Analysis of the Factors Affecting Static In Vitro Pepsinolysis of Food Proteins
Abstract
:1. Introduction
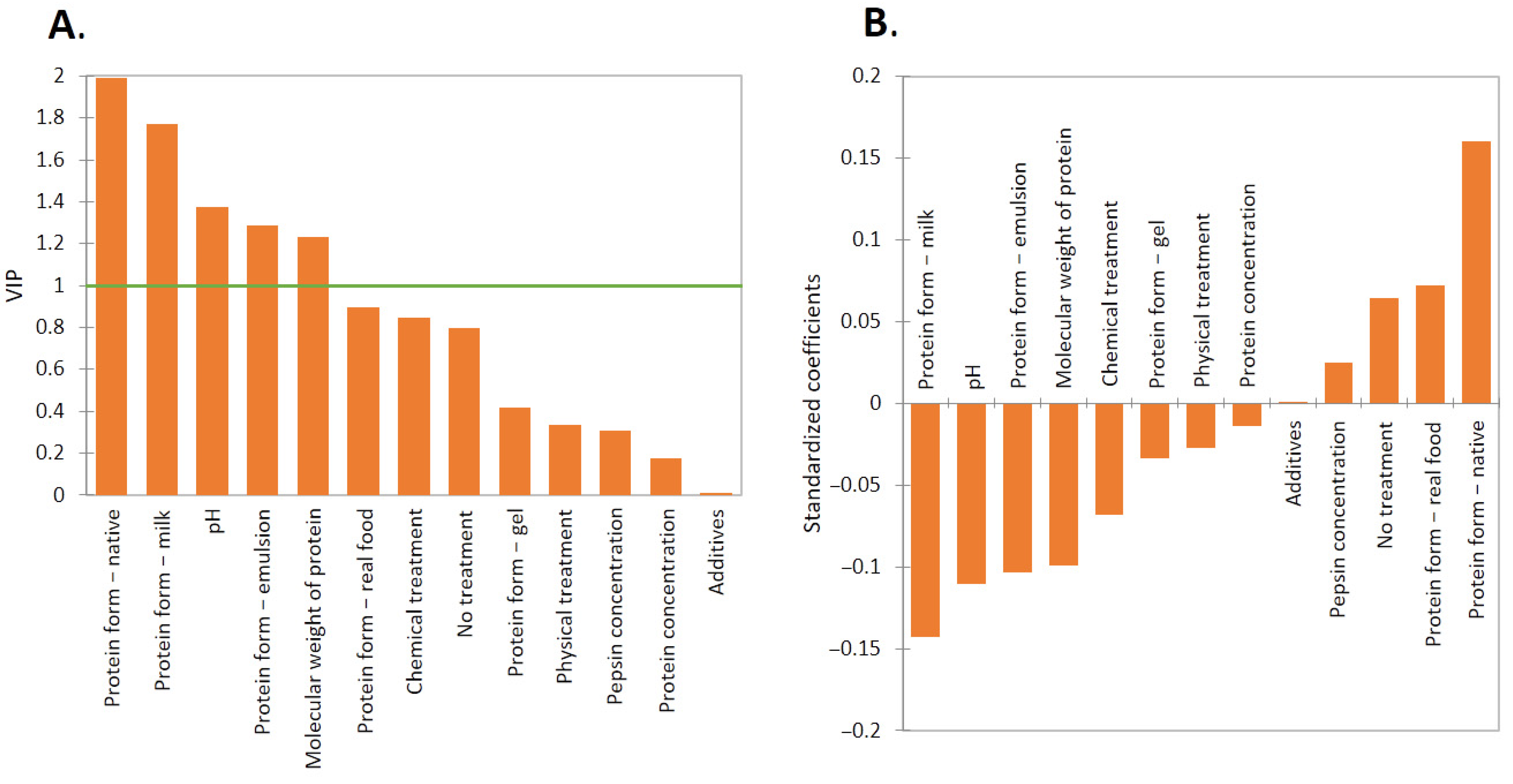
2. Results and Discussion
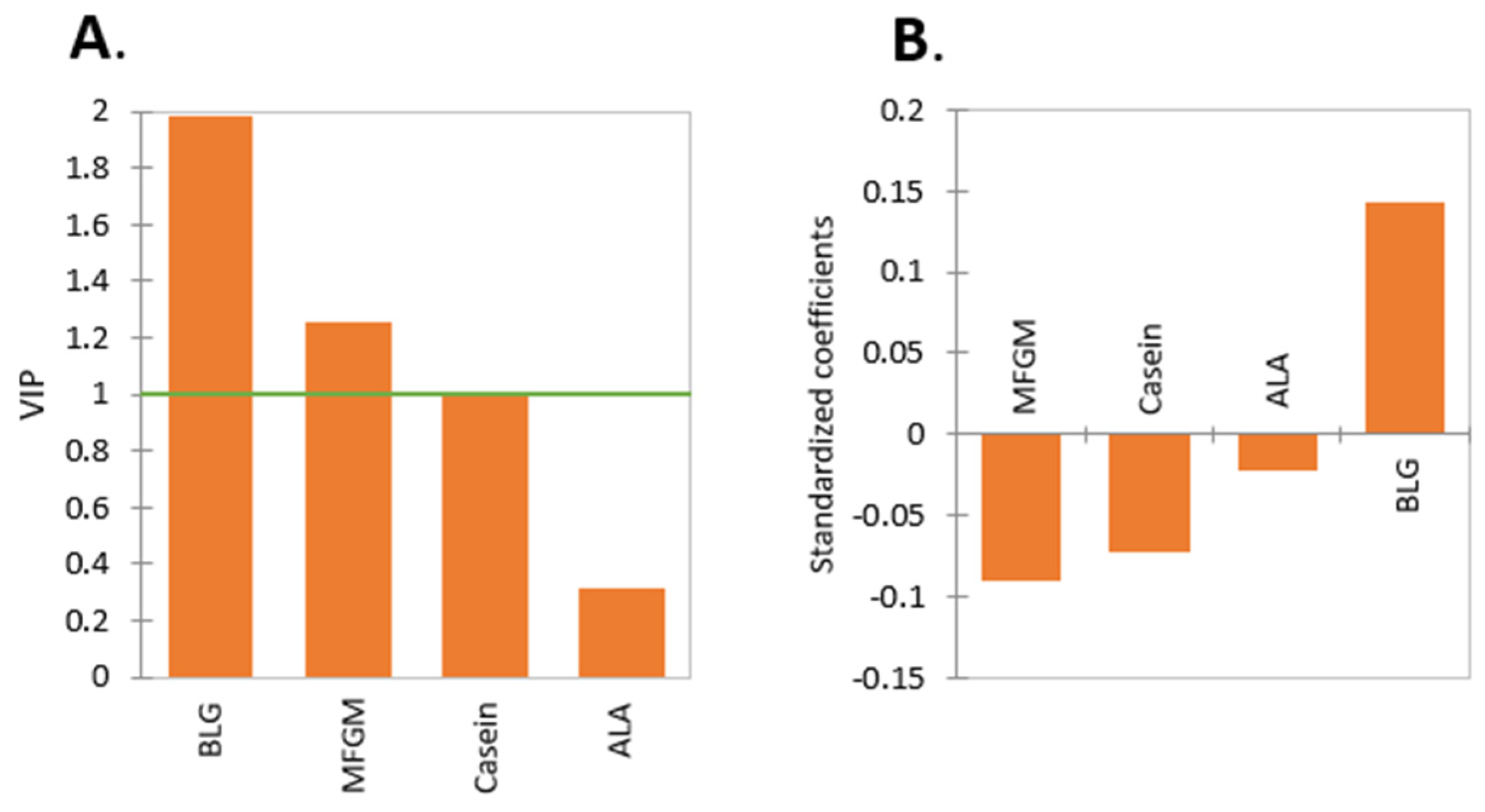
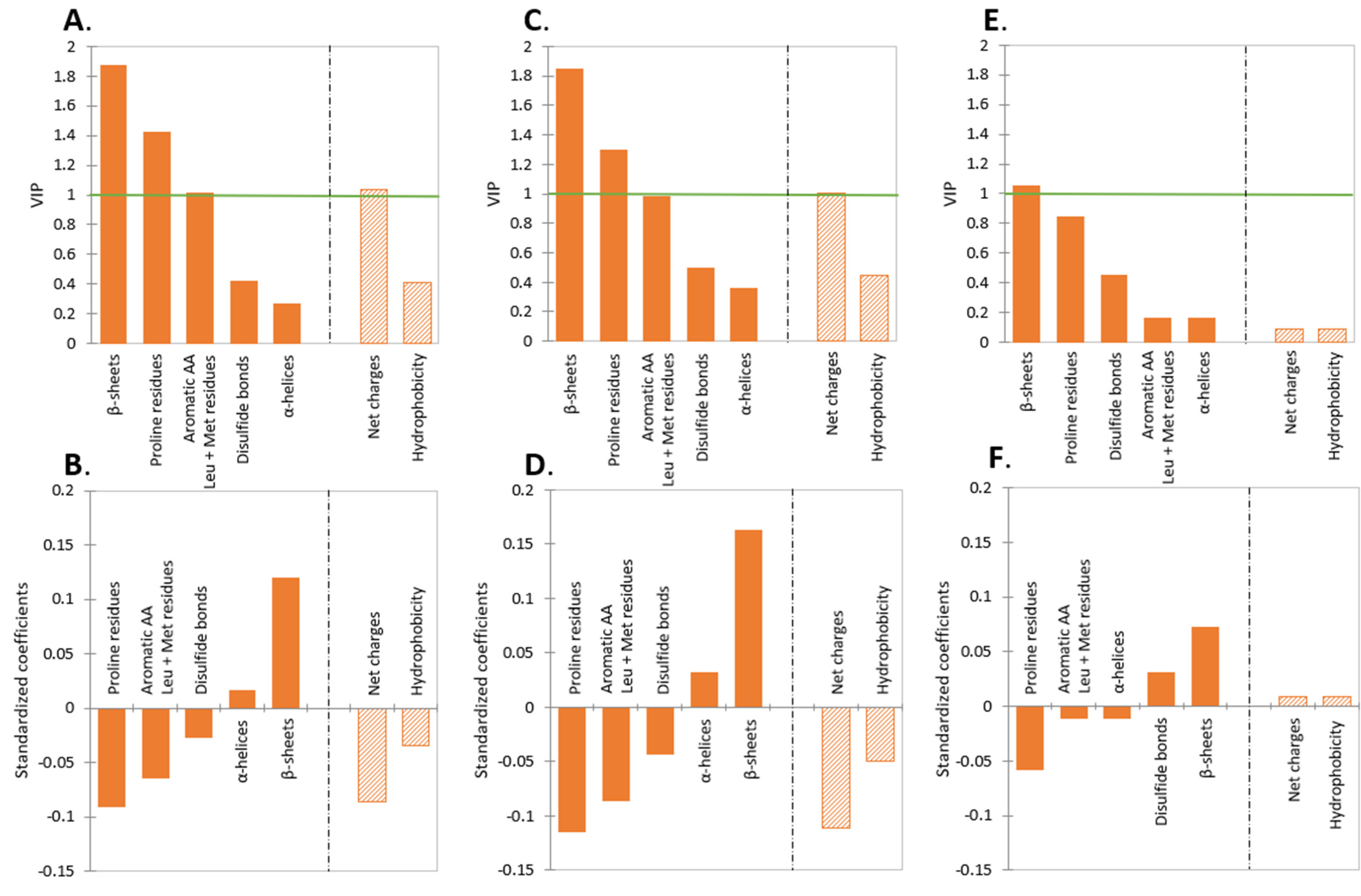
2.1. Effect of Protein Type
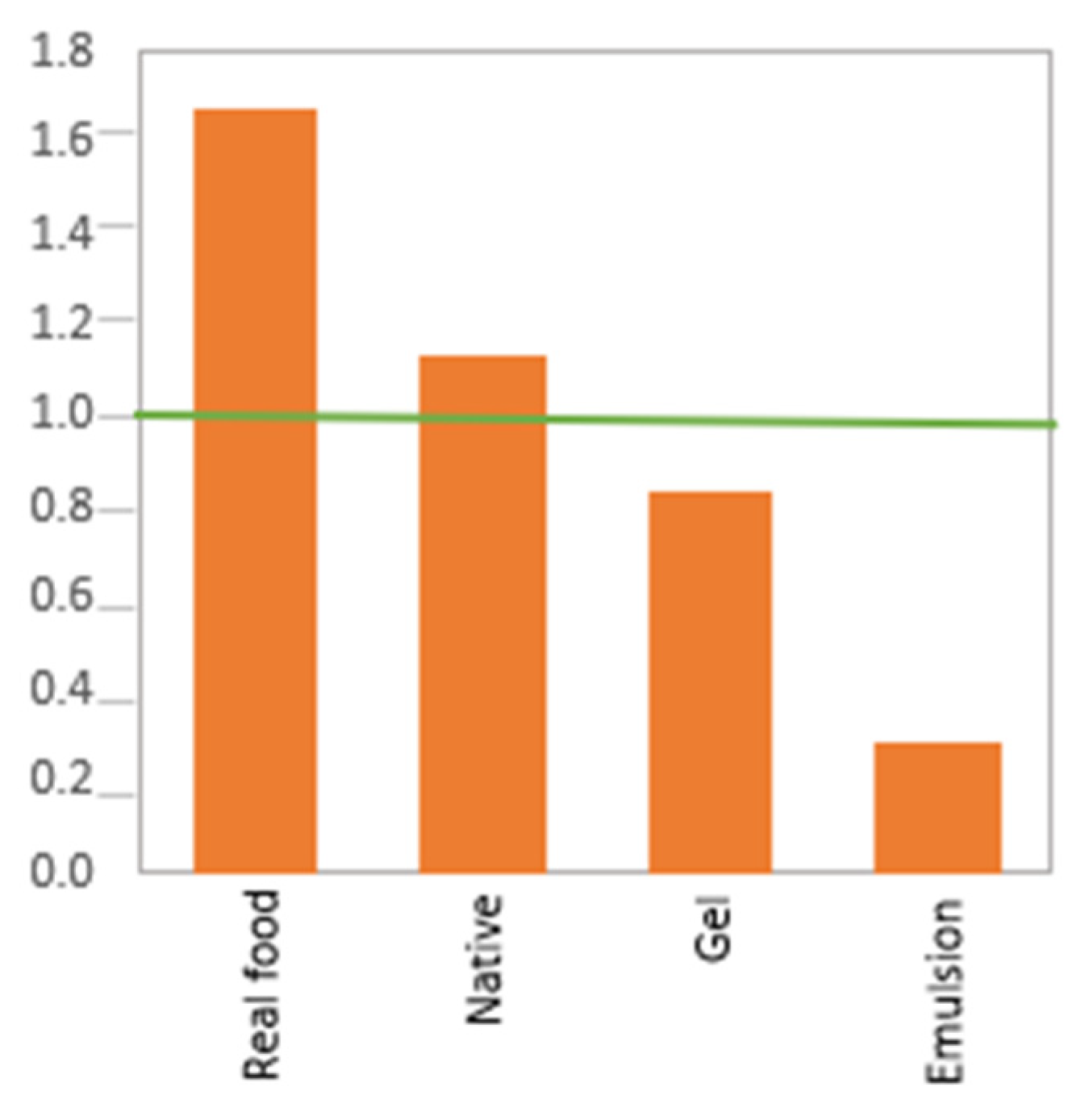
2.2. Effect of Protein Form
2.3. Effect of pH
2.4. Effect of Treatment
2.5. Importance of Additives
2.6. Effect of Molecular Weight, Pepsin, and Protein Concentration
2.7. Possible Improvement for Future Research—Irregular Pepsin Concentration, and Reported pH
3. Methods
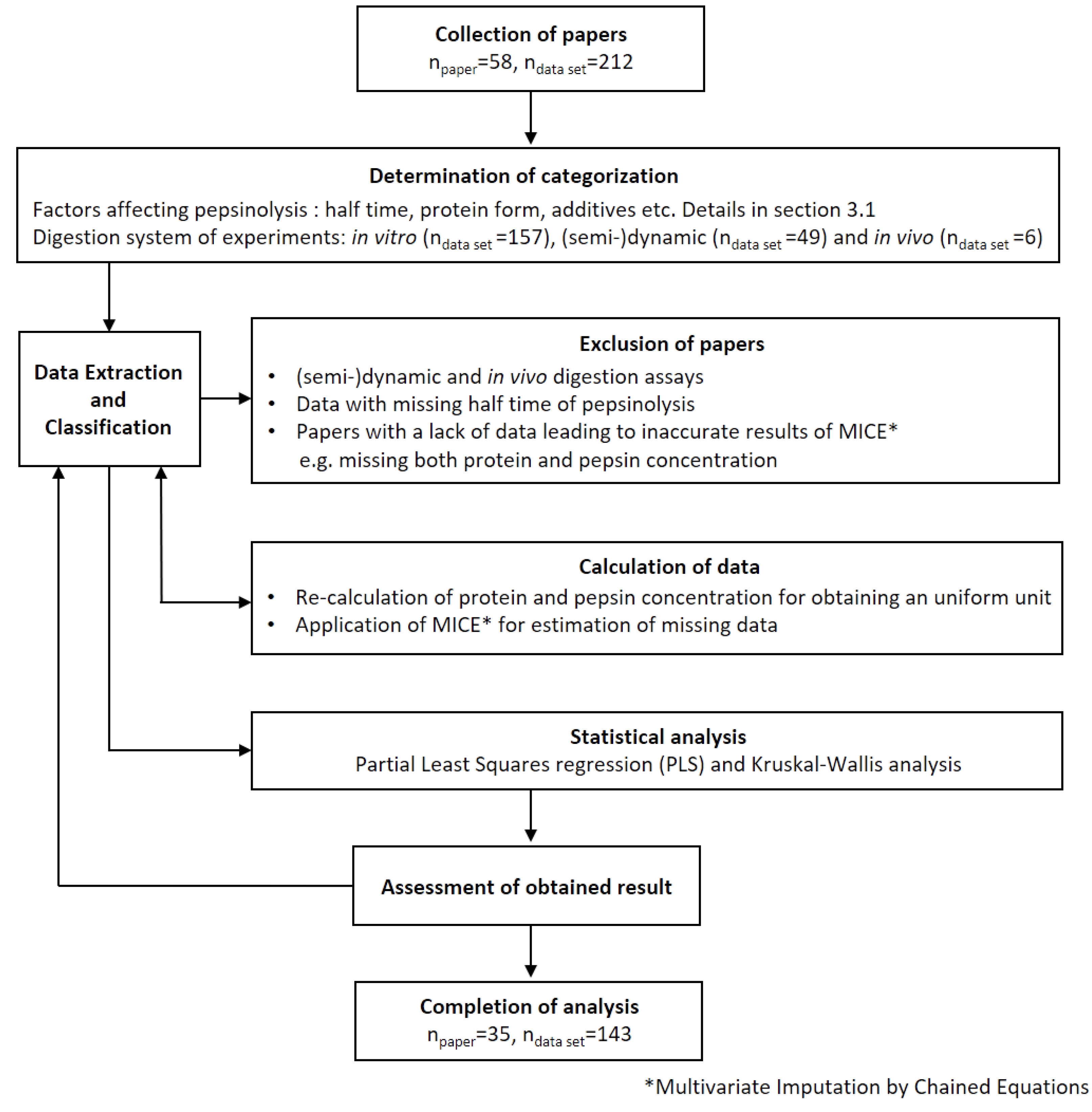
3.1. Data Collection and Extraction
3.2. Numerical Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pasiakos, S.M.; Lieberman, H.R.; Fulgoni, V.L. Higher-protein diets are associated with higher HDL cholesterol and lower BMI and waist circumference in US adults. J. Nutr. 2015, 145, 605–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.H.; Oikawa, S.Y.; Phillips, S.M. Dietary protein to maintain muscle mass in aging: A case for per-meal protein recommendations. J. Frailty Aging 2016, 5, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Loveday, S.M. Food proteins: Technological, nutritional, and sustainability attributes of traditional and emerging proteins. Annu. Rev. Food Sci. Technol. 2019, 10, 311–339. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Helal, A.; Verzelloni, E.; Conte, A. The type and concentration of milk increase the in vitro bioaccessibility of coffee chlorogenic acids. J. Agric. Food Chem. 2012, 60, 11056–11064. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Rioux, L.E.; Britten, M.; Turgeon, S.L. In vitro bioaccessibility of peptides and amino acids from yogurt made with starch, pectin, or β-glucan. Int. Dairy J. 2015, 46, 39–45. [Google Scholar] [CrossRef]
- Chen, M.; Li, B. The effect of molecular weights on the survivability of casein-derived antioxidant peptides after the simulated gastrointestinal digestion. Innov. Food Sci. Emerg. Technol. 2012, 16, 341–348. [Google Scholar] [CrossRef]
