2.1. In Vitro Evaluation of Antimicrobial Activities of Plant Extracts against Potato Phytopathogens
The antimicrobial activity of 22 water plant extracts (WE), 22 water-glycol plant extracts (WGE) and the subcritical CO2 extracts (SCDE) of 3 plants, namely blackseed, thyme, and caraway, were tested against 10 potato phytopathogens.
The susceptibility to phytopathogens varied greatly and was influenced by the pathogen–extract relationship. It was shown that eight out of all tested water extracts inhibited the growth of at least one strain of potato phytopathogen: sage, blackseed, thyme, garlic, clove, onion, turmeric, and bistort (
Figure 1). The zones of phytopathogen growth inhibition ranged from 1.0 ± 0.0 mm (blackseed WE extract against
A. tenuissima) up to 57.6 ± 0.6 mm (clove WE against
R. solani). Garlic and clove WE have demonstrated the broadest spectrum of antimicrobial activity, inhibiting the growth of all 10 tested phytopathogens.
The antibacterial property of garlic extracts against several human pathogens, including Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Salmonella spp., and Streptococcus mutans has already been described [
28,
29,
30,
31,
32]. Although the activity of garlic extracts against potato phytopathogens is under-reported, a garlic WE was active against
Alternaria solani [
33], while chloroform, hexane, methanol [
34], and water [
22] extracts inhibited the growth of
Rhizoctonia solani. In our work, garlic WE was active against
F. oxysporum (19.7 ± 0.6 mm) and against
R. solani (56.0 ± 1.0). To the best of our knowledge, there are no literature data on clove WE activity against potato phytopathogens. Several studies, however, have reported the antifungal activities of clove essential oil and ethanol-water extracts against potato phytopathogens. Clove essential oil exhibited antifungal activity against
F. oxysporum [
19,
21,
35,
36] and
Alternaria alternata [
37], while ethanol-water extract inhibited the growth of
Rhizoctonia solani [
20]. In our study, the phytopathogen growth inhibition zones caused by clove WE ranged from 11.0 ± 0.0 mm against
F. sambucinum to 57.6 ± 0.6 mm against
R. solani.The most susceptible phytopathogens to garlic and clove WE were the moulds
R. solani and
A. solani, while the weakest activities of these extracts were demonstrated against
F. sambucinum and
P. carotovorum. Based on Ponce et al. [
38], garlic and clove extracts were considered to be active (inhibition zone diameter 9–15 mm; clove WE against
F. sambucinum) or very active (inhibition zone diameter >15 mm; the other combinations of extract and phytopathogen). Onion WE against
P. exigua (20.1 ± 0.6 mm) as well as turmeric and bistort WE against
S. scabiei (29.7 ± 0.6 and 24.6 ± 0.6 mm, respectively) were recognized as very active. According to several studies, the growth of
R. solani might be inhibited by turmeric [
25], nettle [
9], sage [
9,
22,
23], and onion water extracts [
22]; however, our study indicates no activity of these extracts against
R. solani.
On the contrary, our observations confirmed Abd-El-Khair et al. [
22], who point out that peppermint WE was inactive against
R. solani. An earlier study also reported the antifungal activity of blackseed ethanol-water extract against
R. solani [
20], probably due to the addition of ethanol as a solvent, enriching the extract in active compounds. Discrepancies in antifungal activity may result from differences in the chemical composition of individual extracts depending on plant variety, storage methods, or the exact extraction procedure. The detailed results of phytopathogen growth inhibition by water extracts are presented in
Figure 1 and
Table S1.
