Elucidating the Color of Rosé Wines Using Polyphenol-Targeted Metabolomics
Abstract
1. Introduction
2. Results
2.1. Rosé Wine Diversity
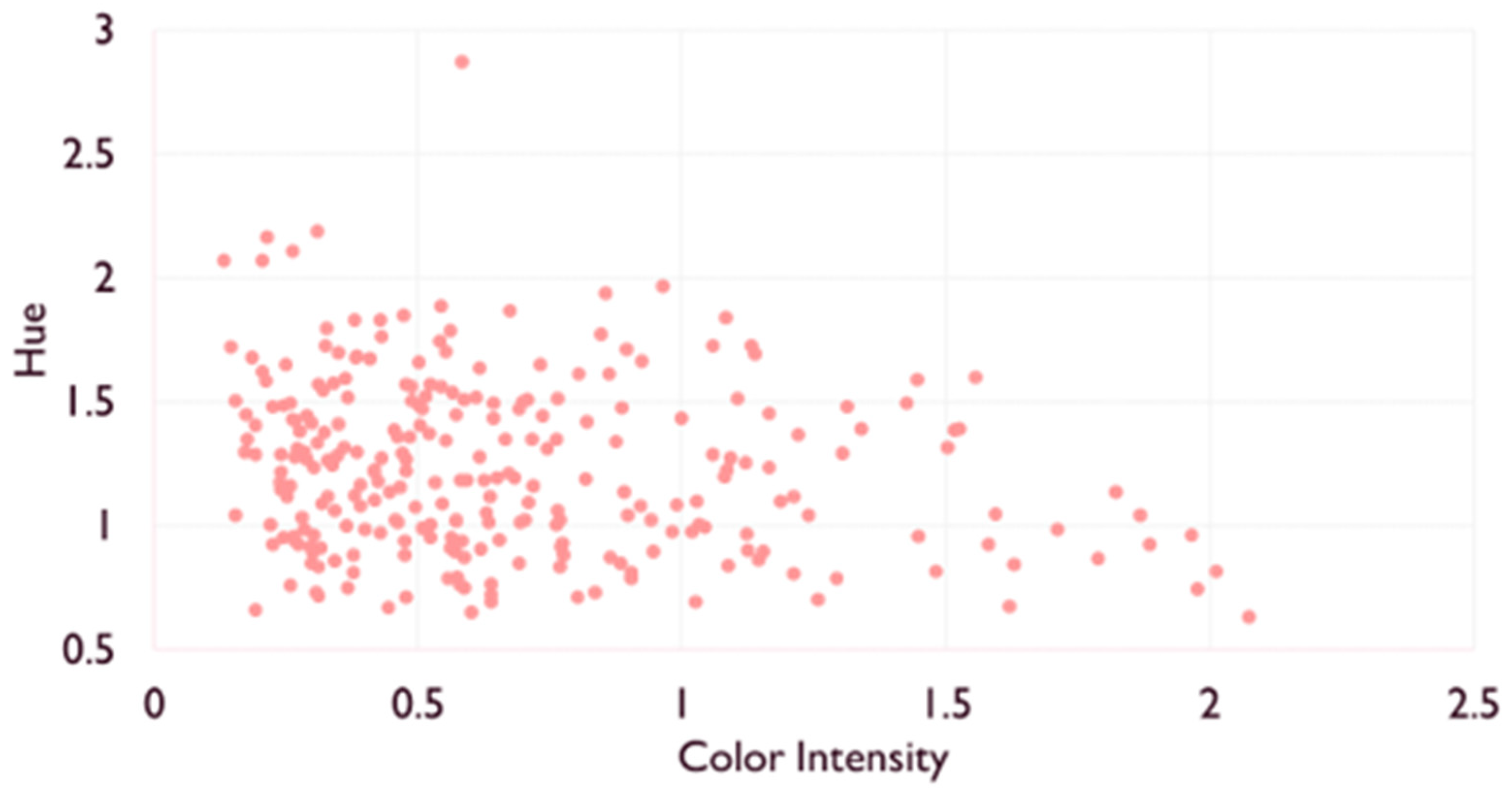
2.1.1. Enological Parameters and Color
2.1.2. Phenolic Composition
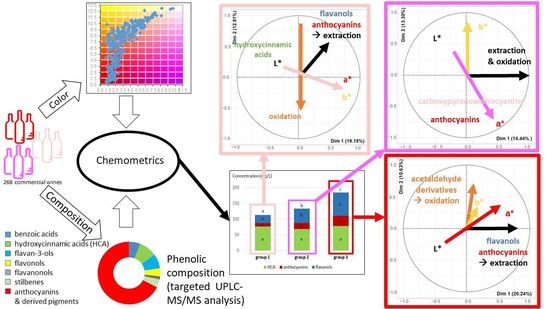
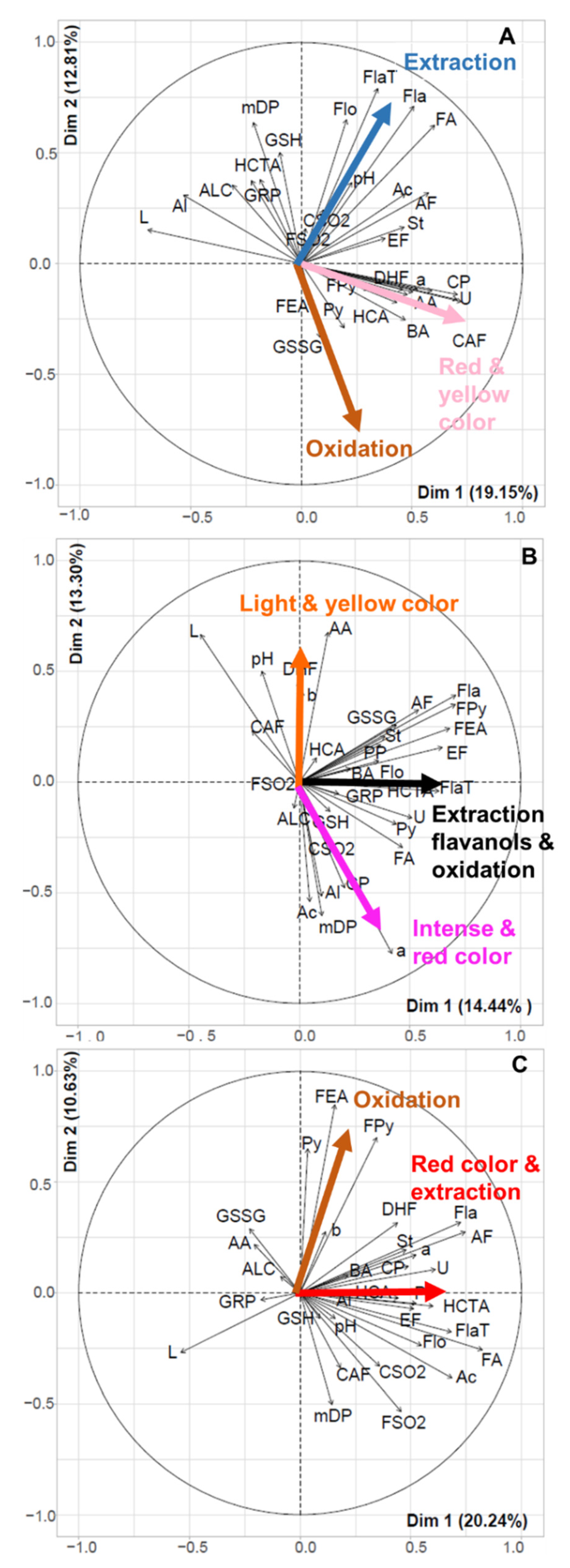
2.2. Links between Color and Phenolic Composition
2.2.1. Multivariate Analysis of the L*a*b*, Enological Analysis, and Phenolic Composition Data
2.2.2. Composition of the Light, Intermediate, and Dark Wine Groups
2.2.3. Links between Color and Phenolic Composition for Light, Dark and Intermediate Wines
3. Discussion
3.1. Rosé Wine(s) Versus Red Wine
3.2. Pigment Composition and Color
3.3. Drivers of Rosé Wine Color
3.3.1. Extraction
3.3.2. Oxidation
4. Materials and Methods
4.1. Chemicals
4.2. Samples and Sampling
4.3. Enological Analysis
4.4. Color Analysis
4.5. Analysis of Phenolic Composition
4.6. Chemometrics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Peres, S.; Giraud-Heraud, E.; Masure, A.-S.; Tempere, S. Rose Wine Market: Anything but Colour? Foods 2020, 9, 1850. [Google Scholar] [CrossRef] [PubMed]
- Brouillard, R.; Dubois, J.-E. Mechanism of the structural transformations of anthocyanins in acidic media. J. Am. Chem. Soc. 1977, 99, 1359–1364. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef]
- Timberlake, C.; Bridle, P. Flavylium salts, anthocyanidins and anthocyanins. II. Reactions with sulphur dioxide. J. Sci. Food Agric. 1967, 18, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Somers, T.C. The polymeric nature of wine pigments. Phytochemistry 1971, 10, 2175–2186. [Google Scholar] [CrossRef]
- Salas, E.; Fulcrand, H.; Meudec, E.; Cheynier, V. Reactions of anthocyanins and tannins in model solutions. J. Agric. Food Chem. 2003, 51, 7951–7961. [Google Scholar] [CrossRef] [PubMed]
- Salas, E.; Le Guernevé, C.; Fulcrand, H.; Poncet-Legrand, C.; Cheynier, V. Structure determination and color properties of a newly synthesized direct-linked flavanol-anthocyanin dimer. Tetrahedron Lett. 2004, 45, 8725–8729. [Google Scholar] [CrossRef]
