Tensiometry as a Simple Analytical Method for Quantification of Solubility and Release of Aroma Molecules in Aqueous Media
Abstract
:1. Introduction
2. Results and Discussion
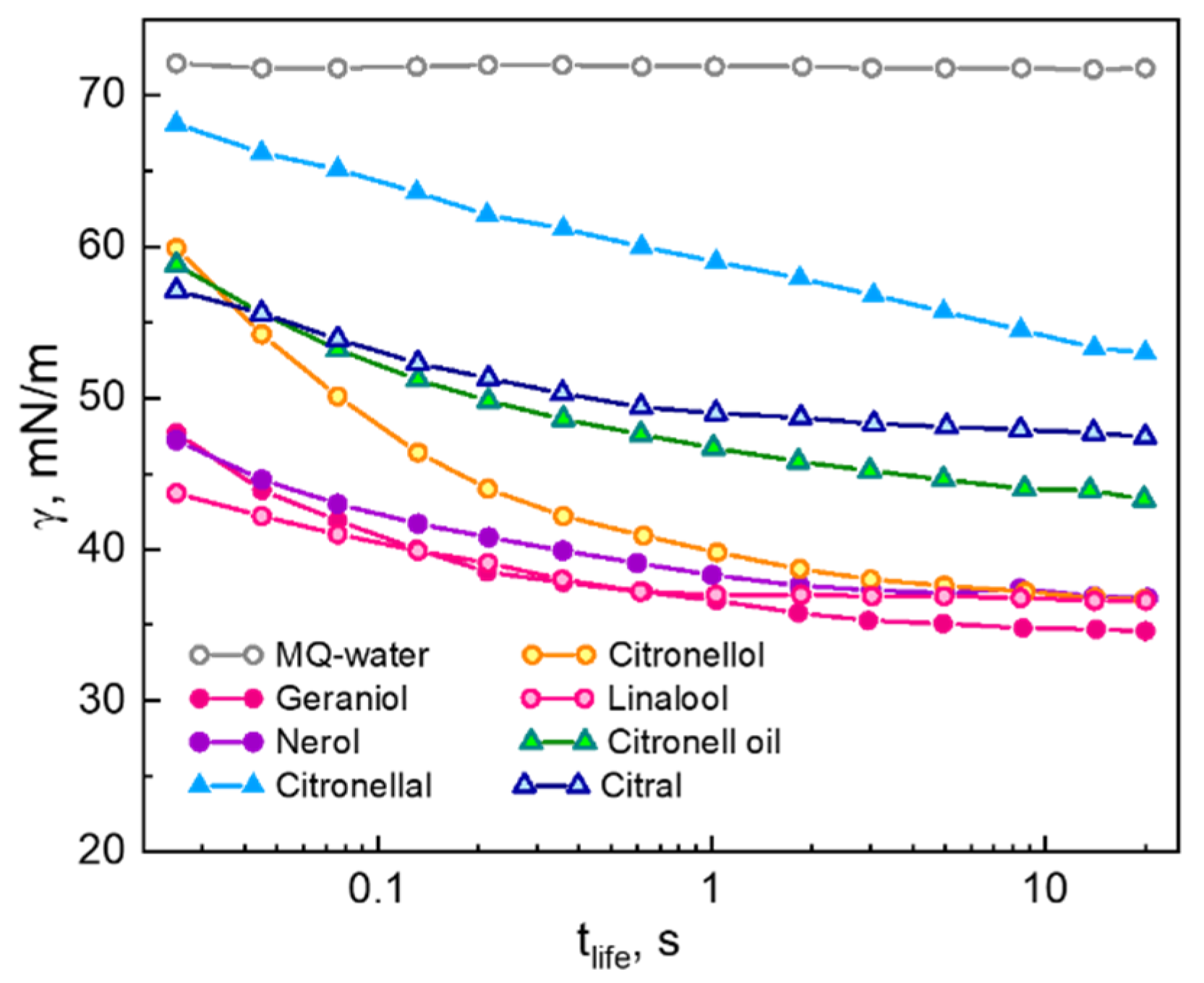
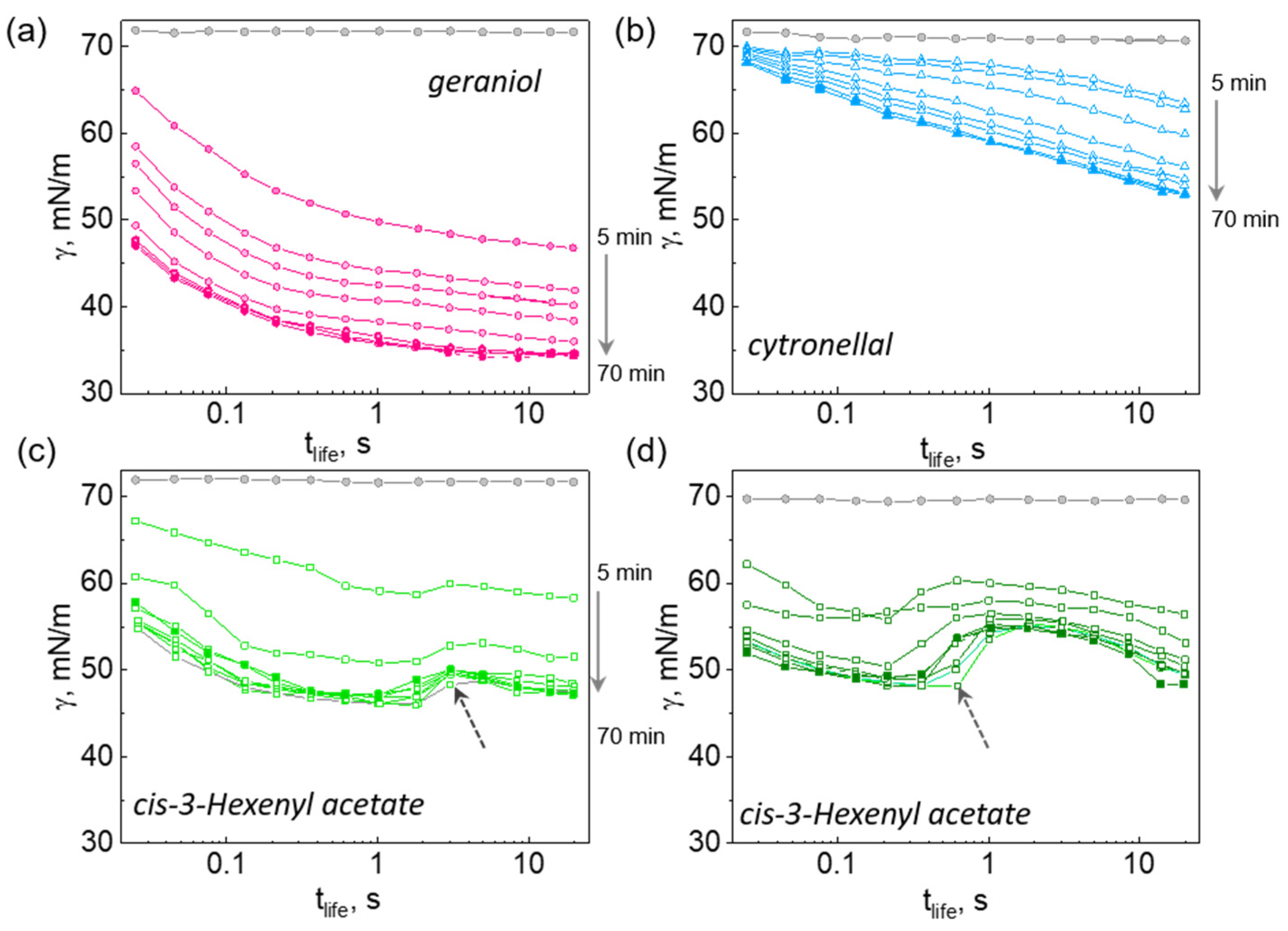
2.1. Dynamic Surface Tension of Saturated Solution of Essential Oils
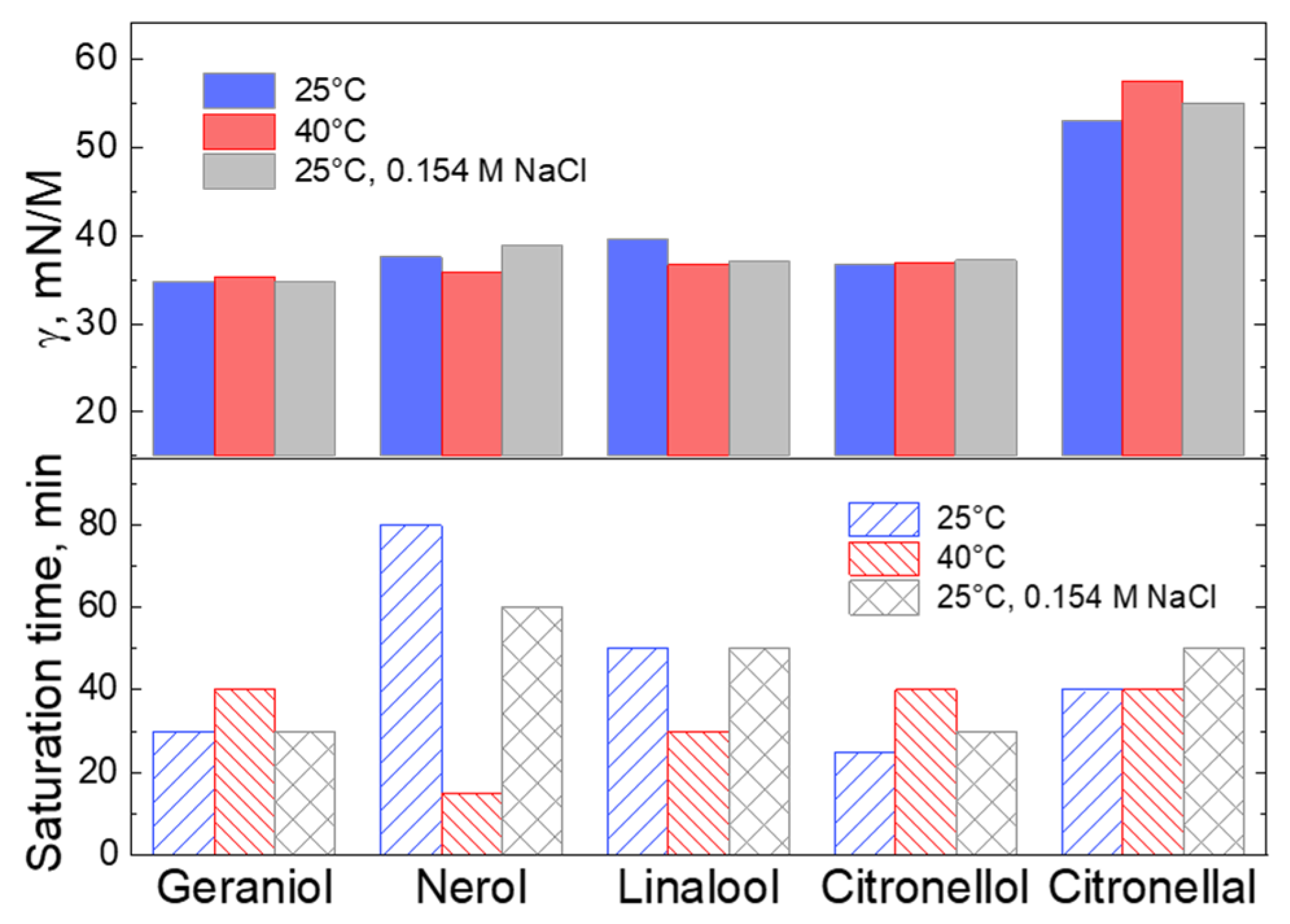
2.2. Kinetics of Solution Saturation under Variation of the Temperature and Presence of the Salt
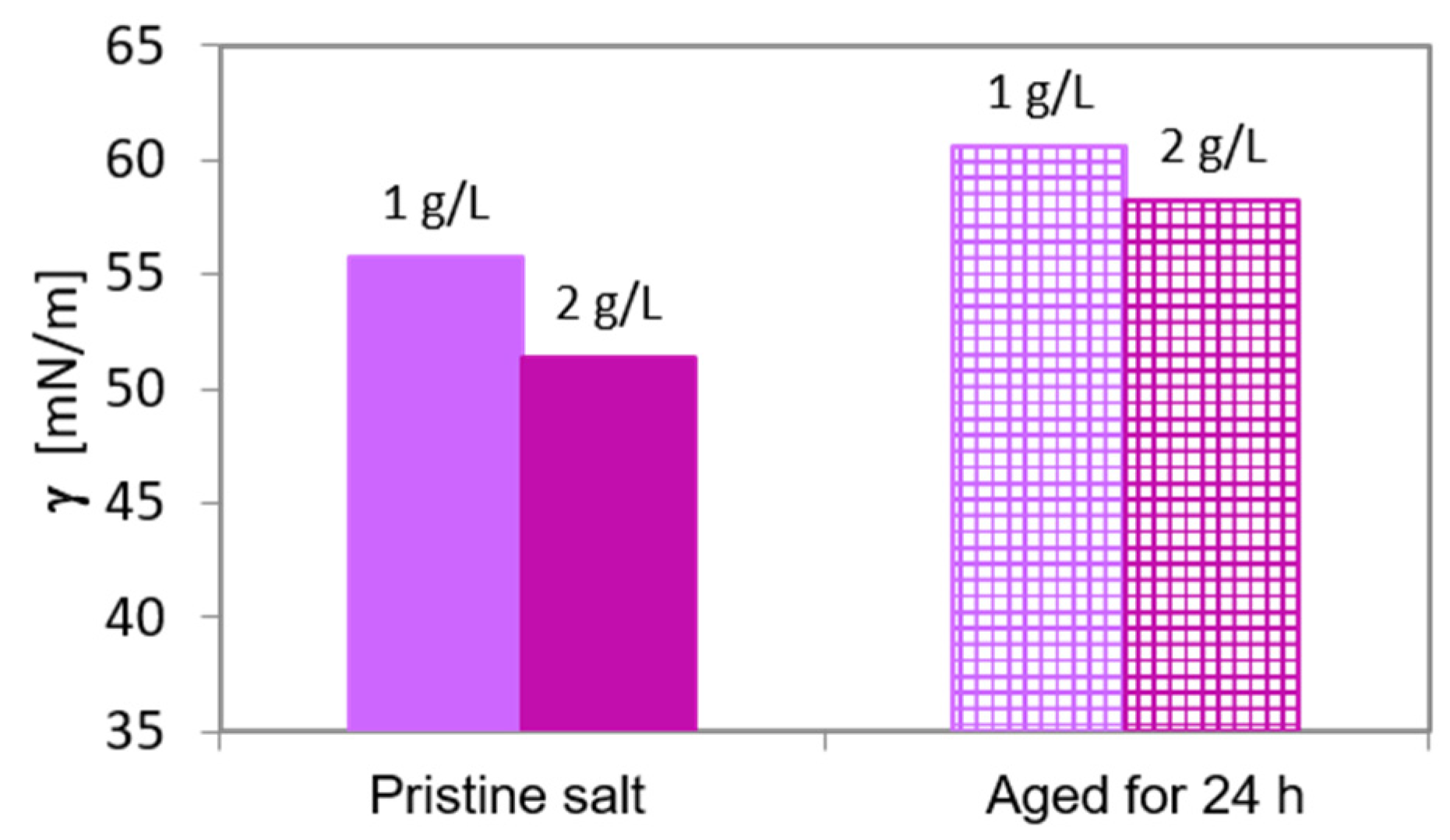
2.3. Assessment of the Fragrance Load and Carrier Aging
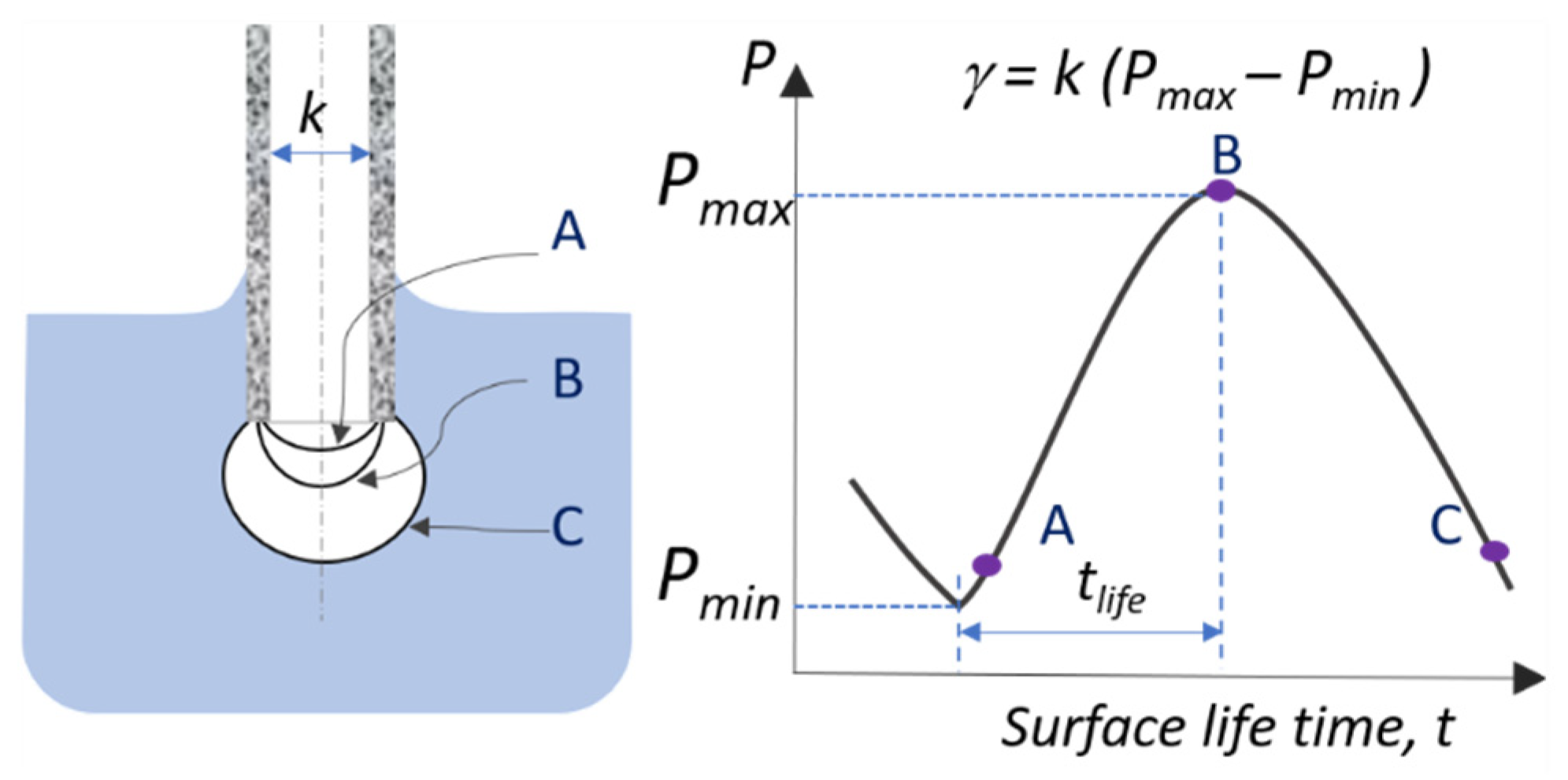
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Firestein, S. How the olfactory system makes sense of scents. Nature 2001, 413, 211–218. [Google Scholar] [CrossRef]
- Wolf, S.; Jovancevic, N.; Gelis, L.; Pietsch, S.; Hatt, H.; Gerwert, K. Dynamical Binding Modes Determine Agonistic and Antagonistic Ligand Effects in the Prostate-Specific G-Protein Coupled Receptor (PSGR). Sci. Rep. 2017, 7, 16007. [Google Scholar] [CrossRef] [Green Version]
- Smyth, H.; Cozzolino, D. Instrumental Methods (Spectroscopy, Electronic Nose, and Tongue) As Tools To Predict Taste and Aroma in Beverages: Advantages and Limitations. Chem. Rev. 2013, 113, 1429–1440. [Google Scholar] [CrossRef]