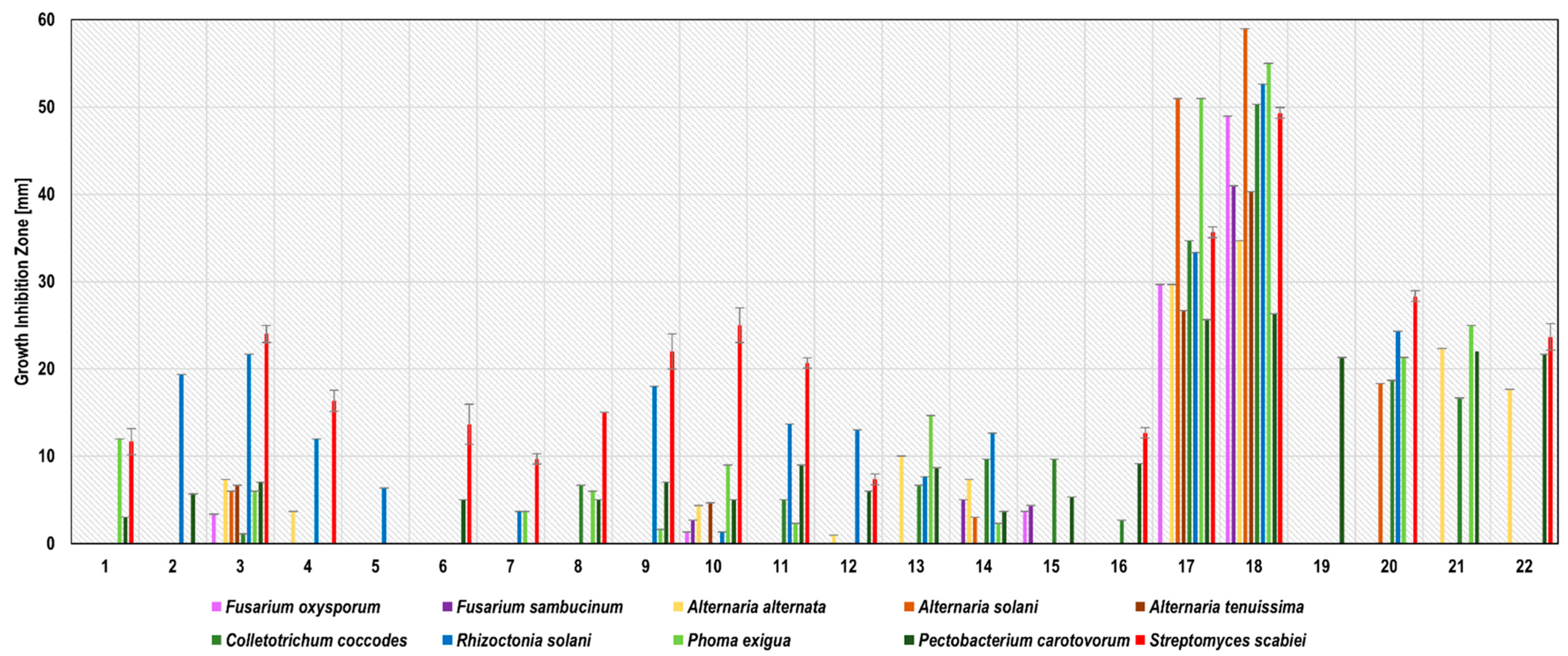
Glycol-water plant extracts expressed a wider range of activity compared to the water extracts of the same plant. All of the 22 glycol-water extracts revealed antimicrobial activity against at least one strain of the phytopathogens tested (
Figure 2). The zones of growth inhibition ranged from 1.0 ± 0.0 mm (caraway WGE against
A. alternata) to 59.0 ± 1.0 mm (clove WGE against
A. solani). As in the case of water extracts, the most active glycol-water extracts were garlic and clove, which inhibited the growth of 9 (no activity against
F. sambucinum) and 10 phytopathogens, respectively. The zones of growth inhibition estimated for garlic WGE ranged from 25.7 ± 0.6 against
P. carotovorum to 51.0 ± 1.0 mm against
P. exigua and
A. solani. For clove WGE, these values ranged from 26.3 ± 0.6 mm against
P. carotovorum to 59.0 ± 1.0 mm against
A. solani. The most sensitive phytopathogens to these two glycol-water extracts were
A. solani,
P. exigua,
R. solani, and
C. coccodes, while the least sensitive were
P. carotovorum and
A. alternata. The following WGE were classified as very active: turmeric against
A. solani,
C. coccodes,
R. solani,
P. exigua, and
S. scabiei; bistort against
A. alternata,
C. coccodes,
P. exigua, and
P. carotovorum; common knotgrass against
A. alternata,
P. carotovorum, and
S. scabiei; sage and rosemary against
R. solani and
S. scabiei; peppermint against
R. solani; horsetail, hop, and summer savoury against
S. scabiei; onion against
P. carotovorum. No studies were found regarding glycol-water extracts against potato phytopathogens. This article is the first such extensive research in this field. Previous studies reported inhibition of
A. alternata growth by peppermint and lavender methanol-water extract [
10] and peppermint essential oil [
26]; however, due to a different type of extract, these findings were not reflected in the present work. The detailed results of phytopathogen growth inhibition by water-glycol extracts are presented in
Figure 2 and
Table S2.