- Remy-Tanneau, S.; Le Guerneve, C.; Meudec, E.; Cheynier, V. Characterization of a colorless anthocyanin-flavan-3-ol dimer containing both carbon-carbon and ether interflavanoid linkages by NMR and mass spectrometries. J. Agric. Food Chem. 2003, 51, 3592–3597. [Google Scholar] [CrossRef]
- Cheynier, V.; Dueñas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.M.; Sarni-Manchado, P.; Fulcrand, H. Structure and properties of wine pigments and tannins. Am. J. Enol. Vitic. 2006, 57, 298–305. [Google Scholar]
- De Freitas, V.; Mateus, N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011, 401, 1463–1473. [Google Scholar] [CrossRef]
- Wirth, J.; Caillé, S.; Souquet, J.M.; Samson, A.; Dieval, J.; Vidal, S.; Fulcrand, H.; Cheynier, V. Impact of post-bottling oxygen exposure on the sensory characteristics and phenolic composition of Grenache rosé wines. Food Chem. 2012, 132, 1861–1871. [Google Scholar] [CrossRef]
- Wirth, J.; Morel-Salmi, C.; Souquet, J.; Dieval, J.; Aagaard, O.; Vidal, S.; Fulcrand, H.; Cheynier, V. The impact of oxygen exposure before and after bottling on the polyphenolic composition of red wines. Food Chem. 2010, 123, 107–116. [Google Scholar] [CrossRef]
- Sarni-Manchado, P.; Cheynier, V.; Moutounet, M. Reaction of enzymically generated quinones with malvidin-3-glucoside. Phytochemistry 1997, 45, 1365–1369. [Google Scholar] [CrossRef]
- Lambert, M.; Meudec, E.; Verbaere, A.; Mazerolles, G.; Wirth, J.; Masson, G.; Cheynier, V.; Sommerer, N. A High-Throughput UHPLC-QqQ-MS Method for Polyphenol Profiling in Rose Wines. Molecules 2015, 20, 7890–7914. [Google Scholar] [CrossRef]
- Hernández, B.; Sáenz, C.; Alberdi, C.; Alfonso, S.; Diñeiro, J.M. Colour Evolution of Rosé Wines after Bottling. S. Afr. J. Enol. Vitic. 2011, 32, 42–50. [Google Scholar] [CrossRef][Green Version]
- Gil, M.; Avila-Salas, F.; Santos, L.S.; Iturmendi, N.; Moine, V.; Cheynier, V.; Saucier, C. Rose Wine Fining Using Polyvinylpolypyrrolidone: Colorimetry, Targeted Polyphenomics, and Molecular Dynamics Simulations. J. Agric. Food Chem. 2017, 65, 10591–10597. [Google Scholar] [CrossRef]