- Brattoli, M.; de Gennaro, G.; de Pinto, V.; Loiotile, A.D.; Lovascio, S.; Penza, M. Odour Detection Methods: Olfactometry and Chemical Sensors. Sensors 2011, 11, 5290–5322. [Google Scholar] [CrossRef] [Green Version]
- Wasilewski, T.; Gębicki, J.; Kamysz, W. Bioelectronic nose: Current status and perspectives. Biosens. Bioelectron. 2017, 87, 480–494. [Google Scholar] [CrossRef]
- Soboleva, O.A.; Protsenko, P.V.; Korolev, V.V.; Viktorova, J.; Yakushenko, A.; Kudla, R.; Gutmann, J.S.; Tsarkova, L.A. Aroma Molecules as Dynamic Volatile Surfactants: Functionality beyond the Scent. ACS Appl. Mater. Interfaces 2019, 11, 40988–40995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowski, A.; Szymczyk, K. Adsorption of monoterpene alcohols at the water-air interface. Adsorption 2019, 25, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Berg, J.C. An Introduction to Interfaces and Colloids: The Bridge to Nanoscience; University of Washington: Washington, WA, USA, 2009. [Google Scholar] [CrossRef] [Green Version]
- Eastoe, J.; Dalton, J.S. Dynamic surface tension and adsorption mechanisms of surfactants at the air–water interface. Adv. Colloid Interface Sci. 2000, 85, 103–144. [Google Scholar] [CrossRef]
- Miller, R.; Aksenenko, E.V.; Fainerman, V.B. Dynamic interfacial tension of surfactant solutions. Adv. Colloid Interface Sci. 2017, 247, 115–129. [Google Scholar] [CrossRef]