- Stojadinovic, M.; Radosavljevic, J.; Ognjenovic, J.; Vesic, J.; Prodic, I.; Stanic-Vucinic, D.; Cirkovic Velickovic, T. Binding affinity between dietary polyphenols and β-lactoglobulin negatively correlates with the protein susceptibility to digestion and total antioxidant activity of complexes formed. Food Chem. 2013, 136, 1263–1271. [Google Scholar] [CrossRef]
- Mandalari, G.; Mackie, A.M.; Rigby, N.M.; Wickham, M.S.J.; Mills, E.N.C. Physiological phosphatidylcholine protects bovine β-lactoglobulin from simulated gastrointestinal proteolysis. Mol. Nutr. Food Res. 2009, 53, S131–S139. [Google Scholar] [CrossRef]
- MacIerzanka, A.; Sancho, A.I.; Mills, E.N.C.; Rigby, N.M.; MacKie, A.R. Emulsification alters simulated gastrointestinal proteolysis of β-casein and β-lactoglobulin. Soft Matter 2009, 5, 538–550. [Google Scholar] [CrossRef]
- Sarkar, A.; Goh, K.K.T.; Singh, R.P.; Singh, H. Behaviour of an oil-in-water emulsion stabilized by β-lactoglobulin in an in vitro gastric model. Food Hydrocoll. 2009, 23, 1563–1569. [Google Scholar] [CrossRef]
- Böttger, F.; Dupont, D.; Marcinkowska, D.; Bajka, B.; Mackie, A.; Macierzanka, A. Which casein in sodium caseinate is most resistant to in vitro digestion? Effect of emulsification and enzymatic structuring. Food Hydrocoll. 2019, 88, 114–118. [Google Scholar] [CrossRef]
- Sams, L.; Amara, S.; Mansuelle, P.; Puppo, R.; Lebrun, R.; Paume, J.; Giallo, J.; Carrière, F. Characterization of pepsin from rabbit gastric extract, its action on β-casein and the effects of lipids on proteolysis. Food Funct. 2018, 9, 5975–5988. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, T.; Britten, M.; Schmitt, C. β-Lactoglobulin and WPI aggregates: Formation, structure and applications. Food Hydrocoll. 2011, 25, 1945–1962. [Google Scholar] [CrossRef]
- Van Vliet, T.; Lakemond, C.M.M.; Visschers, R.W. Rheology and structure of milk protein gels. Curr. Opin. Colloid Interface Sci. 2004, 9, 298–304. [Google Scholar] [CrossRef]