- Salas, E.; Atanasova, V.; Poncet-Legrand, C.; Meudec, E.; Mazauric, J.; Cheynier, V. Demonstration of the occurrence of flavanol-anthocyanin adducts in wine and in model solutions. Anal. Chim. Acta 2004, 513, 325–332. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.; Echavarri, F.; Ferreira, V.; Fernández-Zurbano, P. Pigment composition and color parameters of commercial Spanish red wine samples: Linkage to quality perception. Eur. Food Res. Technol. 2011, 237, 877–887. [Google Scholar] [CrossRef]
- Morel-Salmi, C.; Souquet, J.M.; Bes, M.; Cheynier, V. The effect of flash release treatment on phenolic extraction and wine composition. J. Agric. Food Chem. 2006, 54, 4270–4276. [Google Scholar] [CrossRef]
- Vernhet, A.; Carillo, S.; Rattier, A.; Verbaere, A.; Cheynier, V.; Mekoue Nguela, J. Fate of Anthocyanins and Proanthocyanidins during the Alcoholic Fermentation of Thermovinified Red Musts by Different Saccharomyces Cerevisiae Strains. J. Agric. Food Chem. 2020, 68, 3615–3625. [Google Scholar] [CrossRef]
- Singleton, V.L.; Timberlake, C.F.; Lea, A.G.H. The Phenolic Cinnamates of White Grapes and Wine. J. Sci. Food Agric. 1978, 29, 403–410. [Google Scholar] [CrossRef]
- Chamkha, M.; Cathala, B.; Cheynier, V.; Douillard, R. Phenolic Composition of Champagnes from Chardonnay and Pinot Noir Vintages. J. Agric. Food Chem. 2003, 51, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Souquet, J.-M.; Moutounet, M. Glutathione content and glutathione to hydroxycinnamic acid ratio in Vitis vinifera grapes and musts. Am. J. Enol. Vitic. 1989, 40, 320–324. [Google Scholar]
- Cheynier, V.; Rigaud, J.; Moutounet, M. Oxidation kinetics of trans-caffeoyltartrate and its glutathione derivatives in grape musts. Phytochemistry 1990, 29, 1751–1753. [Google Scholar] [CrossRef]
- Abi-Habib, E.; Poncet-Legrand, C.; Roi, S.; Carrillo, S.; Doco, T.; Vernhet, A. Impact of grape variety, berry maturity and size on the extractability of skin polyphenols during model wine-like maceration experiments. J. Sci. Food Agric. 2021, 101, 3257–3269. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Heras, J.; Callejo, M.; Tesfaye, W.; González, C.; Suárez-Lepe, J. Yeast influence on the formation of stable pigments in red winemaking. Food Chem. 2016, 197, 686–691. [Google Scholar] [CrossRef]