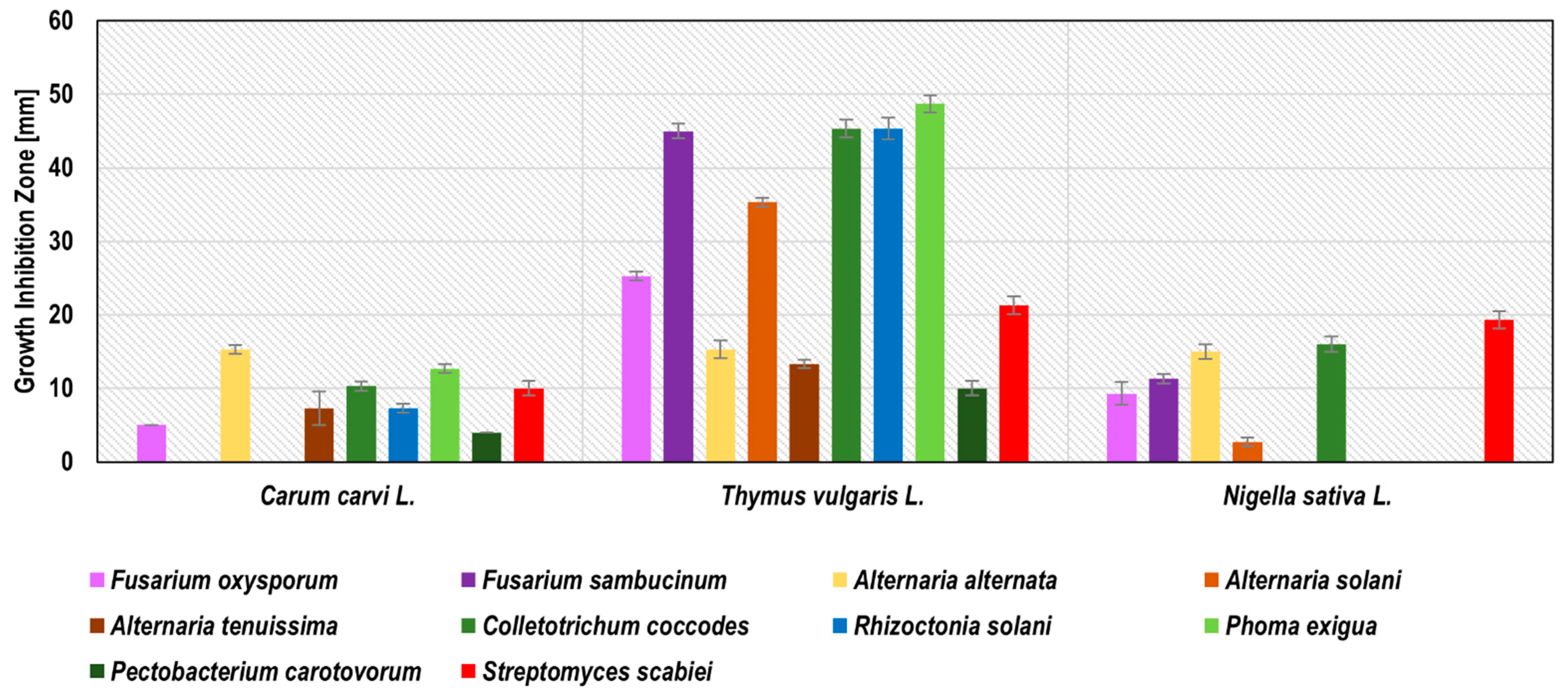
Blackseed, thyme, and caraway subcritical carbon dioxide extracts (SCDE) were tested against potato phytopathogens. Only these three plants were successfully used for subcritical carbon dioxide extraction, probably due to the high level of oil fraction in the seeds of these plants. All of these SCDEs inhibited the growth of at least six phytopathogens (
Figure 3). The growth inhibition zones ranged from 3.1 ± 0.6 mm for the blackseed SCDE against
A. solani to 48.4 ± 1.7 mm for the thyme SCDE against
P. exigua. Thyme SCDE showed the broadest spectrum of antimicrobial activity by inhibiting the growth of all 10 phytopathogens tested. The most susceptible to thyme SCDE were
P. exigua (48.4 ± 1.7 mm),
R. solani,
C. coccodes, and
F. sambucinum (45.2 ± 1.5, 45.2 ± 1.5, and 45.0 ± 0.6 mm, respectively), while the least susceptible were
P. carotovorum and
A. tenuissima (10.0 ± 1.0 and 13.2 ± 0.6 mm, respectively). Our research confirmed the antifungal activity of thyme SCDE against
A. alternata, as previously described by Perina et al. [
39], Soković et al. [
26], and Puškárová et al. [
37] for thyme essential oil/mL. Zambonelli et al. [
40] indicated the antifungal activity of thyme essential oil against
R. solani, which coincides with our results for thyme SCDE. Diánez et al. [
19] present the antifungal activity of thyme essential oil against
F. oxysporum, which is compatible with our research for thyme SCDE. Caraway SCDE inhibited the growth of eight phytopathogens. The best effect was observed against
A. alternata (growth inhibition zone 15.3 ± 0.6 mm) and
P. exigua (growth inhibition zone 12.6 ± 0.6 mm). Convergent results were obtained in previous studies for caraway essential oil, for which antimicrobial activity has been demonstrated against
P. carotovorum [
