Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products
Abstract
1. Introduction
1.1. Historical Aspects of Propolis
1.2. Origin of Propolis
1.3. Extraction Procedures
2. Biologically Active Compounds Presented in Propolis
2.1. Terpenoids
2.1.1. Mono- and Sesquiterpenoids (Volatiles)
2.1.2. Diterpenes
2.1.3. Triterpenoids
2.2. Phenolic Compounds
2.3. Flavonoids
2.4. Phenolic Acids
2.5. Other Organic Compounds
2.5.1. Alkanes and Alkenes
2.5.2. Fatty Acids
3. Main Biological Properties
3.1. Antioxidant Activity
3.2. Antimicrobial Activity
3.2.1. Antibacterial and Antifungal Activities
3.2.2. Antiviral Activity
3.3. Anti-Inflammatory Activity
3.4. Anticancer Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahangari, Z.; Naseri, M.; Vatandoost, F. Propolis: Chemical Composition and its Applications in Endodontics. Iran. Endod. J. 2018, 13, 285–292. [Google Scholar] [CrossRef]
- Hudz, N.; Ivanova, R.; Brindza, J.; Grygorieva, O.; Schubertová, Z.; Ivanišová, E. Approaches to the determination of antioxidant activity of extracts from bee bread and safflower leaves and flowers. Potravin. Slovak J. Food Sci. 2017, 11, 480–487. [Google Scholar] [CrossRef]
- Hudz, N.; Korzeniowska, K.; Wieczorek, P.P.; Schubertová, Z.; Brindza, J.; Ivanišová, E. Approaches to the identification and assay of flavonoids in bee bread extracts by spectrophotometric method. Agrobiod. Improv. Nutr. Health Life Qual. 2017, 1, 168–173. [Google Scholar]
- Hudz, N.; Korytniuk, O.; Yezerska, O.; Motyka, O.; Turkina, V.; Korytniuk, R.; Wieczorek, P.P. Evaluation of the total flavonoid content and antimicrobial activity of the tinctures of propolis of Ukrainian origin. Acta Pol. Pharm. Drug Res. 2020, 77, 897–907. [Google Scholar] [CrossRef]
- Hudz, N.; Yezerska, O.; Grygorieva, O.; Brindza, J.; Felsöciová, S.; Kačániová, M.; Wieczorek, P. Analytical procedure elaboration of total flavonoid content determination and antimicrobial activity of bee bread extracts. Acta Pol. Pharm. Drug Res. 2019, 76, 439–452. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, propolis, and royal jelly: A comprehensive review of their biological actions and health benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef] [PubMed]
- Velychko, S.; Brovarskyi, V.; Brindza, J. Bee stimulation to form protein food reserves. Potravin. Slovak J. Food Sci. 2021, 15, 274–284. [Google Scholar] [CrossRef]
- Zieliński, Ł.; Deja, S.; Jasicka-Misiak, I.; Kafarski, P. Chemometrics as a tool of origin determination of Polish monofloral and multifloral honeys. J. Agric. Food Chem. 2014, 62, 2973–2981. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Makowicz, E.; Stanek, N. Chromatographic fingerprint, antioxidant activity, and colour characteristic of polish goldenrod (Solidago virgaurea L.) honey and flower. Eur. Food Res. Technol. 2018, 244, 1169–1184. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee products in dermatology and skin care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef]
- Castaldo, S.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73, S1–S6. [Google Scholar] [CrossRef]
- Kiziltas, H.; Erkan, C. The effects of different beehives on propolis production and quality. Food Sci. Technol. 2020, 41, 877–883. [Google Scholar] [CrossRef]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical aspects of propolis research in modern times. Evid. Based. Complement. Alternat. Med. 2013, 2013, 964149. [Google Scholar] [CrossRef] [PubMed]
- Neto, M.S.R.; Tintino, S.R.; Silva, A.R.; Costa, M.S.; Boligon, A.A.; Matias, E.E.F.; Balbino, V.Q.; Menezes, I.R.A.; Coutinho, H.D.M. Seasonal variation of Brazilian red propolis: Antibacterial activity, synergistic effect and phytochemical screening. Food Chem. Toxicol. 2017, 107, 572–580. [Google Scholar] [CrossRef]
- Tran, V.H.; Duke, R.K.; Abu-Mellal, A.; Duke, C.C. Propolis with high flavonoid content collected by honey bees from Acacia paradoxa. Phytochemistry 2012, 81, 126–132. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.P.; Wang, K.; Li, G.Q.; Hu, F.L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Santos, L.M.; Fonseca, M.S.; Sokolonski, A.R.; Deegan, K.R.; Araújo, R.P.; UmszaGuez, M.A.; Barbosa, J.D.; Portela, R.D.; Machado, B.A. Propolis: Types, composition, biological activities, and veterinary product patent prospecting. J. Sci. Food Agric. 2020, 100, 136982. [Google Scholar] [CrossRef]