- Ochando, T.; Mouret, J.; Humbert-Goffard, A.; Aguera, E.; Sablayrolles, J.; Farines, V. Comprehensive study of the dynamic interaction between SO2 and acetaldehyde during alcoholic fermentation. Food Res. Int. 2020, 136, 109607. [Google Scholar] [CrossRef]
- Hazelwood, L.; Daran, J.; van Maris, A.; Pronk, J.; Dickinson, J. The ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef]
- Romboli, Y.; Mangani, S.; Buscioni, G.; Granchi, L.; Vincenzini, M. Effect of Saccharomyces cerevisiae and Candida zemplinina on quercetin, vitisin A and hydroxytyrosol contents in Sangiovese wines. World J. Microbiol. Biotechnol. 2015, 31, 1137–1145. [Google Scholar] [CrossRef]
- Remy, S.; Fulcrand, H.; Labarbe, B.; Cheynier, V.; Moutounet, M. First confirmation in red wine of products resulting from direct anthocyanin-tannin reactions. J. Sci. Food Agric. 2000, 80, 745–751. [Google Scholar] [CrossRef]
- Atanasova, V.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Effect of oxygenation on polyphenol changes occurring in the course of wine making. Anal. Chim. Acta 2002, 458, 15–27. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Biler, M.; Meudec, E.; Le Guerneve, C.; Vernhet, A.; Mazauric, J.P.; Legras, J.L.; Loonis, M.; Trouillas, P.; Cheynier, V.; et al. p-Hydroxyphenyl-pyranoanthocyanins: An Experimental and Theoretical Investigation of Their Acid-Base Properties and Molecular Interactions. Int. J. Mol. Sci. 2016, 17, 1842. [Google Scholar] [CrossRef] [PubMed]
- Sarni-Manchado, P.; Fulcrand, H.; Souquet, J.-M.; Cheynier, V.; Moutounet, M. Stability and color of unreported wine anthocyanin-derived pigments. J. Food Sci. 1996, 61, 938–941. [Google Scholar] [CrossRef]
- Poncet-Legrand, C.; Cabane, B.; Bautista-Ortin, A.B.; Carrillo, S.; Fulcrand, H.; Perez, J.; Vernhet, A. Tannin Oxidation: Infra- versus Intermolecular Reactions. Biomacromolecules 2010, 11, 2376–2386. [Google Scholar] [CrossRef] [PubMed]
- Gambuti, A.; Han, G.; Peterson, A.; Waterhouse, A. Sulfur Dioxide and Glutathione Alter the Outcome of Microoxygenation. Am. J. Enol. Vitic. 2015, 66, 411–423. [Google Scholar] [CrossRef][Green Version]
- Hayasaka, Y.; Black, C.; Hack, J.; Smith, P. Structural characterization of reaction products of caftaric acid andbisulfite present in a commercial wine using high resolution massspectrometric and nuclear magnetic resonance techniques. Food Chem. 2017, 230, 99–107. [Google Scholar] [CrossRef]