- Thiele, M.J.; Davari, M.D.; Hofmann, I.; Koenig, M.; Lopez, C.G.; Vojcic, L.; Richtering, W.; Schwaneberg, U.; Tsarkova, L.A. Enzyme-Compatible Dynamic Nanoreactors from Electrostatically Bridged Like-Charged Surfactants and Polyelectrolytes. Angew. Chem. Int. Ed. 2018, 57, 9402–9407. [Google Scholar] [CrossRef]
- Danov, K.D.; Gurkov, T.D.; Stanimirova, R.D.; Uzunova, R.I. Kinetics of transfer of volatile amphiphiles (fragrances) from vapors to aqueous drops and vice versa: Interplay of diffusion and barrier mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2021, 126931. [Google Scholar] [CrossRef]
- Soboleva, O.A.; Tsarkova, L.A. Surface Properties of Aqueous Solutions of Mixtures of Sodium Dodecyl Sulphate and Linalool under Equilibrium and Dynamic Conditions. Colloid J. 2020, 82, 437–447. [Google Scholar] [CrossRef]
- Murphy, R.W.; Zhu, L.; Narsimhan, G.; Jones, O.G. Impacts of Size and Deformability of β-Lactoglobulin Microgels on the Colloidal Stability and Volatile Flavor Release of Microgel-Stabilized Emulsions. Gels 2018, 4, 79. [Google Scholar] [CrossRef] [Green Version]
- Friberg, S.E.; Aikens, P.A. Constant vapor pressure emulsions evaporation: Linalool/water stabilized by Laureth 4. J. Colloid Interface Sci. 2009, 333, 599–604. [Google Scholar] [CrossRef]