- Ikeda, N.Y.; Ambrosio, C.M.S.; Miano, A.C.; Rosalen, P.L.; Gloria, E.M.; Alencar, S.M. Essential oils extracted from organic propolis residues: An exploratory analysis of their antibacterial and antioxidant properties and volatile profile. Molecules 2021, 26, 4694. [Google Scholar] [CrossRef]
- Okińczyc, P.; Szumny, A.; Szperlik, J.; Kulma, A.; Franiczek, R.; Żbikowska, B.; Krzyżanowska, B.; Sroka, Z. Profile of polyphenolic and essential oil composition of Polish propolis, black poplar and aspens buds. Molecules 2018, 23, 1262. [Google Scholar] [CrossRef]
- Farre, R.; Frasquet, I.; Sanchez, A. Propolis and human health. Ars. Pharm. 2004, 45, 21–43. [Google Scholar]
- Rebiai, A.; Belfar, M.L.; Mesbahi, M.A.; Nani, S.; Tliba, A.; Ghamem Amara, D.; Chouikh, A. Fatty acid composition of Algerian propolis. J. Fundam. Appl. Sci. 2017, 9, 656–671. [Google Scholar]
- Roman, A.; Popiela-Pleban, E. Contamination of propolis used as a dietary supplement. Potravin. Slovak J. Food Sci. 2012, 6, 50–52. [Google Scholar] [CrossRef]
- Wang, J.W.; Chen, S.S.; Zhang, Y.M.; Guan, J.; Su, G.Y.; Ding, M.; Li, W.; Zhao, Y.Q. Anti-inflammatory and analgesic activity based on polymorphism of cedrol in mice. Environ. Toxicol. Pharmacol. 2019, 68, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Fokt, H.; Pereira, A.; Ferreira, A.M.; Cunha, A.; Aguiar, C. How do bees prevent hive infection? The antimicrobial properties of propolis. Current Research, Technology and Education. Appl. Microbiol. Biotechnol. 2010, 1, 481–493. [Google Scholar]
- Özkirim, A.; Çelemli, Ö.G.; Schiesser, A.; Charistos, L.; Hatjina, F. A comparison of the activities of Greek and Turkish propolis against Paenibacillus larvae. J. Apic. Res. 2014, 53, 528–536. [Google Scholar] [CrossRef][Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Shanaida, M.; Hudz, N.; Białoń, M.; Kryvtsowa, M.; Svydenko, L.; Filipska, A.; Wieczorek, P.P. Chromatographic profiles and antimicrobial activity of the essential oils obtained from some species and cultivars of the Mentheae tribe (Lamiaceae). Saudi. J. Biol. Sci. 2021, 28, 6145–6152. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Bakdash, A.; Almohammadi, O.H.; Taha, N.A.; Abu-Rumman, A.; Kumar, S. Chemical composition of propolis from the Baha region in Saudi Arabia. Czech J. Food Sci. 2018, 36, 109–118. [Google Scholar]
- Bankova, V.S.; De Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Bouchelaghem, S. Propolis characterization and antimicrobial activities against Staphylococcus aureus and Candida albicans: A review. Saudi J. Biol. Sci. 2022, in press. [CrossRef]
- Šturm, L.; Ulrih, N.P. Advances in the propolis chemical composition between 2013 and 2018: A review. eFood 2019, 1, 24–37. [Google Scholar] [CrossRef]
- Alencar, S.; Oldoni, T.; Castro, M.; Cabral, I.; Costa-Neto, C.; Cury, J.; Rosalen, P.; Ikegaki, M. Chemical composition and biological activity of a new type of Brazilian propolis: Red propolis. J. Ethnopharmacol. 2007, 113, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Cottica, S.M.; Sabik, H.; Antoine, C.; Fortin, J.; Graveline, N.; Visentainer, J.V.; Britten, M. Characterization of Canadian propolis fractions obtained from two-step sequential extraction. LWT–Food Sci. Technol. 2015, 60, 609–614. [Google Scholar] [CrossRef]
- Gardana, C.; Scaglianti, M.; Pietta, P.; Simonetti, P. Analysis of the polyphenolic fraction of propolis from different sources by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007, 45, 390–399. [Google Scholar] [CrossRef]
- Graikou, K.; Popova, M.; Gortzi, O.; Bankova, V.; Chinou, I. Characterization and biological evaluation of selected Mediterranean propolis samples. Is it a new type? LWT–Food Sci. Technol. 2016, 65, 261–267. [Google Scholar] [CrossRef]
- González-Búrquez, M.d.J.; González-Díaz, F.R.; García-Tovar, C.G.; Carrillo-Miranda, L.; Soto-Zárate, C.I.; Canales-Martínez, M.M.; Penieres-Carrillo, J.G.; Crúz-Sánchez, T.A.; Fonseca-Coronado, S. Comparison between in vitro antiviral effect of mexican propolis and three commercial flavonoids against canine distemper virus. Evid. Based. Complement. Alternat. Med. 2018, 2018, 7092416. [Google Scholar] [CrossRef]
- Tatli Seven, P.; Seven, I.; Karakus, S.; Iflazoglu Mutlu, S.; Ozer Kaya, S.; Arkali, G.; Ilgar, M.; Tan, E.; Sahin, Y.M.; Ismik, D.; et al. The in-vivo assessment of Turkish propolis and its nano form on testicular damage induced by cisplatin. J. Integr. Med. 2021, 19, 451–459. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis volatile compounds: Chemical diversity and biological activity: A review. Chem. Cent. J. 2014, 8, 28. [Google Scholar] [CrossRef]