- MacIerzanka, A.; Böttger, F.; Lansonneur, L.; Groizard, R.; Jean, A.S.; Rigby, N.M.; Cross, K.; Wellner, N.; MacKie, A.R. The effect of gel structure on the kinetics of simulated gastrointestinal digestion of bovine β-lactoglobulin. Food Chem. 2012, 134, 2156–2163. [Google Scholar] [CrossRef]
- McSweeney, P.L.H.; Fox, P.F. Advanced Dairy Chemistry: Volume 1A: Proteins: Basic Aspects, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 1A, pp. 1–548. [Google Scholar] [CrossRef]
- Guetouache, M.; Guessas, B.; Medjekal, S. Composition and nutritional value of raw milk. Issues Biol. Sci. Pharm. Res. 2014, 2, 115–122. [Google Scholar]
- Gallier, S.; Vocking, K.; Post, J.A.; Van De Heijning, B.; Acton, D.; Van Der Beek, E.M.; Van Baalen, T. A novel infant milk formula concept: Mimicking the human milk fat globule structure. Colloids Surf. B Biointerfaces 2015, 136, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Record, T.E. Archaebacteria these unusual bacteria are genealogically neither prokaryotes nor eukaryotes. This discovery means there are not two lines of descent but three: The archaebacteria, the true bacteria and the eukaryotes. Sci. Am. 1981, 244, 98–122. [Google Scholar]
- Sobhaninia, M.; Nasirpour, A.; Shahedi, M.; Golkar, A. Oil-in-water emulsions stabilized by whey protein aggregates: Effect of aggregate size, pH of aggregation and emulsion pH. J. Dispers. Sci. Technol. 2017, 38, 1366–1373. [Google Scholar] [CrossRef]
- Fox, P.F. Chapter 1 Milk: An Overview. In Milk Proteins; Academic Press: San Diego, CA, USA, 2008; ISBN 9780123740397. [Google Scholar]
- Horne, D.S. Casein micelle structure: Models and muddles. Curr. Opin. Colloid Interface Sci. 2006, 11, 148–153. [Google Scholar] [CrossRef]
- Singh, H.; Gallier, S. Nature’s complex emulsion: The fat globules of milk. Food Hydrocoll. 2017, 68, 81–89. [Google Scholar] [CrossRef]
- Todeschini, R.; Consonni, V.; Gramatica, P. Chemometrics in QSAR. Compr. Chemom. 2009, 4, 129–172. [Google Scholar] [CrossRef]