- Arapitsas, P.; Ugliano, M.; Perenzoni, D.; Angeli, A.; Pangrazzi, P.; Mattivi, F. Wine metabolomics reveals new sulfonated products in bottled white wines, promoted by small amounts of oxygen. J. Chromatogr. A 2016, 1429, 155–165. [Google Scholar] [CrossRef]
- Arapitsas, P.; Guella, G.; Mattivi, F. The impact of SO2 on wine flavanols and indoles in relation to wine style and age. Sci. Rep. 2018, 8, 858. [Google Scholar] [CrossRef]
- Nikolantonaki, M.; Magiatis, P.; Waterhouse, A. Direct Analysis of Free and Sulfite-Bound Carbonyl Compounds in Wine by Two-Dimensional Quantitative Proton and Carbon Nuclear Magnetic Resonance Spectroscopy. Anal. Chem. 2015, 87, 10799–10806. [Google Scholar] [CrossRef]
- Danilewicz, J. Review of Reaction Mechanisms of Oxygen and Proposed Intermediate Reduction Products in Wine: Central Role of Iron and Copper. Am. J. Enol. Vitic. 2003, 54, 73–85. [Google Scholar]
- Bueno, M.; Marrufo-Curtido, A.; Carrascón, V.; Fernández-Zurbano, P.; Escudero, A.; Ferreira, V. Formation and Accumulation of Acetaldehyde and Strecker Aldehydes during Red Wine Oxidation. Front. Chem. 2018, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Trousdale, E.; Singleton, V.L.; Salgues, M.; Wylde, R. Characterization of 2-S-glutathionylcaftaric acid and its hydrolysis in relation to grape wines. J. Agric. Food Chem. 1986, 34, 217–221. [Google Scholar] [CrossRef]
- Cheynier, V.; Van Hulst, M.W. Oxidation of trans-caftaric acid and 2-S-glutathionyl caftaric acid in model solutions. J. Agric. Food Chem. 1988, 36, 10–15. [Google Scholar] [CrossRef]
- Cheynier, V.; Osse, C.; Rigaud, J. Oxidation of grape juice phenolic compounds in model solutions. J. Food Sci. 1988, 53, 1729–1732. [Google Scholar] [CrossRef]
- Cheynier, V.; Basire, N.; Rigaud, J. Mechanism of trans-caffeoyl tartaric acid and catechin oxidation in model solutions containing grape polyphenoloxidase. J. Agric. Food Chem. 1989, 37, 1069–1071. [Google Scholar] [CrossRef]
- Cheynier, V.; Ricardo Da Silva, J.M. Oxidation of grape procyanidins in model solutions containing trans-caffeoyl tartaric acid and grape polyphenoloxidase. J. Agric. Food Chem. 1991, 39, 1047–1049. [Google Scholar] [CrossRef]