- Popova, M.; Gerginova, D.; Trusheva, B.; Simova, S.; Tamfu, A.N.; Ceylan, O.; Clark, K.; Bankova, V. A preliminary study of chemical profiles of honey, cerumen, and propolis of the african stingless bee Meliponula ferruginea. Foods 2021, 10, 997. [Google Scholar] [CrossRef]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical composition and antioxidant activity of propolis prepared in different forms and in different solvents useful for finished products. Foods 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial activities of European propolis collected from various geographic origins alone and in combination with antibiotics. Medicine 2018, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Tlak Gajger, I.; Pavlović, I.; Bojić, M.; Kosalec, I.; Srečec, S.; Vlainić, T.; Vlainić, J. Components responsible for antimicrobial activity of propolis from continental and Mediterranean regions in Croatian. Czech J. Food Sci. 2017, 35, 376–385. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Lyoussi, B.; Miguel, M.G. Insight on propolis from mediterranean countries: Chemical composition, biological activities and application fields. Chem. Biodivers. 2019, 16, e1900094. [Google Scholar] [CrossRef]
- Cuesta-Rubio, O.; Frontana-Uribe, B.A.; Ramirez-Apan, T.; Cardenas, J. Polyisoprenylated benzophenones in Cuban propolis; biological activity of nemorosone. Z. Naturforsch. 2002, 57, 372–378. [Google Scholar] [CrossRef]
- Ishida, V.F.C.; Negri, G.; Salatino, A.; Bandeira, M.F.C.L. A new type of Brazilian propolis: Prenylated benzophenones in propolis from Amazon and effects against cariogenic bacteria. Food Chem. 2011, 125, 966–972. [Google Scholar] [CrossRef]
- Kumazawa, S.; Yoneda, M.; Shibata, I.; Kanaeda, J.; Hamasaka, T.; Nakayama, T. Direct evidence for the plant origin of Brazilian propolis by observation of honeybee behavior and phytochemical analysis. Chem. Pharm. Bull. 2003, 51, 740–742. [Google Scholar] [CrossRef]
- Salatino, A.; Teixeira, E.W.; Negri, G.; Message, D. Origin and chemical variation of Brazilian propolis. eCAM 2005, 2, 33–38. [Google Scholar] [CrossRef]
- Daugsch, A.; Moraes, C.S.; Fort, P.; Park, Y.K. Brazilian red propolis–chemical composition and botanical origin. eCAM 2008, 5, 435–441. [Google Scholar] [CrossRef]
- Fernandez, M.C.; Cuesta-Rubio, O.; Perez, A.R.; Porto, R.M.O.; Hernandez, I.M.; Piccinelli, A.L.; Rastrelli, L. GC–MS determination of isoflavonoids in seven red Cuban propolis samples. J. Agric. Food Chem. 2008, 56, 9927–9932. [Google Scholar] [CrossRef]
- Lotti, C.; Fernandez, M.C.; Piccinelli, A.L.; Cuesta-Rubio, O.; Hernandez, I.M.; Rastrelli, L. Chemical constituents of red Mexican propolis. J. Agric. Food Chem. 2010, 58, 2209–2213. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, C.; Shy, H.; Lin, J. Cytotoxic prenylflavanones from Taiwanese propolis. J. Nat. Prod. 2003, 66, 503–506. [Google Scholar] [CrossRef]
- Kumazawa, S.; Nakamura, J.; Murase, M.; Miyagawa, M.; Ahn, M.R.; Fukumoto, S. Plant origin of Okinawan propolis: Honeybee behavior observation and phytochemical analysis. Naturwissenchaften 2008, 95, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Trusheva, B.; Antonova, D.; Cutajar, S.; Mifsud, D.; Farrugia, C.; Tsvetkova, I.; Najdensky, H.; Bankova, V. The specific chemical profile of Mediterranean propolis from Malta. Food Chem. 2011, 126, 1431–1435. [Google Scholar] [CrossRef]
- Popova, M.P.; Chinou, I.B.; Marekov, I.N.; Bankova, V.S. Terpenes with antimicrobial activity from Cretan propolis. Phytochem. 2009, 70, 1262–1271. [Google Scholar] [CrossRef]
- Fernandes-Silva, C.C.; Salatino, A.; Salatino, M.L.F.; Breyer, E.D.H.; Negri, G. Chemical profiling of six samples of Brazilian propolis. Química Nova 2013, 36, 237–240. [Google Scholar] [CrossRef]
- Machado, J.L.; Assunçao, A.K.M.; da Silva, M.C.P.; Reis, A.S.D.; Costa, G.C.; Arruda, D.D.S.; Nascimento, F.R.F.D. Brazilian green propolis: Anti-inflammatory property by an immunomodulatory activity. Evid.-Based Complement. Altern. Med. 2012, 2012, 157652. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Shimamura, Y.; Masuda, S.; Shirafuji, K.; Moli, R.T.; Kumazawa, S. A new prenylflavonoid isolated from propolis collected in the Solomon Islands. Biosci. Biotechnol. Biochem. 2012, 76, 1038–1040. [Google Scholar] [CrossRef]