27] and
A. alternata [
24]. Blackseed SCDE inhibited the growth of six phytopathogens, with the largest inhibition zones against
S. scabiei (19.2 ± 1.2 mm) and
C. coccodes (16.1 ± 1.2 mm). Based on Ponce et al. [
28], thyme SCDE was considered as active against eight tested pathogens (
F. oxysporum,
F. sambucinum,
A. alternaria,
A. solani,
C. coccodes,
R. solani,
P. exigua, and
S. scabiei); caraway SDCE was recognised as very active against
A. alternata, and blackseed SCDE against
C. coccodes and
S. scabiei. The detailed results of phytopathogen growth inhibitions by SCDE are presented in
Figure 3 and
Table S3.
Glycol-water extracts exhibited in general a wider spectrum of activity against the potato phytopathogens than water extracts. The exceptions were garlic and clove extracts, which inhibited a similar number of pathogens regardless of the solvent used. The greatest differences in antimicrobial activity were observed for SCDE thyme extracts compared to both WE and WGE. Thyme SCDE was recognized as active or very active against all 10 tested phytopathogens, while both WGE and WE only against 2 pathogens. These differences were similar for caraway and blackseed SCDE (active or very active against 4 and 5, respectively) in comparison to WE (not active or very active), but smaller for WGE (active against 1 and 3 pathogens, respectively).
The Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal/Fungicidal Concentration (MBC/MFC) were determined for garlic and clove water extracts and for thyme and caraway subcritical CO
2 extracts that were the most effective against the phytopathogens. Further experiments on glycol-water extracts were abandoned due to their negative impact on the morphological features of seed potatoes (data not published). The detailed results are presented in
Table 1.