- Belloque, J.; Chicón, R.; López-Fandiño, R. Unfolding and refolding of β-lactoglobulin subjected to high hydrostatic pressure at different pH values and temperatures and its influence on proteolysis. J. Agric. Food Chem. 2007, 55, 5282–5288. [Google Scholar] [CrossRef] [PubMed]
- Mohan Reddy, I.; Kella, N.K.D.; Kinsella, J.E. Structural and conformational basis of the resistance of β-lactoglobulin to peptic and chymotryptic digestion. J. Agric. Food Chem. 1988, 36, 737–741. [Google Scholar] [CrossRef]
- Mouécoucou, J.; Villaume, C.; Sanchez, C.; Méjean, L. β-Lactoglobulin/polysaccharide interactions during in vitro gastric and pancreatic hydrolysis assessed in dialysis bags of different molecular weight cut-offs. Biochim. Biophys. Acta Gen. Subj. 2004, 1670, 105–112. [Google Scholar] [CrossRef]
- Hamuro, Y.; Coales, S.J.; Molnar, K.S.; Tuske, S.J.; Morrow, J.A. Specificity of immobilized porcine pepsin in H/D exchange compatible conditions. Rapid Commun. Mass Spectrom. 2008, 22, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Perticaroli, S.; Nickels, J.D.; Ehlers, G.; Sokolov, A.P. Rigidity, secondary structure, and the universality of the boson peak in proteins. Biophys. J. 2014, 106, 2667–2674. [Google Scholar] [CrossRef] [Green Version]
- Imai, K.; Mitaku, S. Mechanisms of secondary structure breakers in soluble proteins. Biophysics 2005, 1, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Baglieri, A.; Mahe, S.; Benamouzig, R.; Savoie, L.; Tome, D. Digestion patterns of endogenous and different exogenous proteins affect the composition of intestinal effluents in humans. J. Nutr. 1995, 125, 1894–1903. [Google Scholar] [CrossRef]
- Piper, D.W.; Fenton, B.H. pH stability and activity curves of pepsin with special reference to their clinical importance. Gut 1965, 6, 506–508. [Google Scholar] [CrossRef] [Green Version]