- Šuran, J.; Cepanec, I.; Mašek, T.; Radić, B.; Radić, S.; Tlak Gajger, I.; Vlainić, J. Propolis extract and its bioactive compounds–from traditional to modern extraction technologies. Molecules 2021, 26, 2930. [Google Scholar] [CrossRef]
- Coelho, G.R.; Mendonça, R.Z.; Vilar, K.D.S.; Figueiredo, C.A.; Badari, J.C.; Taniwaki, N.; Namiyama, G.; Oliveira, M.I.; Curti, S.P.; Silva, P.E.; et al. Antiviral action of hydromethanolic extract of geopropolis from Scaptotrigona postica against antiherpes simplex virus (HSV-1). J. Evid. Based Complement. Altern. Med. 2015, 2015, 296086. [Google Scholar]
- Kročko, M.; Bobko, M.; Bučko, O.; Čanigová, M.; Ducková, V. Sensory quality, colour and oxidative stability of cured cooked ham with propolis extract. Potravin. Slovak J. Food Sci. 2014, 8, 102–106. [Google Scholar] [CrossRef]
- Woźniak, M.; Mrówczyńska, L.; Kwaśniewska-Sip, P.; Waśkiewicz, A.; Nowak, P.; Ratajczak, I. Effect of the solvent on propolis phenolic profile and its antifungal, antioxidant, and in vitro cytoprotective activity in human erythrocytes under oxidative stress. Molecules 2020, 25, 4266. [Google Scholar] [CrossRef] [PubMed]
- Rushdi, A.I.; Adgaba, N.; Bayaqoob, N.I.; Al-Khazim, A.; Simoneit, B.I.; El-Mubarak, A.H.; Al-Mutlaq, K.F. Characteristics and chemical compositions of propolis from Ethiopia. Springerplus 2014, 3, 253. [Google Scholar] [CrossRef] [PubMed]
- Lisbona-González, M.J.; Muñoz-Soto, E.; Reyes-Botella, C.; Olmedo-Gaya, M.V.; Diaz-Castro, J.; Moreno-Fernandez, J. Study of the antimicrobial effect of an ethanolic extract of propolis in periodontal disease. Appl. Sci. 2021, 11, 7463. [Google Scholar] [CrossRef]
- Nichitoi, M.M.; Josceanu, A.M.; Isopescu, R.D.; Isopencu, G.O.; Geana, E.I.; Ciucure, C.T.; Lavric, V. Polyphenolics profile effects upon the antioxidant and antimicrobial activity of propolis extracts. Sci. Rep. 2021, 11, 20113. [Google Scholar] [CrossRef] [PubMed]
- Boulechfar, S.; Zellagui, A.; Asan-Ozusaglam, M.; Bensouici, C.; Erenler, R.; Yildiz, I.; Tacer, S.; Boural, H.; Demirtas, I. Chemical composition, antioxidant, and antimicrobial activities of two essential oils from Algerian propolis. Z. Naturforsch. C. J. Biosci. 2021, 10. [Google Scholar] [CrossRef]
- Mohtar, L.G.; Rodríguez, S.A.; Nazareno, M.A. Comparative analysis of volatile compound profiles of propolis from different provenances. J. Sci. Food Agric. 2018, 98, 3409–3415. [Google Scholar] [CrossRef]
- Chi, Y.; Luo, L.; Cui, M.; Hao, Y.; Liu, T.; Huang, X.; Guo, X. Chemical composition and antioxidant activity of essential oil of Chinese propolis. Chem. Biodivers. 2020, 17, e1900489. [Google Scholar] [CrossRef]
- Felipe, C.; Albuquerque, A.; de Pontes, J.; de Melo, J.; Rodrigues, T.; de Sousa, A.; Monteiro, Á.B.; Ribeiro, A.; Lopes, J.P.; de Menezes, I.; et al. Comparative study of alpha- and beta-pinene effect on PTZ-induced convulsions in mice. Fundam. Clin. Pharmacol. 2019, 33, 181–190. [Google Scholar] [CrossRef]
- Wagh, V.D. Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharmacol. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A multifunctional compound with potent therapeutic effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.; Chaijaroenkul, W.; Na-Bangchang, K. Therapeutic potential and pharmacological activities of β-eudesmol. Chem. Biol. Drug Des. 2021, 97, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Greay, S.J.; Hammer, K.A. Recent developments in the bioactivity of mono- and diterpenes: Anticancer and antimicrobial activity. Phytochem. Rev. 2015, 14, 1–6. [Google Scholar] [CrossRef]
- Aminimoghadamfarouj, N.; Nematollahi, A. Propolis diterpenes as a remarkable bio-source for drug discovery development: A review. Int. J. Mol. Sci. 2017, 18, 1290. [Google Scholar] [CrossRef] [PubMed]
- Mahamat, A.A.; Nyemb, J.N.; Gade, I.S.; Ngenge, A.T.; Talla, E.; Céline, H.; Sophie, L.; Mbafor, J.T. A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts. Open Chem. 2020, 18, 239–243. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F. Antioxidant activity in bee products: A review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Touzani, S.; Imtara, H.; Katekhaye, S.; Mechchate, H.; Ouassou, H.; Alqahtani, A.S.; Noman, O.M.; Nasr, F.A.; Fearnley, H.; Fearnley, J.; et al. Determination of phenolic compounds in various propolis samples collected from an African and an Asian region and their impact on antioxidant and antibacterial activities. Molecules 2021, 26, 4589. [Google Scholar] [CrossRef]