MIC values of garlic WE vary between 6.3 and 25 mg/mL, and the most sensitive were the moulds
A. solani and
P. exigua (MIC 6.3 mg/mL), followed by
F. oxysporum,
F. sambucinum,
C. coccodes,
R. solani and Gram-positive bacteria
S. scabiei (MIC 12.5 mg/mL). The growth inhibition of two moulds of the genus
Alternaria:
A. alternata and
A. tenuissima, as well as bacteria
P. carotovorum, was possible at a garlic WE concentration not lower than 25 mg/mL. The lower MIC value of garlic WE compared to Gram-negative bacteria
P. carotovorum was noted against other representatives of the
Enterobacteriaceae family,
E. coli and
Salmonella typhi, by Iwalokun et al. [
41] (MIC 20.8 and 21.8 mg/mL, respectively). In the same study, MIC values for
S. aureus and
Streptococcus pneumoniae were 27.1 and 30.3 mg/mL, respectively, which is more than two times higher than those obtained in our work for
S. scabiei, another representative of Gram-positive bacteria. In the present work, MBC/MFC values of garlic WE ranged from 12.5 to 25 mg/mL against the tested microorganisms, with the exception of
A. tenuissima, for which no MFC was detected.
MIC values of clove WE were found to be 50 to 75% lower than those of garlic WE and vary between 3.1 and 6.3 mg/mL for all the phytopathogens tested. On the contrary, the MBC/MFC values were more diversified. The lowest were recorded against
A. solani,
R. solani, and
P. exigua (6.3 mg/mL), and the highest against
P. carotovorum (25 mg/mL). As mentioned above, there are no previously reported data on clove WE activity against potato phytopathogens. There are, however, some reports on clove essential oil action against selected fungi. Puškárová et al. [
37] have not estimated the MFC value (MIC was equal to 0.025%) of clove essential oil against
A. alternata, which is similar to our results on MFC determination obtained for clove WE (in our work, MIC was 252 times higher). Rana et al. [
36] determined the MIC of clove essential oil against
F. oxysporum as equal to 10 µL/mL, about 630 times lower than that observed in our work for clove WE. In another study, Sharma et al. [
21] reported MIC 31.25 ppm and MFC 125 ppm of clove essential oil against
F. oxysporum, 2016 and 1000 times lower than the values obtained in the present work for clove WE, respectively. The weaker antifungal action of clove WE compared to clove essential oil may be attributed to the higher concentration of antifungal components in pure essential oil than in water extract.
MIC values of thyme SCDE ranged between 2.9 and 11.5 mg/mL. The lowest MIC values were recorded against
C. coccodes and
S. scabiei (2.9 mg/mL), while the highest were against
A. alternata,
A. tenuissima, and
P. exigua (11.5 mg/mL). In the earlier studies of Puškárová et al. [
37] and Soković et al. [
26], however, thyme essential oil revealed antifungal activity at a much lower concentration (MIC 0.025% and MIC 0.25 μL/mL, respectively). It can be assumed that this is related to the differences in the qualitative and quantitative composition of the essential oil and the subcritical carbon dioxide extract.
The MIC values for caraway SCDE were the highest of all the extracts tested. The growth of five phytopathogens (
A. alternata,
A. tenuissima,
P. exigua,
P. carotovorum, and
S. scabiei) was inhibited by only 0.9 mg/mL undiluted extract. The lowest value was observed against
F. oxysporum, and the minimum inhibitory concentration was 22.5 mg/mL. The MIC values of caraway SCDE against
P. carotovorum and
A. alternata recorded in this work were significantly higher than the values of caraway essential oil obtained in the work of Iacobellis et al. [
27] (910 µg) and Begum et al. [
24] (50 ppm).
Due to the high activity and broad spectrum of action, in terms of future research on seed potatoes, we recommend the use of water extracts from garlic and clove, as well as caraway and thyme subcritical carbon dioxide extracts. The use of other extracts that exhibited high antimicrobial activity but not against such a wide phytopathogen spectrum is also worth considering, e.g., onion WE (antifungal activity against P. exigua), turmeric and bistort WE (against S. scabiei), and blackseed SCDE (against C. coccodes and S. scabiei).