- Cheynier, V.; Rigaud, J.; Souquet, J.M.; Barillère, J.M.; Moutounet, M. Effect of pomace contact and hyperoxidation on the phenolic composition and quality of Grenache and Chardonnay wines. Am. J. Enol. Vitic. 1989, 40, 36–42. [Google Scholar]
- Simpson, R.F. Factors affecting oxidative browning of white wine. Vitis 1982, 21, 233–239. [Google Scholar]
- Guyot, S.; Vercauteren, J.; Cheynier, V. Colourless and yellow dimers resulting from (+)-catechin oxidative coupling catalysed by grape polyphenoloxidase. Phytochemistry 1996, 42, 1279–1288. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Guerneve, C.L.; Fulcrand, H.; Cheynier, V.; Moutounet, M. New polyphenolic compounds with xanthylium skeletons formed through reaction between (+)-catechin and glyoxylic acid. J. Agric. Food Chem. 1999, 47, 5211–5217. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Cheynier, V.; Moutounet, M. Study of the reactions between (+)-catechin and furfural derivatives in the presence or absence of anthocyanins and their implication in food color change. J. Agric. Food Chem. 2000, 48, 5946–5954. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Meudec, M.; Eder, M.; Lamuela-Raventos, R.; Sommerer, N.; Cheynier, V. Targeted filtering reduces the complexity of routine UHPLC-HRMS data to decipher polyphenol polymerization. Food Chem. 2017, 227, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Ontañón, I.; Sánchez, D.; Sáez, V.; Mattivi, F.; Ferreira, V.; Arapitsas, P. Liquid Chromatography−Mass Spectrometry-Based Metabolomics for Understanding the Compositional Changes Induced by Oxidative or Anoxic Storage of Red Wines. J. Agric. Food Chem. 2020, 68, 13367–13379. [Google Scholar] [CrossRef]
- Glories, Y. La couleur des vins rouges. 1ere partie. Les équilibres des anthocyanes et des tanins. OENO One 1984, 18, 195–217. [Google Scholar] [CrossRef]
- Kennedy, J.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Pinasseau, L.; Verbaere, A.; Roques, M.; Meudec, E.; Vallverdu-Queralt, A.; Terrier, N.; Boulet, J.C.; Cheynier, V.; Sommerer, N. A fast and robust UHPLC-MRM-MS method to characterize and quantify grape skin tannins after chemical depolymerization. Molecules 2016, 21, 1409. [Google Scholar] [CrossRef]