- Yarnykh, T.; Yuryeva, G.; Rukhmakova, O.; Buryak, M.; Herasymova, I. Evolution of propolis standardization methods. Ukr. Biopharm. J. 2020, 2, 4–13. [Google Scholar] [CrossRef]
- Pellati, F.; Prencipe, F.P.; Bertelli, D.; Benvenuti, S. An efficient chemical analysis of phenolic acids and flavonoids in raw propolis by microwave-assisted extraction combined with high-performance liquid chromatography using the fused-core technology. J. Pharm. Biomed. Anal. 2013, 81, 126–132. [Google Scholar] [CrossRef]
- Ghosh, S.; McArthur, R.; Guo, Z.C.; McKerchar, R.; Donkor, K.; Xu, J.; Cheeptham, N. Evidence for Anti-Pseudogymnoascus destructans (Pd) activity of propolis. Antibiotics 2017, 21, 2. [Google Scholar] [CrossRef]
- Funakoshi-Tago, M.; Okamoto, K.; Izumi, R.; Tago, K.; Yanagisawa, K.; Narukawa, Y.; Kiuchi, F.; Kasahara, T.; Tamura, H. Anti-inflammatory activity of flavonoids in Nepalese propolis is attributed to inhibition of the IL-33 signaling pathway. Int Immunopharmacol. 2015, 25, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Üstün, Y.; Arslan, S.; Aslan, T. Effects of calcium hydroxide and propolis intracanal medicaments on bond strength of resin-based endodontic sealer as assessed by push-out test. Dent. Mater. J. 2013, 32, 913–919. [Google Scholar] [CrossRef] [PubMed][Green Version]
- da Silva, S.S.; Thomé, G.; Cataneo, A.H.; Miranda, M.M.; Felipe, I.; Andrade, C.G.; Watanabe, M.A.; Piana, G.M.; Sforcin, J.M.; Pavanelli, W.R.; et al. Brazilian propolis antileishmanial and immunomodulatory effects. Evid. Based Complement. Alternat. Med. 2013, 2013, 673058. [Google Scholar] [CrossRef] [PubMed]
- Cedikova, M.; Miklikova, M.; Stachova, L.; Grundmanova, M.; Tuma, Z.; Vetvicka, V.; Zech, N.; Kralickova, M.; Kuncova, J. Effects of the Czech propolis on sperm mitochondrial function. Evid. Based Complement. Alternat. Med. 2014, 2014, 248768. [Google Scholar] [CrossRef]
- Rebiai, A.; Ben Seghir, B.; Hemmami, H.; Zeghoud, S.; Belfar, M.L.; Kouadri, I. Determination of some phenolic acids in Algerian propolis. Ovidius Univ. Ann. Chem. 2021, 32, 120–124. [Google Scholar] [CrossRef]
- Aga, H.; Shibuya, T.; Sugimoto, T.; Kurimoto, M.; Nakajima, S. Isolation and identification of antimicrobial compounds in Brazilian Propolis. Biosci. Biotechnol. Biochem. 1994, 58, 945–956. [Google Scholar] [CrossRef]
- Koo, H.; Hayacibara, M.F.; Schobel, B.D.; Cury, J.A.; Rosalen, P.L.; Park, Y.K. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J. Antimicrob. Chemother. 2001, 52, 782–789. [Google Scholar] [CrossRef]
- Siqueira, A.B.S.; Rodrigues, L.R.N.A.; Santos, R.K.B.; Marinho, R.R.; Abreu, S.; Peixoto, R.F.; Gurgel, B.C. Antifungal activity of propolis against Candida species isolated from cases of chronic periodontitis. Braz. Oral Res. 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Doğru, F.; Parlakpınar, H.; Duman, Y.; Özhan, O.; Keskin, M.; Polat, A. Propolis Ve Perganın Antimikrobiyal Etkilerinin İn-vitro Olarak Araştırılmasi. İnönü Üniv. Sağlık Hizmetleri Mesl. Yüksekokulu Derg. 2021, 9, 1084–1093. [Google Scholar] [CrossRef]
- Fosquiera, E.C.; Steffens, J.P.; Reinke, S.M.G.; Possagno, R.C.; Kozlowski, J.V.A.; Rezende, E.C.D.; Santos, E.B.D. Efeito da própolis no crescimento in vitro de microrganismos associados à periodontite em pacientes HIV-positivos. Periodontia 2008, 18, 77–82. [Google Scholar]
- Ota, C.; Unterkircher, C.; Fantinato, V.; Shimizu, M.T. Antifungal activity of propolis on different species of Candida. Mycoses 2001, 44, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.J.; Mohammadi, F.; Bayat, M.; Gema, S.M.; Ghadirian, H.; Seifi, H.; Bayat, H.; Bahrami, N. Applications of propolis in dentistry: A review. Ethiop. J. Health Sci. 2018, 28, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Walczyńska-Dragon, K.; Felitti, R.; Nitecka-Buchta, A.; Baron, S.; Olczyk, P. The influence of propolis on dental plaque reduction and the correlation between dental plaque and severity of COVID-19 complications–A Literature Review. Molecules 2021, 26, 5516. [Google Scholar] [CrossRef]
- Ahangari, Z.; Naseri, M.; Jalili, M.; Mansouri, Y.; Mashhadiabbas, F.; Torkaman, A. Effect of propolis on dentin regeneration and the potential role of dental pulp stem cell in guinea pigs. Cell J. 2012, 13, 223–228. [Google Scholar]
- Dziedzic, A.; Kubina, R.; Wojtyczka, R.D.; Kabała-Dzik, A.; Tanasiewicz, M.; Morawiec, T. The Antibacterial Effect of Ethanol Extract of Polish Propolis on Mutans Streptococci and Lactobacilli Isolated from Saliva. Evid. Based Complement. Alternat. Med. 2013, 2013, 681891. [Google Scholar] [CrossRef] [PubMed]
