.2 | 0.316 ± 0.036 | 11.34 ± 0.32 | 6.18 ± 0.17 | 14.04 ± 0.61 |
| Tumour | 17.1 ± 0.84 | 176.6 ± 4.12 | 117.8 ± 1.91 | 0.438 ± 0.06 | 12.22 ± 0.372 | 5.82 ± 0.27 | 16.18 ± 0.79 |
| CISP | 3.6 ± 0.36 ab | 82.2 ± 2.44 ab | 55 ± 4.21 ab | 1.482 ± 0.12 ab | 23.28 ± 1.44 ab | 13.68 ± 0.36 ab | 64.06 ± 4.05 ab |
| CISP + PE-NPs | 17.84 ± 1.25 ac | 183 ± 7.2 ac | 119.6 ± 1.12 c | 0.732 ± 0.12 ac | 12.9 ± 0.46 c | 6.2 ± 0.35 c | 14.46 ± 0.71 c |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harakeh, S.; Almuhayawi, M.S.; Akefe, I.O.; Saber, S.H.; Al Jaouni, S.K.; Alzughaibi, T.; Almehmadi, Y.; Ali, S.S.; Bharali, D.J.; Mousa, S. Novel Pomegranate-Nanoparticles Ameliorate Cisplatin-Induced Nephrotoxicity and Improves Cisplatin Anti-Cancer Efficacy in Ehrlich Carcinoma Mice Model. Molecules 2022, 27, 1605. https://doi.org/10.3390/molecules27051605
Harakeh S, Almuhayawi MS, Akefe IO, Saber SH, Al Jaouni SK, Alzughaibi T, Almehmadi Y, Ali SS, Bharali DJ, Mousa S. Novel Pomegranate-Nanoparticles Ameliorate Cisplatin-Induced Nephrotoxicity and Improves Cisplatin Anti-Cancer Efficacy in Ehrlich Carcinoma Mice Model. Molecules. 2022; 27(5):1605. https://doi.org/10.3390/molecules27051605
Chicago/Turabian StyleHarakeh, Steve, Mohammed S. Almuhayawi, Isaac O. Akefe, Saber H. Saber, Soad K. Al Jaouni, Torki Alzughaibi, Yousef Almehmadi, Soad Shaker Ali, Dhruba J. Bharali, and Shaker Mousa. 2022. "Novel Pomegranate-Nanoparticles Ameliorate Cisplatin-Induced Nephrotoxicity and Improves Cisplatin Anti-Cancer Efficacy in Ehrlich Carcinoma Mice Model" Molecules 27, no. 5: 1605. https://doi.org/10.3390/molecules27051605
APA StyleHarakeh, S., Almuhayawi, M. S., Akefe, I. O., Saber, S. H., Al Jaouni, S. K., Alzughaibi, T., Almehmadi, Y., Ali, S. S., Bharali, D. J., & Mousa, S. (2022). Novel Pomegranate-Nanoparticles Ameliorate Cisplatin-Induced Nephrotoxicity and Improves Cisplatin Anti-Cancer Efficacy in Ehrlich Carcinoma Mice Model. Molecules, 27(5), 1605. https://doi.org/10.3390/molecules27051605