- Morgan, A.A.; Rubenstein, E. Proline: The distribution, frequency, positioning, and common functional roles of proline and polyproline sequences in the human proteome. PLoS ONE 2013, 8, e53785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.C.; Chen, W.L.; Mao, S.J.T. Antioxidant nature of bovine milk β-lactoglobulin. J. Dairy Sci. 2007, 90, 547–555. [Google Scholar] [CrossRef]
- Farrell, H.M.; Qi, P.X.; Brown, E.M.; Cooke, P.H.; Tunick, M.H.; Wickham, E.D.; Unruh, J.J. Molten globule structures in milk proteins: Implications for potential new structure-function relationships. J. Dairy Sci. 2002, 85, 459–471. [Google Scholar] [CrossRef]
- Lucia, G.; Melissa, Y.; Eugenio, C.; Yovanna, C. Relationship between kappa casein genes (CSN3) and industrial yield in holstein cows in Nariño-Colombia. In Milk Protein; Hurley, W.L., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Creamer, L.K.; Plowman, J.E.; Liddell, M.J.; Smith, M.H.; Hill, J.P. Micelle stability: κ-casein structure and function. J. Dairy Sci. 1998, 81, 3004–3012. [Google Scholar] [CrossRef]
- Hoagland, P.D.; Unruh, J.J.; Wickham, E.D.; Farrell, H.M. Secondary structure of bovine αS2-casein: Theoretical and experimental approaches. J. Dairy Sci. 2001, 84, 1944–1949. [Google Scholar] [CrossRef]
- Creamer, L.K.; Parry, D.A.D.; Malcolm, G.N. Secondary structure of bovine β-lactoglobulin B. Arch. Biochem. Biophys. 1983, 227, 98–105. [Google Scholar] [CrossRef]
- Spöttel, J.; Brockelt, J.; Falke, S. Benzyl isothiocyanate—Effects on molecular structure and proteolytic stability. Molecules 2021, 26, 6247. [Google Scholar] [CrossRef] [PubMed]
- Michael Byler, D.; Farrell, H.M.; Susi, H. Raman spectroscopic study of casein structure. J. Dairy Sci. 1988, 71, 2622–2629. [Google Scholar] [CrossRef]
- Singh, H. Interactions of milk proteins during the manufacture of milk powders. Dairy Sci. Technol. 2007, 87, 413–423. [Google Scholar] [CrossRef]
- UniProt The UniProt Consortium. UniProt: The Universal Protein Knowledgebase. Nucleic Acids Res. 2021, 46, 2699. Available online: https://www.uniprot.org/. (accessed on 1 October 2021).