- Stähli, A.; Schröter, H.; Bullitta, S.; Serralutzu, F.; Dore, A.; Nietzsche, S.; Milia, E.; Sculean, A.; Eick, S. In vitro activity of propolis on oral microorganisms and biofilms. Antibiotics 2021, 10, 1045. [Google Scholar] [CrossRef]
- El-Tayeb, M.M.; Abu-Seida, A.M.; El Ashry, S.H.; El-Hady, S.A. Evaluation of antibacterial activity of propolis on regenerative potential of necrotic immature permanent teeth in dogs. BMC Oral. Health 2019, 19, 174. [Google Scholar] [CrossRef]
- Listyasari, N.A.; Santoso, O. Inhibition of dental plaque formation by toothpaste containing propolis. Dent. J. (Maj. Kedokt. Gigi) 2012, 45, 208–211. [Google Scholar] [CrossRef][Green Version]
- Amoros, M.; Sauvager, F.; Girre, L.; Cormier, M. In vitro antiviral activity of propolis. Apidologie 1992, 23, 231–240. [Google Scholar] [CrossRef]
- Debiaggi, M.; Tateo, F.; Pagani, L.; Luini, M.; Romero, E. Effects of propolis flavonoids on virus infectivity and replication. Microbiologica 1990, 13, 207–213. [Google Scholar]
- Syed, S.; Saleem, A. Severe acute respiratory syndrome epidemiology and control. Lab. Med. 2004, 35, 112–116. [Google Scholar] [CrossRef]
- Amoros, M.; Lurton, F.; Boustie, J.; Girre, L.; Sauvager, F.; Cormier, M. Comparison of the antiherpes simplex virus activities of propolis and 3-methyl-but-2-enyl caffeate. J. Nat. Prod. 1994, 57, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 2010, 24, 673–679. [Google Scholar] [CrossRef]
- Kumar, V.; Dhanjal, J.K.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (Mpro) of SARS-CoV-2 and inhibit its activity. J. Biomol. Struct. Dyn. 2020, 39, 3842–3854. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Rosalen, P.L.; Alencar, S.M.; Mayer, M.P.A. Anti-inflammatory mechanisms of neovestitol from Brazilian red propolis in LPS-activated macrophages. J. Funct. Foods 2017, 36, 440–447. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, D.; Hu, Y.; Huang, Y.; Yu, Y.; Wang, D. The immunological enhancement activity of propolis flavonoids liposome in vitro and in vivo. Evid. Based Complement. Alternat. Med. 2014, 2014, 483513. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, P.; Wisowski, G.; Komosinska-Vassev, K.; Stojko, J.; Klimek, K.; Olczyk, M.; Kozma, E.M. Propolis Modifies Collagen Types I and III Accumulation in the Matrix of Burnt Tissue. Evid. Based Complement. Alternat. Med. 2013, 2013, 423809. [Google Scholar] [CrossRef]
- Sawicka, D.; Borawska, M.H. The use of propolis in skin diseases. Derm. Estet. 2013, 1, 13–17. [Google Scholar]
- Shinmei, Y.; Yano, H.; Kagawa, Y.; Izawa, K.; Akagi, M.; Inoue, T.; Kamei, C. Effect of Brazilian propolis on sneezing and nasal rubbing in experimental allergic rhinitis of mice. Immunopharmacol. Immunotoxicol. 2009, 31, 688–693. [Google Scholar] [CrossRef]
- Hellgren, J.; Cervin, A.; Nordling, S.; Bergman, A.; Cardell, L.O. Allergic rhinitis and the common cold–high cost to society. Allergy 2010, 65, 776–783. [Google Scholar] [CrossRef]
- Jung, W.-K.; Lee, D.-Y.; Choi, Y.H.; Yea, S.S.; Choi, I.; Park, S.G.; Seo, S.K.; Lee, S.W.; Lee, C.M.; Kim, S.K.; et al. Caffeic acid phenethyl ester attenuates allergic airway inflammation and hyperresponsiveness in murine model of ovalbumin-induced asthma. Life Sci. 2008, 82, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Khayyal, M.T.; el-Ghazaly, M.A.; el-Khatib, A.S.; Hatem, A.M.; de Vries, P.J.; el-Shafei, S.; Khattab, M.M. A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients. Fundam. Clin. Pharmacol. 2003, 17, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Maffia, P.; Pinto, L.; Ianaro, A.; Russo, A.; Capasso, F.; Ialenti, A. Phytochemical compounds involved in the anti-inflammatory effect of propolis extract. Fitoterapia 2002, 73, 53–63. [Google Scholar] [CrossRef]
- Patel, S. Emerging adjuvant therapy for cancer: Propolis and its constituents. J. Diet. Suppl. 2016, 13, 245–268. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Gao, R.; Shah, N.; Bhargava, P.; Furune, T.; Kaul, S.; Terao, K.; Wadhwa, R. Anticancer activity in honeybee propolis: Functional insights to the role of caffeic acid phenethyl ester and its complex with γ-cyclodextrin. Integr. Cancer Ther. 2018, 17, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wang, Y.; Yin, X.; Liu, X.; Xuan, H. Ethanol extract of propolis and its constituent caffeic acid phenethyl ester inhibit breast cancer cells proliferation in inflammatory microenvironment by inhibiting TLR4 signal pathway and inducing apoptosis and autophagy. BMC Complement. Altern. Med. 2017, 17, 471. [Google Scholar] [CrossRef]