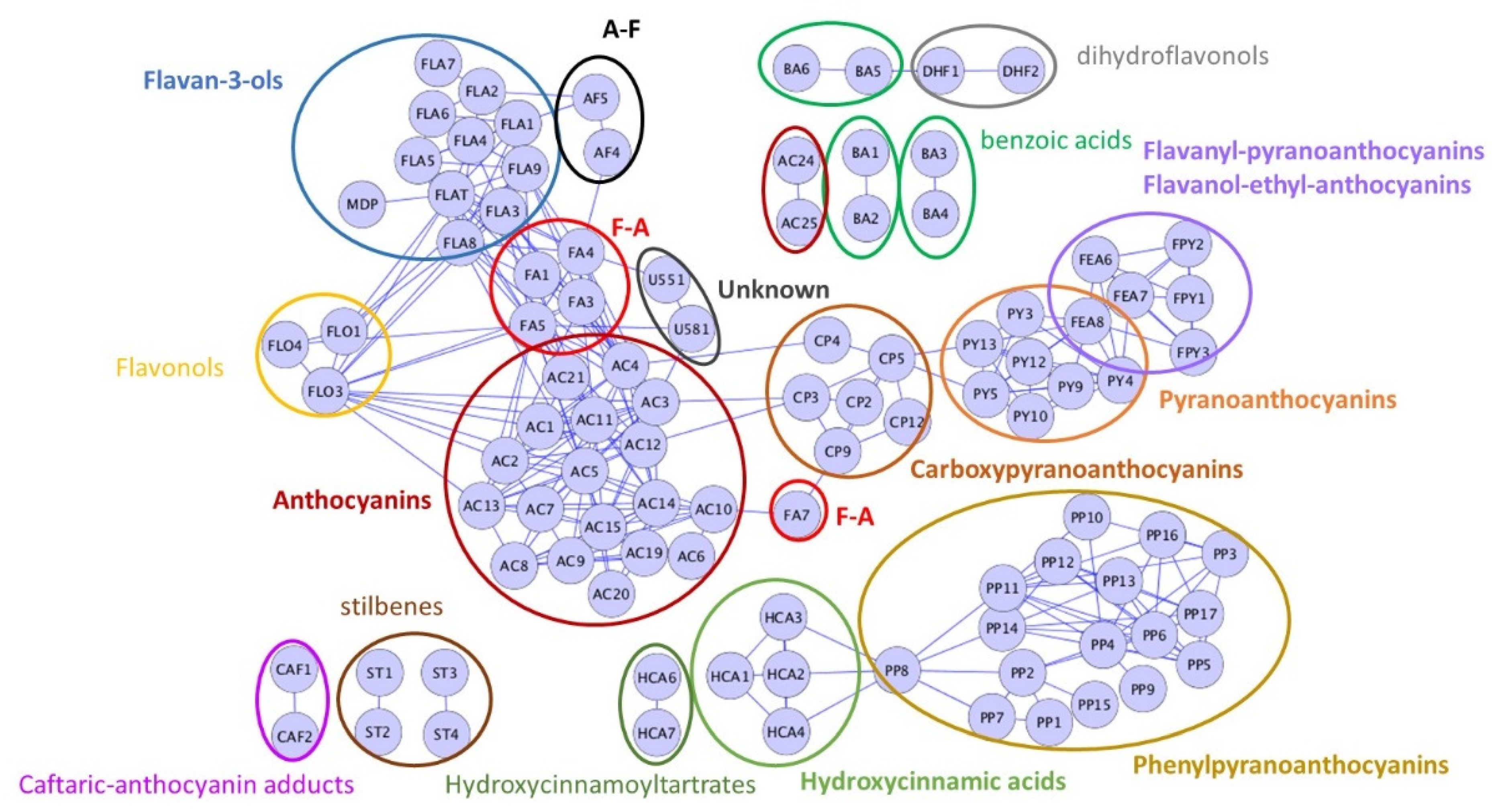
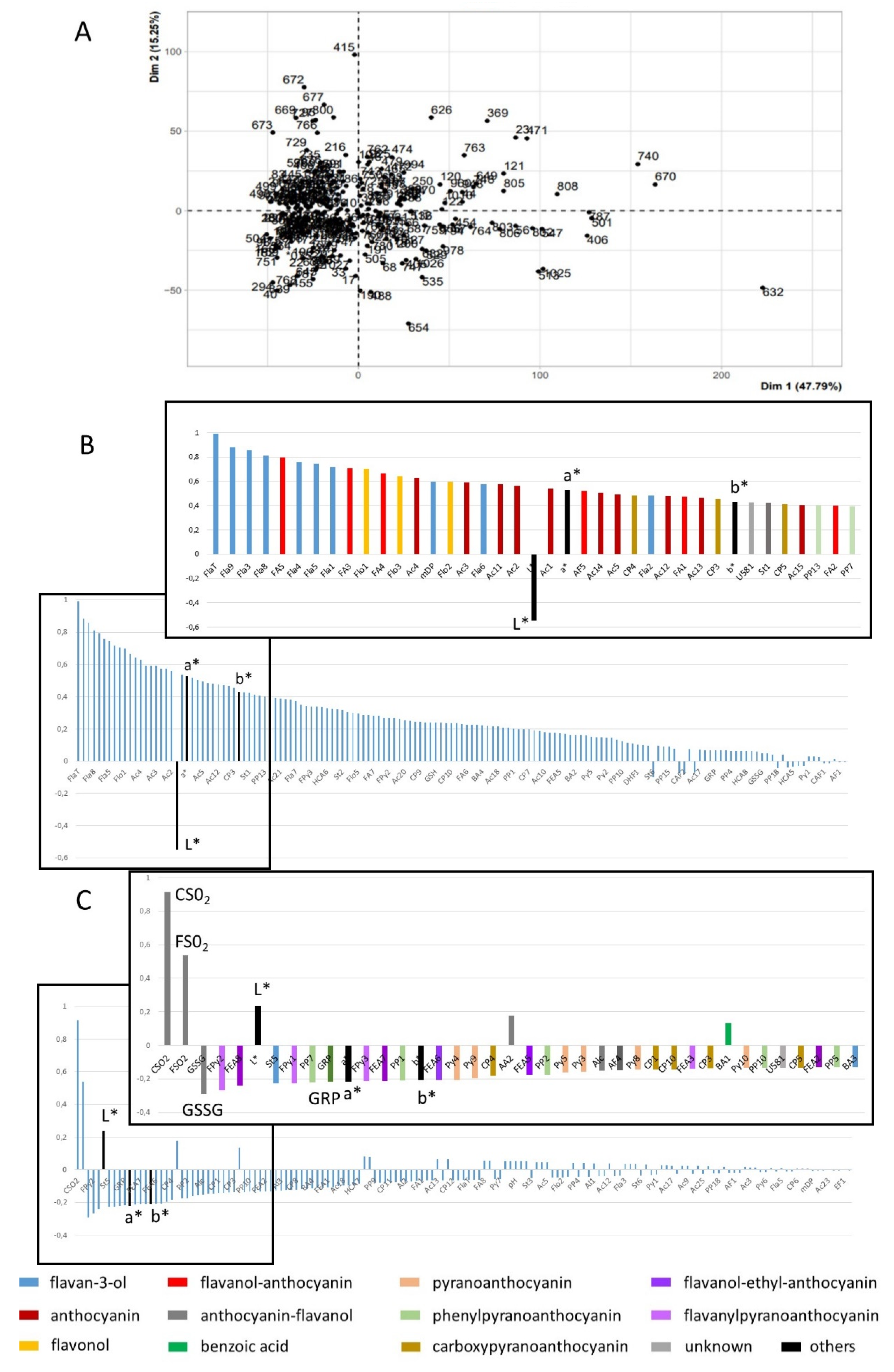
| Variable | Light | Intermediate | Dark |
|---|---|---|---|
| Phenolic Compounds (mg·L−1) | Mean ± SD (n = 114) | Mean ± SD (n = 101) | Mean ± SD (n = 53) |
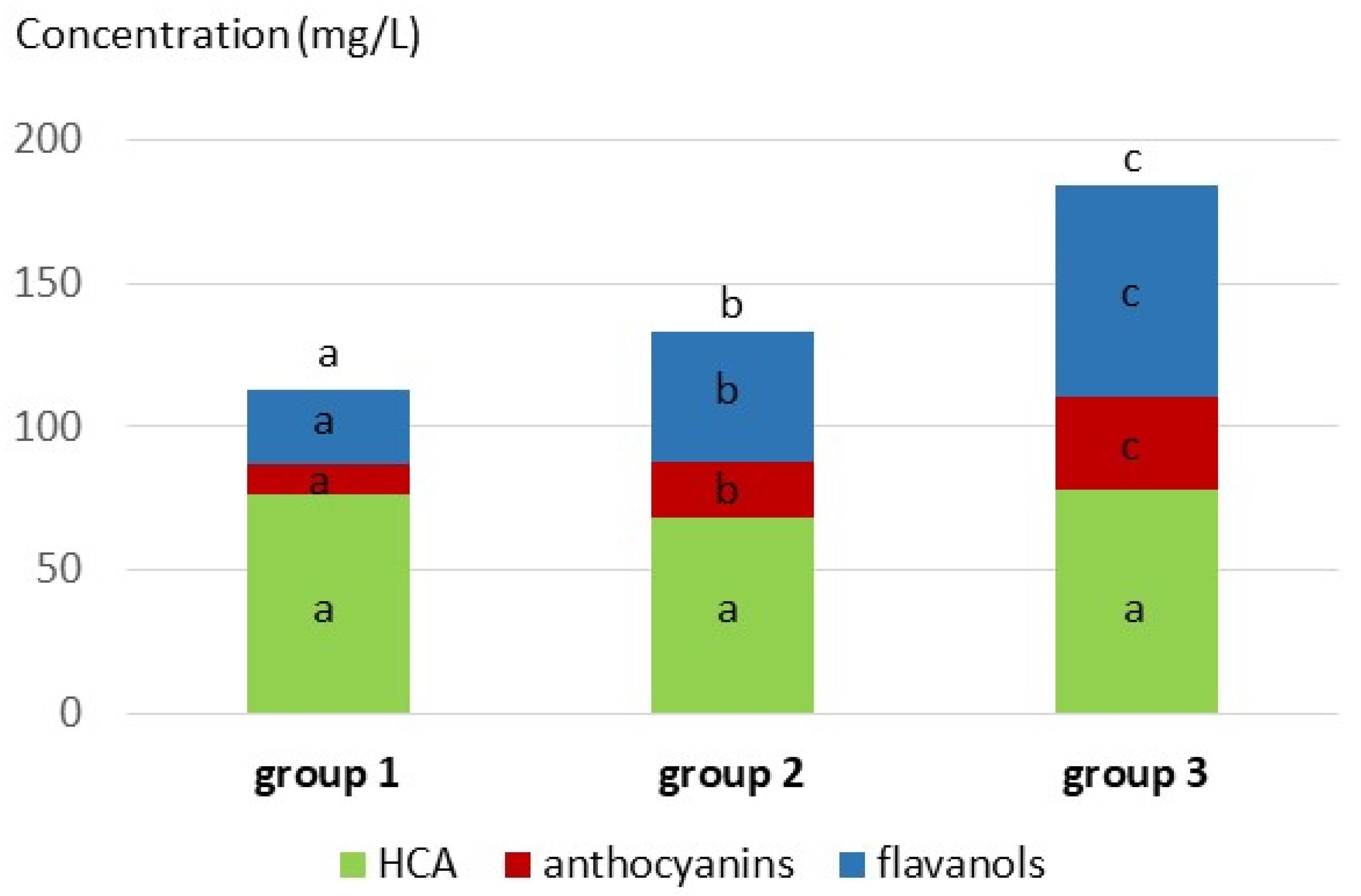
| Benzoic acids and ethyl esters (BA 1–6) | 6.2 ± 6.0 a | 9.9 ± 7.1 b | 13.2 ± 7.5 c |
| Hydroxycinnamic acids and ethyl esters (HCA 1–5) | 3.6 ± 2.8 a | 4.2 ± 5.5 a | 3.9 ± 3.6 a |
| Hydroxycinnamoyl tartaric acids (HCA 6–8) | 53.4 ± 27.2 a | 47.3 ± 21.0 a | 50.0 ± 26.3 a |
| 2-S-glutathionylcaffeoyltartaric acid (GRP) | 19.8 ± 12.5 a | 16.8 ± 10.7 a | 24.6 ± 18.6 b |
| Stilbenoids (St 1–6) | 3.2 ± 2.4 a | 4.6 ± 3.4 b | 5.9 ± 6.4 c |
| Flavonols (Flo 1–5) | 0.7 ± 1.5 a | 1.4 ± 1.7 b | 2.1 ± 1.6 c |
| Dihydroflavonols (DHF 1–2) | 0.9 ± 0.8 a | 1.6 ± 1.8 b | 0.9 ± 0.7 a |
| Flavanols monomers and oligomers (Fla 1–9) | 19.2 ± 19.6 a | 32.3 ± 26.7 b | 33.6 ± 22.7 b |
| Total flavanol units after phloroglucinolysis (FlaT) | 25.3 ± 27.6 a | 45.3 ± 31.8 b | 73.5 ± 45.5 c |
| Mean degree of polymerisation (mDP) | 2.4 ± 0.6 a | 2.7 ± 0.9 a | 3.5 ± 1.3 b |
| Anthocyanins (Ac 1–25) | 10.6 ± 6.2 a | 19.2 ± 12.7 b | 32.2 ± 19.1 c |
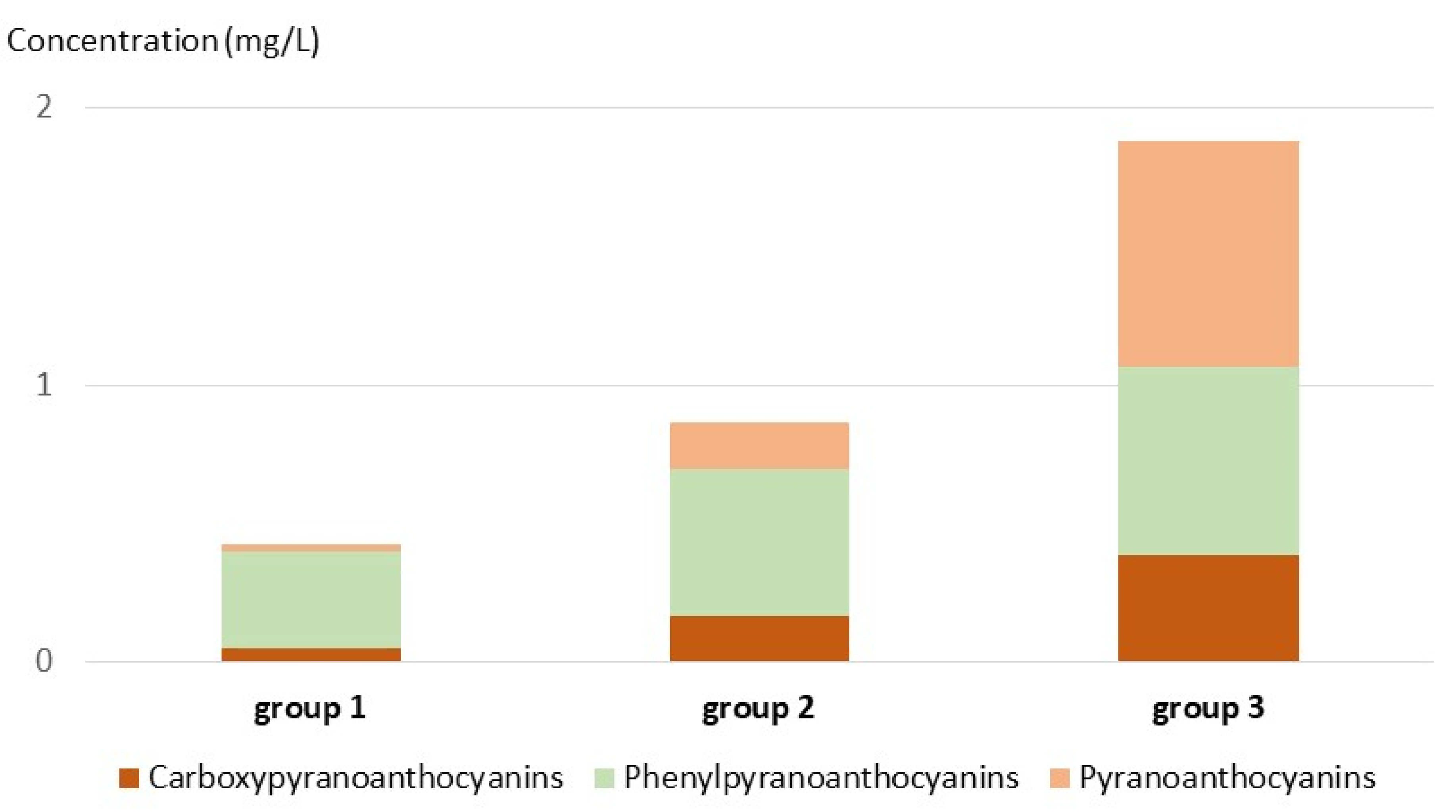
| Pyranoanthocyanins (Py 1–13) | 0.02 ± 0.05 a | 0.17 ± 0.30 b | 0.82 ± 0.95 c |
| Carboxypyranoanthocyanins (CP 1–12) | 0.05 ± 0.04 a | 0.17 ± 0.15 b | 0.39 ± 0.25 c |
| Flavanol-anthocyanin adducts (FA 1–8) | 0.05 ± 0.07 a | 0.13 ± 0.11 b | 0.30 ± 0.26 c |
| Anthocyanin-flavanol adducts (A-type) (AF 1–5) | 0.001 ± 0.005 a | 0.005 ± 0.011 b | 0.010 ± 0.015 c |
| Caftaric-anthocyanin adducts (CAF 1–2) | 0.02 ± 0.04 a | 0.03 ±0.04 a | 0.02 ±0.03 a |
| Ethyl-flavanol dimers (EF 1–2) | 0.001 ± 0.003 a | 0.006 ± 0.024 ab | 0.008 ± 0.011 b |
| Flavanol-ethyl-anthocyanins (FEA 1–8) | 0.003 ± 0.023 a | 0.064 ± 0.319 a | 0.205 ± 0.351 b |
| Phenylpyranoanthocyanins (PP 1–18) | 0.35 ± 0.41 a | 0.53 ± 0.48 b | 0.68 ± 0.59 c |
| Flavanyl-pyranoanthocyanins (FPy 1–3) | 0.002 ± 0.007 a | 0.010 ± 0.037 ab | 0.016 ± 0.044 b |