- Osorio, D.; Rondón-Villarreal, P.; Torres, R. Peptides: A package for data mining of antimicrobial peptides. R. J. 2015, 7, 4–14. [Google Scholar] [CrossRef]
- Potočnik, K.; Gantner, V.; Kuterovac, K.; Cividini, A. Mare’s milk: Composition and protein fraction in comparison with different milk species. Mljekarstvo Čas. Unaprjeđenje Proizv. Prerade Mlijeka 2011, 61, 107–113. [Google Scholar]
- Ye, A.; Cui, J.; Singh, H. Proteolysis of milk fat globule membrane proteins during in vitro gastric digestion of milk. J. Dairy Sci. 2011, 94, 2762–2770. [Google Scholar] [CrossRef] [PubMed]
- Inglingstad, R.A.; Devold, T.G.; Eriksen, E.K.; Holm, H.; Jacobsen, M.; Liland, K.H.; Rukke, E.O.; Vegarud, G.E. Comparison of the digestion of caseins and whey proteins in equine, bovine, caprine and human milks by human gastrointestinal enzymes. Dairy Sci. Technol. 2010, 90, 549–563. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Xu, W.; Cui, J.; Ma, Y.; Zhou, S. Goat and buffalo milk fat globule membranes exhibit better effects at inducing apoptosis and reduction the viability of HT-29 cells. Sci. Rep. 2019, 9, 2577. [Google Scholar] [CrossRef] [Green Version]
- Zhai, J.L.; Day, L.; Aguilar, M.I.; Wooster, T.J. Protein folding at emulsion oil/water interfaces. Curr. Opin. Colloid Interface Sci. 2013, 18, 257–271. [Google Scholar] [CrossRef]
- Taulier, N.; Chalikian, T.V. Characterization of pH-induced transitions of β-lactoglobulin: Ultrasonic, densimetric, and spectroscopic studies. J. Mol. Biol. 2001, 314, 873–889. [Google Scholar] [CrossRef] [Green Version]
- Halabi, A.; Croguennec, T.; Bouhallab, S.; Dupont, D.; Deglaire, A. Modification of protein structures by altering the whey protein profile and heat treatment affects: In vitro static digestion of model infant milk formulas. Food Funct. 2020, 11, 6933–6945. [Google Scholar] [CrossRef]
- Peram, M.R.; Loveday, S.M.; Ye, A.; Singh, H. In vitro gastric digestion of heat-induced aggregates of β-lactoglobulin. J. Dairy Sci. 2013, 96, 63–74. [Google Scholar] [CrossRef] [Green Version]
- De Wit, J.N.; Swinkels, G.A.M. A differential scanning calorimetric study of the denaturation of bovine β-lactoglobulin. Biochim. Biophys. Acta 1980, 624, 40–50. [Google Scholar] [CrossRef]
- Bax, M.L.; Aubry, L.; Ferreira, C.; Daudin, J.D.; Gatellier, P.; Rémond, D.; Santé-Lhoutellier, V. Cooking temperature is a key determinant of in vitro meat protein digestion rate: Investigation of underlying mechanisms. J. Agric. Food Chem. 2012, 60, 2569–2576. [Google Scholar] [CrossRef]
- Manjunath, S.; Satish Rao, B.S.; Satyamoorthy, K.; Mahato, K.K. Laser induced autofluorescence in the monitoring of β-mercaptoethanol mediated photo induced proton coupled electron transfer in proteins. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Almarza, J.; Rincon, L.; Bahsas, A.; Brito, F. Molecular mechanism for the denaturation of proteins by urea. Biochemistry 2009, 48, 7608–7613. [Google Scholar] [CrossRef] [PubMed]
- Lalithapriya, U.; Mariajenita, P.; Renuka, V.; Sudharsan, K.; Karthikeyan, S.; Sivarajan, M.; Murugan, D.; Sukumar, M. Investigation of natural extracts and sodium bisulfite impact on thermal signals and physicochemical compositions of Litopenaeus vannamei during chilled storage. J. Aquat. Food Prod. Technol. 2019, 28, 609–623. [Google Scholar] [CrossRef]
- Rom, D.L.; Shull, J.M.; Chandrashekar, A.; Kirleis, W. Effects of cooking and treatment with sodium bisulfite on in vitro protein digestibility and microstructure of sorghum flour. Cereal Chem. 1992, 69, 178–181. [Google Scholar]
- Samanta, U.; Bahadur, R.P.; Chakrabarti, P. Quantifying the accessible surface area of protein residues in their local environment. Protein Eng. 2002, 15, 659–667. [Google Scholar] [CrossRef] [Green Version]
- Dee, K.C.; Puleo, D.A.; Bizios, R. An Introduction to Tissue-Biomaterial Interactions; Wiley-Liss Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Egger, L.; Ménard, O.; Delgado-Andrade, C.; Alvito, P.; Assunção, R.; Balance, S.; Barberá, R.; Brodkorb, A.; Cattenoz, T.; Clemente, A.; et al. The harmonized INFOGEST in vitro digestion method: From knowledge to action. Food Res. Int. 2016, 88, 217–225. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A istandardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Lasda Bergman, E.M. Finding citations to social work literature: The relative benefits of using web of science, scopus, or google scholar. J. Acad. Libr. 2012, 38, 370–379. [Google Scholar] [CrossRef] [Green Version]
- Falagas, M.E.; Pitsouni, E.I.; Malietzis, G.A.; Pappas, G. Comparison of PubMed, scopus, web of science, and google scholar: Strengths and weaknesses. FASEB J. 2008, 22, 338–342. [Google Scholar] [CrossRef]
- Bramer, W.M.; Rethlefsen, M.L.; Kleijnen, J.; Franco, O.H. Optimal database combinations for literature searches in systematic reviews: A prospective exploratory study. Syst. Rev. 2017, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing Version 4.1.0; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Łozińska, N.; Głowacz-Różyńska, A.; Artichowicz, W.; Lu, Y.; Jungnickel, C. Microencapsulation of fish oil—Determination of optimal wall material and encapsulation methodology. J. Food Eng. 2020, 268, 109730. [Google Scholar] [CrossRef]
- Tobias, R.D. An Introduction to partial least squares regression. In Proceedings of the Twentieth Annual SAS Users Group International Conference, Orlando, FL, USA, 2–5 April 1995; SAS Institute: Cary, NC, USA, 1995; pp. 1250–1257. [Google Scholar]
- Addinsoft. Addinsoft XLSTAT Statistical and Data Analysis Solution Version 2021.4.1 (1201); Addinsoft: Paris, France, 2021. [Google Scholar]
- Vinzi, V.E.; Trinchera, L.; Amato, S. Handbook of Partial Least Squares; Springer: Berlin, Germany, 2010; ISBN 9783540328278. [Google Scholar]
- Farrés, M.; Platikanov, S.; Tsakovski, S.; Tauler, R. Comparison of the variable importance in projection (VIP) and of the selectivity ratio (SR) methods for variable selection and interpretation. J. Chemom. 2015, 29, 528–536. [Google Scholar] [CrossRef]
- Sakai, K.; Yoshino, K.; Satter, M.A.; Ota, F.; Nii, Y.; Fukuta, K.; Ueda, N.; Shimizu5, Y.; Yamamoto1, S. Note Effects of pH Variation and NaCl on In Vitro Digestibility of Cow’s Milk Proteins in Commercially Available Infant Formulas. J. Nutr. Sci. Vitaminol. 2000, 46, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Bonfatti, V.; Di Martino, G.; Carnier, P. Effectiveness of mid-infrared spectroscopy for the prediction of detailed protein composition and contents of protein genetic variants of individual milk of Simmental cows. J. Dairy Sci. 2011, 94, 5776–5785. [Google Scholar] [CrossRef]
- Gellrich, K.; Meyer, H.H.D.; Wiedemann, S. Composition of major proteins in cow milk differing in mean protein concentration during the first 155 days of lactation and the influence of season as well as shortterm restricted feeding in early and mid-lactation. Czech J. Anim. Sci. 2014, 59, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Schopen, G.C.B.; Visker, M.H.P.W.; Koks, P.D.; Mullaart, E.; van Arendonk, J.A.M.; Bovenhuis, H. Whole-genome association study for milk protein composition in dairy cattle. J. Dairy Sci. 2011, 94, 3148–3158. [Google Scholar] [CrossRef] [Green Version]
- Lazar, I., Jr.; Lazar, I., Sr. GelAnalyzer 19.1 2010. Available online: www.gelanalyzer.com (accessed on 10 January 2022).