- Campoccia, D.; Ravaioli, S.; Santi, S.; Mariani, V.; Santarcangelo, C.; De Filippis, A.; Montanaro, L.; Arciola, C.R.; Daglia, M. Exploring the anticancer effects of standardized extracts of poplar-type propolis: In vitro cytotoxicity toward cancer and normal cell lines. Biomed. Pharmacother. 2021, 141, 111895. [Google Scholar] [CrossRef]
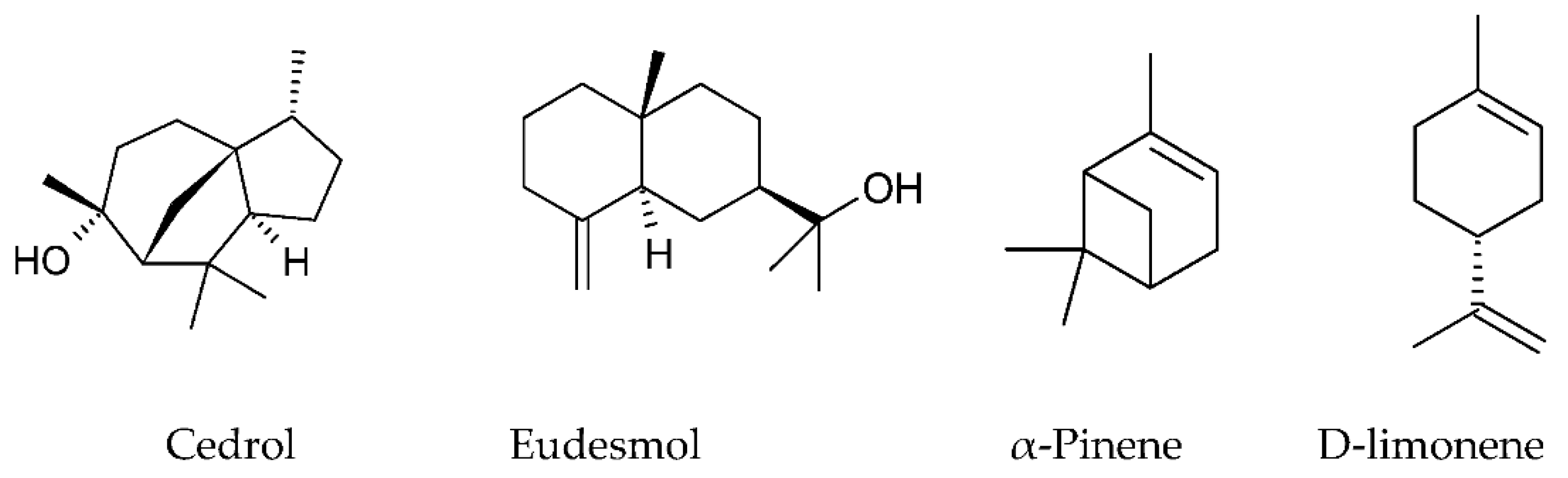
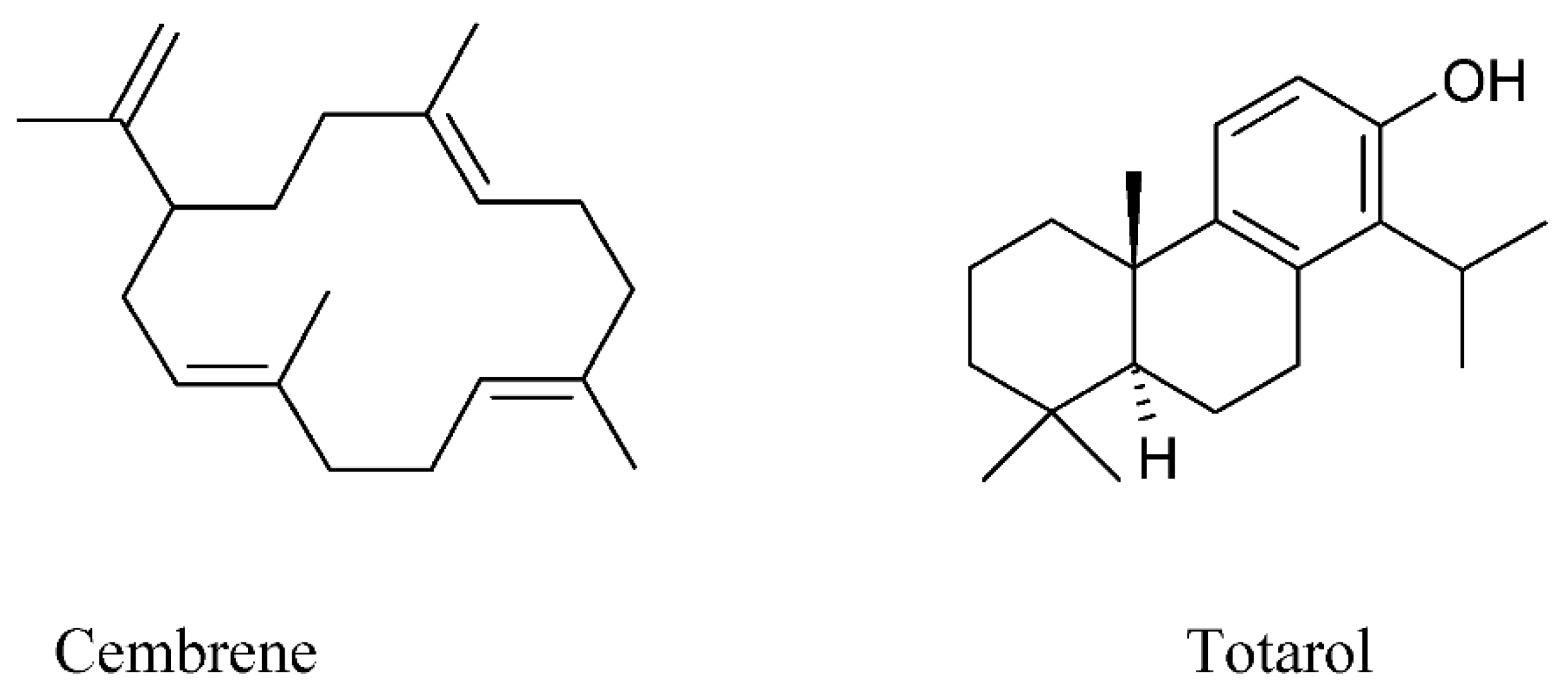
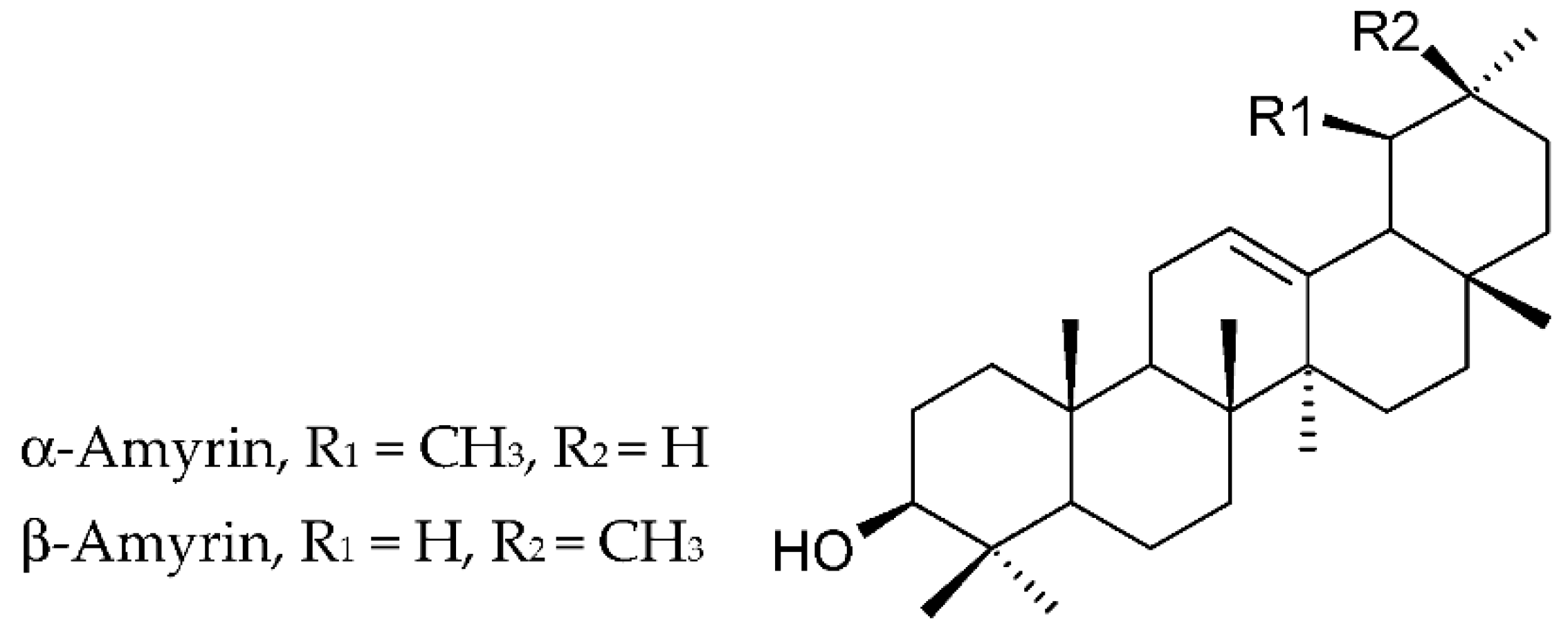

| Group | Representatives | Propolis Type | Reference |
|---|---|---|---|
| Terpenoids | |||
| Mono- and sesquiterpenoids | Cedrol (17.0%) α-Pinene (56.1%) | Algerian, Oum El Bouaghi Batna | [67] |
| α-Pinene and β-pinene | Brazilian | [18] | |
| Limonene | Venezuelan | [68] | |
| β-Caryophyllene and nerolidol | Argentinian green | [68] | |
| Cedrol, γ-eudesmol, phenethyl alcohol, benzyl alcohol, 2-3,4-dimethoxystyrene, methoxy-4-vinylphenol, and guaiol | Chinese | [69] | |
| Diterpenes | Clerodane diterpenes | Brazilian | [75] |
| Cembrene (C20H32) and totarol (C20H30O) | Saudi Arabia (Baha) | [30] | |
| Triterpenoids | α-Amyrin | Ethiopian propolis | [64] |
| Lupenone, α-amyrin and β-amyrin | Cameroonian | [76] | |
| Fatty acids | |||
| Unsaturated fatty acids | cis-11-Eicosenoic acid (C20:1, n-9), cis-11,14-eicosadienoic acid (C20:2, n-6), cis-5,8,11,14,17-eicosapentaenoic acid (C20:5), arachidonic acid (C20:4, n-6), linoleic acid (C18:2, c + t n-6), palmitoleic acid (C16:1), and γ-linolenic acid (C18:3, n-6) | Algerian | [21] |
| Saturated fatty acids | Oleic acid, nonanoic acid (C9H18O2), decanoic acid (C10H20O2), dodecanoic acid (C12H24O2) | Saudi Arabia | [30] |
| Hexatriacontanoic acid (C36H72O2) | Cameroonian | [76] | |
| Flavonoids | |||
| Flavanols | Catechin | Mexican | [38] |
| Flavanones | Pinocembrin | Mexican | [38] |
| European brown poplar | [42] | ||
| Turkish (1.22 mg/g) | [39] | ||
| Croatian (0–6.39 mg/mL) | [44] | ||
| Irish, Czech, German | [43] | ||
| Romanian poplar | [66] | ||
| Polish | [63] | ||
| Naringenin | Mexican | [38] | |
| Pinostrobin | Turkish (2.93 mg/g) | [39] | |
| Plathymenin | Nepalese | [82] | |
| Flavanonols | Pinobanksin 3-acetate | Australian | [15] |
| Pinobanksin-3-O-propionate, pinobanksin-3-O-butyrate, pinobanksyn-3-O-pentenoate | European brown poplar | [42] | |
| Pinobanksin | Turkish | [39] | |
| Polish | [63] | ||
| Flavones | Chrysin | Polish | [19,63] |
| Mexican | [38] | ||
| European brown poplar | [42] | ||
| Turkish (2.94 mg/g) | [39] | ||
| Croatian (0–8.02 mg/mL) | [44] | ||
| Irish, Czech | [43] | ||
| Romanian poplar | [66] | ||
| Apigenin | Brazilian red | [14] | |
| European brown poplar | [42] | ||
| Croatian (0–1.23 mg/mL) | [44] | ||
| Polish | [63] | ||
| Luteolin | Turkish | [12] | |
| Brazilian red | [14] | ||
| Tectochrysyn | Croatian (0–16.07 mg/mL) | [44] | |
| C-glycosyl flavones | Apigenin-6,8-di-C-malonyl glucoside dihexoside isomer, apigenin-di-C-malonyl trihexoside isomer | Geopropolis from Scaptotrigona postica | [61] |
| Flavonols | Quercetin | Brazilian red | [14] |
| Mexican | [38] | ||
| European brown poplar | [42] | ||
| Romanian poplar | [66] | ||
| Polish | [63] | ||
| Kaempferol | Mexican | [38] | |
| European brown poplar | [42] | ||
| Croatian (0–0.672 mg/mL) | [44] | ||
| Polish | [63] | ||
| Galangin | Polish | [19,63] | |
| Turkish (0.09 mg/g) | [39] | ||
| Croatian (0–8.71 mg/mL) | [44] | ||
| Irish, Czech | [43] | ||
| Romanian poplar | [66] | ||
| Rutin | Turkish | [12] | |
| Brazilian red | [14] | ||
| Isoflavones | Homopterocarpin, medicarpin, 4,7-dimethoxy-2-isoflavonol | Brazilian red | [34] |
| Medicarpin | Nepalese | [82] | |
| Chalcons | 2′,3′,4′-Trimethoxychalcone, 2′-hydroxy-3′,4′-dimethoxychal-cone, 2′,4′-dihydroxy-3′-methoxychalcone | Australian | [15] |
| Phenolic acids | Caffeic acid | Turkish (0.17 mg/g) | [12,39] |
| Algerian | [21] | ||
| Polish | [63] | ||
| Ferulic acid | Turkish (0.36 mg/g) | [12,39] | |
| Croatian (0–1.370 mg/mL) | [44] | ||
| Romanian poplar | [66] | ||
| Polish | [63] | ||
| t-Cinnamic acid | Turkish (0.05–0.14 mg/g or 3.95 mg/g) | [12,39] | |
| p-Coumaric acid | Croatian (0–1.031 mg/mL) | [44] | |
| Romanian poplar | [66] | ||
| Polish | [19,63] | ||
| Algerian | [21] | ||
| Turkish (0.07–0.24 mg/g) | [12] | ||
| Chlorogenic acid | Algerian | [21] | |
| Gallic acid | Algerian | [21] | |