| Unknown 551 & 581 (U 551 & 581) | 0.016 ± 0.020 a | 0.038 ± 0.037 b | 0.092 ± 0.066 c |
| Alcohols (Al 1–3) | 13.6 ± 6.3 a | 11.5 ± 6.1 a | 15.8 ± 9.3 b |
| Amino acids (AA 1–2) | 7.9 ± 3.5 a | 8.6 ± 4.1 a | 6.4 ± 3.3 b |
| Glutathione (GSH) | 0.06 ± 0.05 a | 0.06 ± 0.07 a | 0.09 ± 0.09 b |
| Oxidized glutathione (GSSG) | 0.06 ± 0.13 a | 0.18 ± 0.55 a | 0.91 ± 3.21 b |
| Enological analysis | |||
| pH | 3.4 ± 0.1 a | 3.4 ± 0.2 a | 3.4 ± 0.2 a |
| Free sulfites content (FSO2) | 25 ± 20 a | 21 ± 13 a | 16 ± 9 b |
| Combined sulfites content (CSO2) | 75 ± 21 a | 78 ± 23 a | 74 ± 23 a |
| % alcohol (ALC) | 12.7 ± 0.9 a | 12.7 ± 1.2 a | 13.1 ± 0.8 b |
| Color analysis | |||
| L* | 93.2 ± 2.2 a | 84.6 ± 3.5 b | 70.6 ± 5.5 c |
| a* | 7.0 ± 3.2 a | 17.3 ± 5.71 b | 34.7 ± 7.6 c |
| b* | 10.30 ± 2.68 a | 15.62 ± 3.32 b | 20.0 ± 4.2 c |
| Color intensity (CI) | 0.33 ± 0.10 a | 0.69± 0.14 b | 1.36 ± 0.31 c |
| Hue (H) | 1.50 ± 0.47 a | 1.16 ± 0.26 b | 0.88 ± 0.16 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leborgne, C.; Lambert, M.; Ducasse, M.-A.; Meudec, E.; Verbaere, A.; Sommerer, N.; Boulet, J.-C.; Masson, G.; Mouret, J.-R.; Cheynier, V. Elucidating the Color of Rosé Wines Using Polyphenol-Targeted Metabolomics. Molecules 2022, 27, 1359. https://doi.org/10.3390/molecules27041359
Leborgne C, Lambert M, Ducasse M-A, Meudec E, Verbaere A, Sommerer N, Boulet J-C, Masson G, Mouret J-R, Cheynier V. Elucidating the Color of Rosé Wines Using Polyphenol-Targeted Metabolomics. Molecules. 2022; 27(4):1359. https://doi.org/10.3390/molecules27041359
Chicago/Turabian StyleLeborgne, Cécile, Marine Lambert, Marie-Agnès Ducasse, Emmanuelle Meudec, Arnaud Verbaere, Nicolas Sommerer, Jean-Claude Boulet, Gilles Masson, Jean-Roch Mouret, and Véronique Cheynier. 2022. "Elucidating the Color of Rosé Wines Using Polyphenol-Targeted Metabolomics" Molecules 27, no. 4: 1359. https://doi.org/10.3390/molecules27041359
APA StyleLeborgne, C., Lambert, M., Ducasse, M.-A., Meudec, E., Verbaere, A., Sommerer, N., Boulet, J.-C., Masson, G., Mouret, J.-R., & Cheynier, V. (2022). Elucidating the Color of Rosé Wines Using Polyphenol-Targeted Metabolomics. Molecules, 27(4), 1359. https://doi.org/10.3390/molecules27041359