- Luo, C.; Guo, Y.; Li, Z.; Ahmed, I.; Pramod, S.N.; Gao, X.; Lv, L.; Lin, H. Lipid emulsion enhances fish allergen parvalbumin’s resistance to in vitro digestion and IgG/IgE binding capacity. Food Chem. 2020, 302, 125333. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Valderrama, J.; Wilde, P.J.; Mulholland, F.; Morris, V.J. Protein unfolding at fluid interfaces and its effect on proteolysis in the stomach. Soft Matter 2012, 8, 4402–4414. [Google Scholar] [CrossRef]
- Sarkar, A.; Zhang, S.; Murray, B.; Russell, J.A.; Boxal, S. Modulating in vitro gastric digestion of emulsions using composite whey protein-cellulose nanocrystal interfaces. Colloids Surf. B 2017, 158, 137–146. [Google Scholar] [CrossRef]
- Parris, N.; Cooke, P.H.; Hicks, K.B. Encapsulation of essential oils in zein nanospherical particles. J. Agric. Food Chem. 2005, 53, 4788–4792. [Google Scholar] [CrossRef]
- Sarkar, A.; Goh, K.K.; Singh, H. Properties of oil-in-water emulsions stabilized by β-lactoglobulin in simulated gastric fluid as influenced by ionic strength and presence of mucin. Food Hydrocolloid 2010, 24, 534–541. [Google Scholar] [CrossRef]
- Taylor, J.; Taylor, J.R.; Belton, P.S.; Minnaar, A. Kafirin microparticle encapsulation of catechin and sorghum condensed tannins. J. Agric. Food Chem. 2009, 57, 7523–7528. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Sathe, S.K. Phaseolin in vitro pepsin digestibility: Role of acids and phenolic compounds. J. Agric. Food Chem. 2003, 51, 3466–3472. [Google Scholar] [CrossRef]
- Nyemb, K.; Guérin-Dubiard, C.; Dupont, D.; Jardin, J.; Rutherfurd, S.M.; Nau, F. The extent of ovalbumin in vitro digestion and the nature of generated peptides are modulated by the morphology of protein aggregates. Food Chem. 2014, 157, 429–438. [Google Scholar] [CrossRef]
- Tantoush, Z.; Apostolovic, D.; Kravic, B.; Prodic, I.; Mihajlovic, L.; Stanic-Vucinic, D.; Velickovic, T.C. Green tea catechins of food supplements facilitate pepsin digestion of major food allergens. but hampers their digestion if oxidized by phenol oxidase. J. Funct. Foods 2012, 4, 650–660. [Google Scholar] [CrossRef]
- Sarkar, A.; Ademuyiwa, V.; Stubley, S.; Esa, N.H.; Goycoolea, F.M.; Qin, X.; Gonzalez, F.; Olvera, C. Pickering emulsions co-stabilized by composite protein/polysaccharide particle-particle interfaces: Impact on in vitro gastric stability. Food Hydrocolloid 2018, 84, 282–291. [Google Scholar] [CrossRef]
- Araiza-Calahorra, A.; Sarkar, A. Designing biopolymer-coated Pickering emulsions to modulate in vitro gastric digestion: A static model study. Food Funct. 2019, 10, 5498–5509. [Google Scholar] [CrossRef] [PubMed]
- Floury, J.; Bianchi, T.; Thévenot, J.; Dupont, D.; Jamme, F.; Lutton, E.; Panouille, M.; Boue, F.; Le Feunteun, S. Exploring the breakdown of dairy protein gels during in vitro gastric digestion using time-lapse synchrotron deep-UV fluorescence microscopy. Food Chem. 2018, 239, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Yim, S.G.; Choi, Y.; Ha, T.V.A.; Ko, S. Physiochemical properties and prolonged release behaviours of chitosan-denatured β-lactoglobulin microcapsules for potential food applications. Food Chem. 2012, 134, 992–998. [Google Scholar] [CrossRef]
- Dupont, D.; Mandalari, G.; Molle, D.; Jardin, J.; Léonil, J.; Faulks, R.M.; Wickham, M.S.J.; Mills, E.N.C.; Mackie, A.R. Comparative resistance of food proteins to adult and infant in vitro digestion models. Mol. Nutr. Food Res. 2010, 54, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Ofori-Anti, A.O.; Ariyarathna, H.; Chen, L.; Lee, H.L.; Pramod, S.N.; Goodman, R.E. Establishing objective detection limits for the pepsin digestion assay used in the assessment of genetically modified foods. Regul. Toxicol. Pharmacol. 2008, 52, 94–103. [Google Scholar] [CrossRef]
- Takagi, K.; Teshima, R.; Okunuki, H.; Sawada, J.I. Comparative study of in vitro digestibility of food proteins and effect of preheating on the digestion. Biol. Pharm. Bull. 2003, 26, 969–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loveday, S.M.; Peram, M.R.; Singh, H.; Ye, A.; Jameson, G.B. Digestive diversity and kinetic intrigue among heated and unheated β-lactoglobulin species. Food Funct. 2014, 5, 2783. [Google Scholar] [CrossRef]
- Jeong, H.J.; Jeong, J.B.; Kim, D.S.; de Lumen, B.O. Inhibition of core histone acetylation by the cancer preventive peptide lunasin. J. Agric. Food Chem. 2007, 55, 632–637. [Google Scholar] [CrossRef]