| Turkish (0.015–0.025 mg/g) | [12] | ||
| Syringic acid | Turkish | [12] | |
| Polish | [63] | ||
| Vanilic acid | Polish | [63] | |
| 3,5-Diprenyl-4-hydroxycinnamic acid, 3-prenyl-4-dihydrocinnamoloxy-cinnamic acid (C23H24O4) | Brazilian propolis | [87] | |
| Product | Composition and Brief Technology | Activity | Reference |
|---|---|---|---|
| Toothpaste with propolis | There is no information in the paper | Reducing dental plaque formation | [105] |
| Propolis extract (Spanish propolis) | The propolis extract was prepared under aseptic conditions; 20 g of unrefined propolis was crushed and dissolved in 100 mL of 66% ethanol. The mixture was kept at room temperature for 28 days and subsequently filtered | Antibacterial activity against anaerobic bacteria (Porphyromonas gingivalis and Tannerella forsythensis) using sublingual administration | [65] |
| Nanoform of Turkish propolis | A total of 3.5 g of chitosan was dissolved in 230 mL of 2% aqueous acetic acid solution (v/v) in an ultrasonic bath; 1 g of tween 80 was added into the chitosan solution which was mixed by magnetic stirring at a temperature of 25 °C for 30 min. Then, 840 mg of propolis was dissolved in 120 mL of ethanol. This propolis solution was added into the chitosan/tween 80 blend, and sonicated for 10 min in order obtain NP-10 | Propolis-bearing polymeric nanoparticles can mitigate the side effects of cisplatin | [39] |
| Liposoms for subcutaneous administration | Liposoms contains a complex mixture which principally contains rutin, myricetin, quercetin, kaempferol, apigenin, pinocembrin, chrysin, and galanigin. Propolis was extracted with 95% ethanol three times, and the ethanol solution was retrieved. Then, the precipitation was extracted with ethyl acetate three times, and ethyl acetate was retrieved. At the end, the precipitation was dried in vacuum, and propolis flavonoids were obtained | Immunological enhancement activity | [107] |
| Propolis apitherapeutic ointment | There is no information in the paper | Propolis burn treatment led to enhanced collagens and its components expression | [108] |
| Propolis granular (Yamada Apiculture Center, Inc., Okayama, Japan) and propolis ethanol extract 55 wt.%/vol.% (for oral administration) | Propolis granular was dissolved in 5% gum arabic, and propolis ethanol extract was dissolved in 1% ethanol | The relief of symptoms of allergic rhinitis through inhibition of histamine release | [110] |
| Dried 13% solution of the aqueous extract of propolis | A 13% solution of the aqueous extract of propolis was supplied by Propharma (Stenlose, Denmark), which was prepared by aqueous decoction of crude propolis, collected from Denmark, China, Uruguay, and Brazil. This extract was standardized to contain not less than 0.05% of organic aromatic acids, chiefly caffeic, ferulic, isoferulic, cinnamic, and 3,4-dimethoxy-cinnamic acids in addition to trace amounts of various flavonoids. The aqueous extract was first concentrated, then spray-dried under high pressure before being incorporated into the milk formula. The sachets were intended to be given suspended in water as a milk drink orally once a day for two months. | Marked reduction in the incidence and severity of nocturnal attacks and improvement of ventilatory functions | [113] |
| propolis-γ CD powder | The technology is not described | Anticancer | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieczorek, P.P.; Hudz, N.; Yezerska, O.; Horčinová-Sedláčková, V.; Shanaida, M.; Korytniuk, O.; Jasicka-Misiak, I. Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products. Molecules 2022, 27, 1600. https://doi.org/10.3390/molecules27051600
Wieczorek PP, Hudz N, Yezerska O, Horčinová-Sedláčková V, Shanaida M, Korytniuk O, Jasicka-Misiak I. Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products. Molecules. 2022; 27(5):1600. https://doi.org/10.3390/molecules27051600
Chicago/Turabian StyleWieczorek, Piotr Paweł, Nataliia Hudz, Oksana Yezerska, Vladimira Horčinová-Sedláčková, Mariia Shanaida, Oleksii Korytniuk, and Iza Jasicka-Misiak. 2022. "Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products" Molecules 27, no. 5: 1600. https://doi.org/10.3390/molecules27051600
APA StyleWieczorek, P. P., Hudz, N., Yezerska, O., Horčinová-Sedláčková, V., Shanaida, M., Korytniuk, O., & Jasicka-Misiak, I. (2022). Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products. Molecules, 27(5), 1600. https://doi.org/10.3390/molecules27051600