- Binks, B.P.; Fletcher, P.D.I.; Holt, B.L.; Beaussoubre, P.; Wong, K. Selective Retardation of Perfume Oil Evaporation from Oil-in-Water Emulsions Stabilized by Either Surfactant or Nanoparticles. Langmuir 2010, 26, 18024–18030. [Google Scholar] [CrossRef] [PubMed]
- Qi, N.; Sun, H.; Zhao, H.; Li, Y. Achieving foaming control smartly: Pre-solubilized flavor oil serves as an in situ homogeneous defoamer. Soft Matter 2018, 14, 2059–2067. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, Y.; Zhu, X. Inclusion of Hydrophobic Liquids in Silica Aerogel Microparticles in an Aqueous Process: Microencapsulation and Extra Pore Creation. ACS Appl. Mater. Interfaces 2021, 13, 12230–12240. [Google Scholar] [CrossRef]
- Ly, H.V.; Longo, M.L. The influence of short-chain alcohols on interfacial tension, mechanical properties, area/molecule, and permeability of fluid lipid bilayers. Biophys. J. 2004, 87, 1013–1033. [Google Scholar] [CrossRef] [Green Version]
- Pereira, I.; Zielinska, A.; Ferreira, N.R.; Silva, A.M.; Souto, E.B. Optimization of linalool-loaded solid lipid nanoparticles using experimental factorial design and long-term stability studies with a new centrifugal sedimentation method. Int. J. Pharm. 2018, 549, 261–270. [Google Scholar] [CrossRef]