- Baderschneider, B.; Crevel, R.W.R.; Earl, L.K.; Lalljie, A.; Sanders, D.J.; Sanders, I.J. Sequence analysis and resistance to pepsin hydrolysis as part of an assessment of the potential allergenicity of ice structuring protein type III HPLC 12. Food Chem. Toxicol. 2002, 40, 965–978. [Google Scholar] [CrossRef]
- Yao, X.; Bunt, C.; Cornish, J.; Quek, S.Y.; Wen, J. Improved RP-HPLC method for determ inationof bovine lactoferrin and its proteolyticdegradation in simulated gastrointestinal fluids. Biomed. Chromatogr. 2012, 27, 197–202. [Google Scholar] [CrossRef]
- Miralles, B.; Del Barrio, R.; Cueva, C.; Recio, I.; Amigo, L. Dynamic gastric digestion of a commercial whey protein concentrate. J. Agric. Food Chem. 2018, 98, 1873–1879. [Google Scholar] [CrossRef]
- Foster, E.S.; Kimber, I.; Dearman, R.J. Relationship between protein digestibility and allergenicity: Comparisons of pepsin and cathepsin. Toxicology 2013, 309, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Lau, T.; Cai, N.; Singh, J.; Pedersen, J.F.; Vensel, W.H.; Hurkman, W.J.; Wilson, J.D.; Lemaux, P.G.; Buchanan, B.B. Digestibility of protein and starch from sorghum (Sorghum bicolor) is linked to biochemical and structural features of grain endosperm. J. Cereal Sci. 2009, 49, 73–82. [Google Scholar] [CrossRef]
- Arbab, M.E.; El Tinay, A.H. Effect of cooking and treatment with sodium bisulphite or ascorbic acid on the in vitro protein digestibility of two sorghum cultivars. Food Chem. 1997, 59, 339–343. [Google Scholar] [CrossRef]

| Minimum | Maximum | 1st Quartile | Median | 3rd Quartile | Mean | Standard Deviation (n) | |
|---|---|---|---|---|---|---|---|
| Additives | −1 | 1 | 0.00 | 0 | 0.00 | 0.02 | 0.51 |
| Molecular weight (kDa) | 5.80 | 270.00 | 18.00 | 24.00 | 51.11 | 41.18 | 44.47 |
| Protein concentration (mg/mL) | 1.00 × 10−3 | 54.25 | 0.25 | 0.60 | 1.00 | 2.09 | 5.44 |
| Pepsin concentration (mg/mL) | 5.00 × 10−5 | 3.05 | 0.24 | 0.80 | 1.42 | 0.84 | 0.66 |
| pH | 1.20 | 5.50 | 1.55 | 2.00 | 2.50 | 2.14 | 0.90 |
| HT (min) | 0.50 | 630.00 | 4.65 | 17.80 | 73.50 | 57.57 | 104.19 |
| Primary Structure | Secondary Structure | Tertiary Structure | Properties | |||||
|---|---|---|---|---|---|---|---|---|
| Aromatic AA Residues + Leu and Met | Proline Residues | Total Residues | β-Sheet (%) | α-Helix (%) | Disulfide Bond (Excluding Free Cys) | Net-Charge (at pH 3) | Hydrophobicity | |
| BLG | 36 a | 8 a | 44 | 50 f | 15 f | 2 a | 18.5 | 0.037 |
| ALA | 24 b | 2 b | 26 | 14 g | 26 g | 4 b | 9.5 | 0.041 |
| αs1-casein | 42 c | 17 c | 59 | 20 h | 13 h | 0 c | 23.2 | −0.052 |
| αs2-casein | 37 c | 10 c | 47 | 30 e | 24–32 e | 1 i | 31.5 | −0.17 |
| β-casein | 43 c | 34 c | 77 | 22 h | 13 h | 0 c | 19.0 | 0.06 |
| κ-casein | 24 c | 20 c | 44 | 10 d | 30 d | 0 c | 16.5 | −0.00042 |
| Xanthine oxidase | 261 | 71 | 332 | 25 | 32 | 1 | 166.9 | 0.038 |
| Lactadherin (PAS VI/VII) | 90 | 21 | 111 | 20 | 3 | 9 | 44.3 | 0.064 |
| Butyrophilin (BTN1A1) | 111 | 37 | 148 | 24 | 2 | 2 | 61.1 | 0.024 |
| Emulsion | Gel | Milk | Native | Real Food | |
|---|---|---|---|---|---|
| Emulsion | 1 | 0.001 | <0.0001 | <0.0001 | <0.0001 |
| Gel | 1 | 0.000 | 0.170 | 0.057 | |
| Milk | 1 | 0.009 | 0.075 | ||
| Native | 1 | 0.443 | |||
| Real food | 1 |
| Emulsion | Gel | Milk | Native | Real Food | |
|---|---|---|---|---|---|
| Emulsion | 1 | 0.079 | 0.010 | 0.300 | 0.001 |
| Gel | 1 | 0.333 | 0.004 | <0.0001 | |
| Milk | 1 | 0.000 | <0.0001 | ||
| Native | 1 | 0.012 | |||
| Real food | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, N.; Dulko, D.; Macierzanka, A.; Jungnickel, C. Analysis of the Factors Affecting Static In Vitro Pepsinolysis of Food Proteins. Molecules 2022, 27, 1260. https://doi.org/10.3390/molecules27041260
Maeda N, Dulko D, Macierzanka A, Jungnickel C. Analysis of the Factors Affecting Static In Vitro Pepsinolysis of Food Proteins. Molecules. 2022; 27(4):1260. https://doi.org/10.3390/molecules27041260
Chicago/Turabian StyleMaeda, Natsumi, Dorota Dulko, Adam Macierzanka, and Christian Jungnickel. 2022. "Analysis of the Factors Affecting Static In Vitro Pepsinolysis of Food Proteins" Molecules 27, no. 4: 1260. https://doi.org/10.3390/molecules27041260
APA StyleMaeda, N., Dulko, D., Macierzanka, A., & Jungnickel, C. (2022). Analysis of the Factors Affecting Static In Vitro Pepsinolysis of Food Proteins. Molecules, 27(4), 1260. https://doi.org/10.3390/molecules27041260