2.2. Chemical Composition of Selected Extracts
The chemical composition of garlic and clove water extracts, as well as that of thyme and caraway subcritical carbon dioxide extracts, was identified. The results are presented in
Table 2,
Table 3,
Table 4 and
Table 5.
A total of 33 (97.54%) compounds of clove water extract were identified and eugenol (allylbenzene class of chemical compounds) was determined to be the main component (82.39%). This result is in accordance with the results reported for different types of clove extracts by many authors. In other studies, eugenol constituted 53.9% [
42], 75.41% [
21], or even 85% [
35] of clove oil and 52.88% of subcritical CO
2 extract [
43] and was the principal compound in all of them. The eugenol content indicated in the literature was a result of both the origin of the plant material and the fact that the analytical method used (GC-MS) gives the relative content of the ingredients, and not the actual one. This is confirmed by the lower content of eugenol in carbon dioxide extract (52,88%) because this extraction method shows the full organic profile of the plant matrix and consequently a greater number of compounds. The relative content of eugenol was therefore lower in carbon dioxide extract than in essential oils of clove. The presented investigation also revealed the presence of eugenol acetate (4.56%) and caryophyllene (2.49%). These compounds are also reported in the above-mentioned studies of clove extracts in different concentrations. It is well documented that the antimicrobial activity of clove extracts is mostly related to high eugenol content, and the mode of action is based on cytoplasmic membrane disruption, inhibition of some microbial enzymes, and the negative effect of ions and ATP transport [
44].
A total of 42 (83.01%) components of garlic water extract were identified, and 5-hydroxymethylfurfural (33.24%) was observed in the highest quantity. This compound was previously found in black garlic subjected to heat treatment [
45] and in three Chinese varieties of fresh garlic (4.88, 26.78 and 47.10%); however, no antimicrobial activity has yet been reported for 5-hydroxymethylfurfural. No allicin was observed in our garlic WE, probably due to its instability and quick transformation into other sulphur components [
46]. Small quantities of diallyl disulphide (1.58%), dimethyl trisulphide (0.95%), 3-vinyl-1,2-dithiacyclohex-4-ene (0.44%), allyl methyl trisulphide (0.41%), and others were determined and might be jointly responsible for the strong antimicrobial activity of garlic WE against the tested potato phytopathogens [
13].
In total, 63 (95.26%) of the components of thyme subcritical CO
2 extract were identified and thymol (monoterpenoid phenol derivative of
p-cymene; 48.54%) was determined to be dominant. This result is confirmed by numerous studies, which verify the presence of thymol in thyme essential oils at a level of 39.14 [
47], 47.9 [
48], and 48.9% [
26]. Some studies [
49] reported another monoterpenoid phenol, thymol isomer carvacrol, as a major component of thyme essential oil, which in the current study was present at a level of 3.16%. Also, the presence of
p-cymene (8.53%) was reported in the above-mentioned studies. The antimicrobial activity of thymol and carvacrol is revealed in the work of Perina et al. [
39] and Sim et al. [
50]. Their mode of antimicrobial action included the disruption of cell walls, leading to leakage of cell components and, consequently, to lysis. Other reported mechanisms include disturbances in protein synthesis and quorum sensing [
51].
Based on 33 (99.19%) of the compounds identified in caraway subcritical CO
2 extract, (+)-carvone (terpenoid; 52,14%) and
d-limonene (cyclic monoterpene; 37.17%) were determined as the two main components. These results are similar to those obtained by Iacobellis et al. [
27], where carvone (23.3%) and limonene (18.2%) were detected as the principal components of caraway essential oil; however, other compounds found in high quantities in this work, such as germacrene and trans-dihydrocarvone, were found only in traces in the caraway SCDE in the present study. Also, Argañosa et al. [
52] and Putievsky et al. [
53] indicated carvone and limonene as the dominant components in caraway essential oil. A different composition, however, was reported by Begum et al. [
24], where thymol (48.20%), o-cymene (19.29%), and γ-terpinen (17.61%) were the main components.