- Popadyuk, N.; Popadyuk, A.; Kohut, A.; Voronov, A. Thermoresponsive latexes for fragrance encapsulation and release. Int. J. Cosmet. Sci. 2016, 38, 139–147. [Google Scholar] [CrossRef]
- Fieber, W.; Scheklaukov, A.; Kunz, W.; Pleines, M.; Benczédi, D.; Zemb, T. Towards a general understanding of the effects of hydrophobic additives on the viscosity of surfactant solutions. J. Mol. Liq. 2021, 329, 115523. [Google Scholar] [CrossRef]
- Penfold, J.; Tucker, I.; Green, A.; Grainger, D.; Jones, C.; Ford, G.; Roberts, C.; Hubbard, J.; Petkov, J.; Thomas, R.K.; et al. Impact of Model Perfumes on Surfactant and Mixed Surfactant Self-Assembly. Langmuir 2008, 24, 12209–12220. [Google Scholar] [CrossRef] [PubMed]
- Pleines, M.; Kunz, W.; Zemb, T.; Benczédi, D.; Fieber, W. Molecular factors governing the viscosity peak of giant micelles in the presence of salt and fragrances. J. Colloid Interface Sci. 2019, 537, 682–693. [Google Scholar] [CrossRef]
- Tchakalova, V.; Lutz, E.; Lamboley, S.; Moulin, E.; Benczédi, D.; Giuseppone, N.; Herrmann, A. Design of Stimuli-Responsive Dynamic Covalent Delivery Systems for Volatile Compounds (Part 2): Fragrance-Releasing Cleavable Surfactants in Functional Perfumery Applications. Chem.—A Eur. J. 2021, 27, 13468–13476. [Google Scholar] [CrossRef]
- Soboleva, O.A.; Gurkov, T.D.; Stanimirova, R.D.; Protsenko, P.V.; Tsarkova, L.A. Volatile aroma surfactants: Evaluation of the adsorption-evaporation behavior under dynamic and equilibrium conditions. 2021; submitted. [Google Scholar]
- Rak, D.; Sedlak, M. On the Mesoscale Solubility in Liquid Solutions and Mixtures. J. Phys. Chem. B 2019, 123, 1365–1374. [Google Scholar] [CrossRef]
- Miller, R.; Dutschk, V.; Fainerman, V.B. Influence of molecular processes at liquid interfaces on dynamic surface tensions and wetting kinetics. J. Adhes. 2004, 80, 549–561. [Google Scholar] [CrossRef]
- Covarrubias-Cervantes, M.; Champion, D.; Debeaufort, F.; Voilley, A. Translational Diffusion Coefficients of Volatile Compounds in Various Aqueous Solutions at Low and Subzero Temperatures. J. Agric. Food Chem. 2005, 53, 6771–6776. [Google Scholar] [CrossRef] [PubMed]


| Volatile Amphiphile | Purity (GC)% | Molecular Weight, g/moL | Solubility in Water at 20 °C g/L | logPb at 20 °C | Boiling Point, °C |
|---|---|---|---|---|---|
| Geraniol 98 | 98.7 | 154.25 | 0.686 | 3.28 | 229–230 |
| Nerol | 98.9 | 154.25 | --- | 3.56 | 224–225 |
| Linalool | 98.8 | 154.25 | 1.45 | 2.44 | 198–200 |
| Citronellol | 96.8 | 156.27 | - | 3.91 | 224 |
| Citronellal | 98.8 | 154.25 | 0.07 | 3.53 | 208 |
| Citral | 97.8 | 152.23 | 0.42 | 2.33 | 225 |
| Citronell oil | Geraniol (25–45%) and Citronellal (25–54%), Citral, Eugenol and Vanillin | 154.25 | --- | --- | 208–230 |
| Cis-3Hexenyl acetate | 98.6 | 142.20 | 11.1 | 1.77 | 172 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudla, R.; Gutmann, J.S.; Tsarkova, L.A. Tensiometry as a Simple Analytical Method for Quantification of Solubility and Release of Aroma Molecules in Aqueous Media. Molecules 2021, 26, 7655. https://doi.org/10.3390/molecules26247655
Kudla R, Gutmann JS, Tsarkova LA. Tensiometry as a Simple Analytical Method for Quantification of Solubility and Release of Aroma Molecules in Aqueous Media. Molecules. 2021; 26(24):7655. https://doi.org/10.3390/molecules26247655
Chicago/Turabian StyleKudla, Ruth, Jochen S. Gutmann, and Larisa A. Tsarkova. 2021. "Tensiometry as a Simple Analytical Method for Quantification of Solubility and Release of Aroma Molecules in Aqueous Media" Molecules 26, no. 24: 7655. https://doi.org/10.3390/molecules26247655
APA StyleKudla, R., Gutmann, J. S., & Tsarkova, L. A. (2021). Tensiometry as a Simple Analytical Method for Quantification of Solubility and Release of Aroma Molecules in Aqueous Media. Molecules, 26(24), 7655. https://doi.org/10.3390/molecules26247655







