Striatal Cholinergic Signaling in Time and Space
Abstract
:1. Introduction
2. Firing Patterns of Cholinergic Interneurons and Their Behavioral Correlates
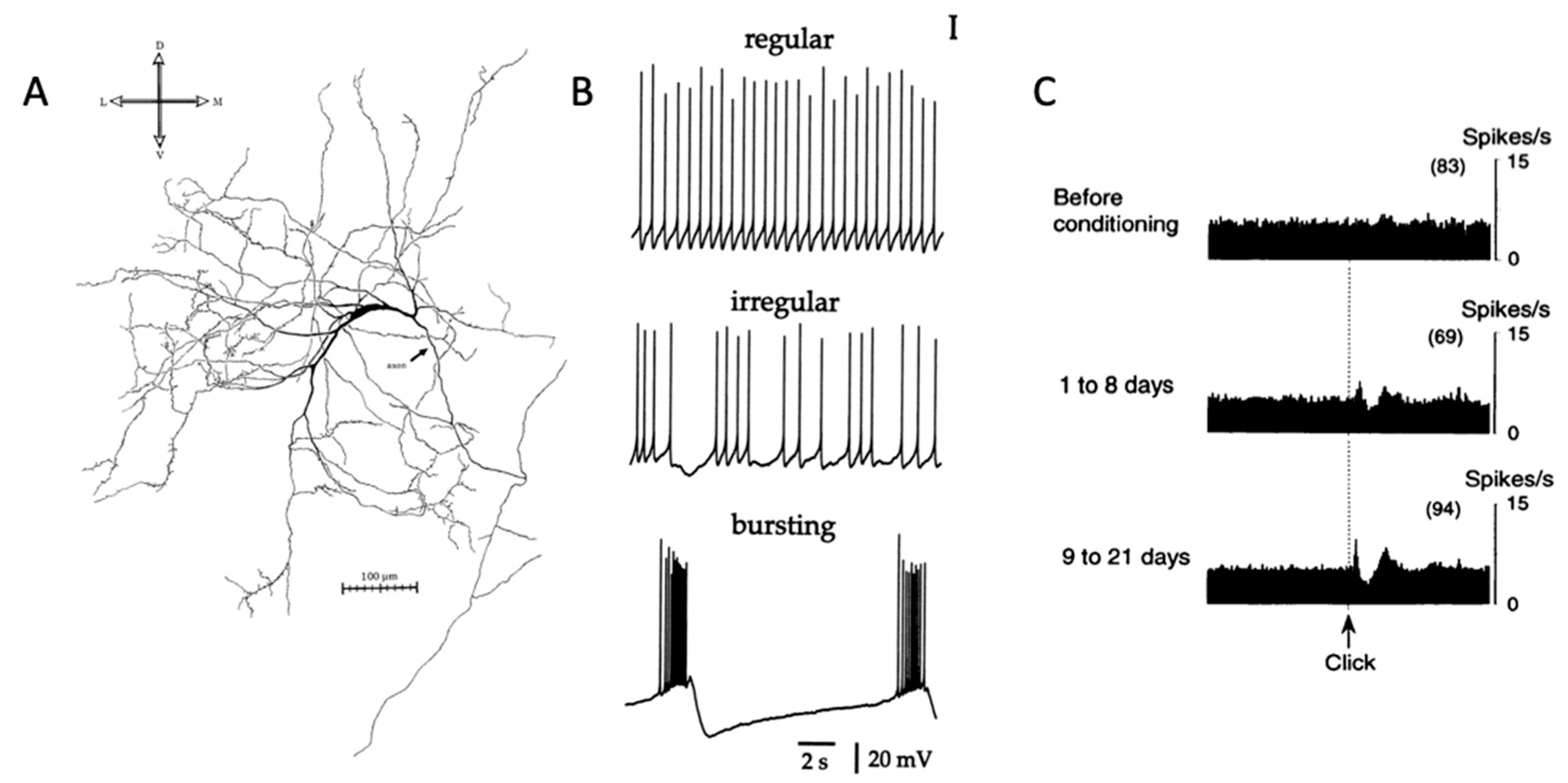
3. Striatal Cholinergic Transmission
3.1. Cholinergic Release Sites: Axons, Varicosities, and Synapses
3.2. Acetylcholine Concentration in Vesicles and Rate of Release
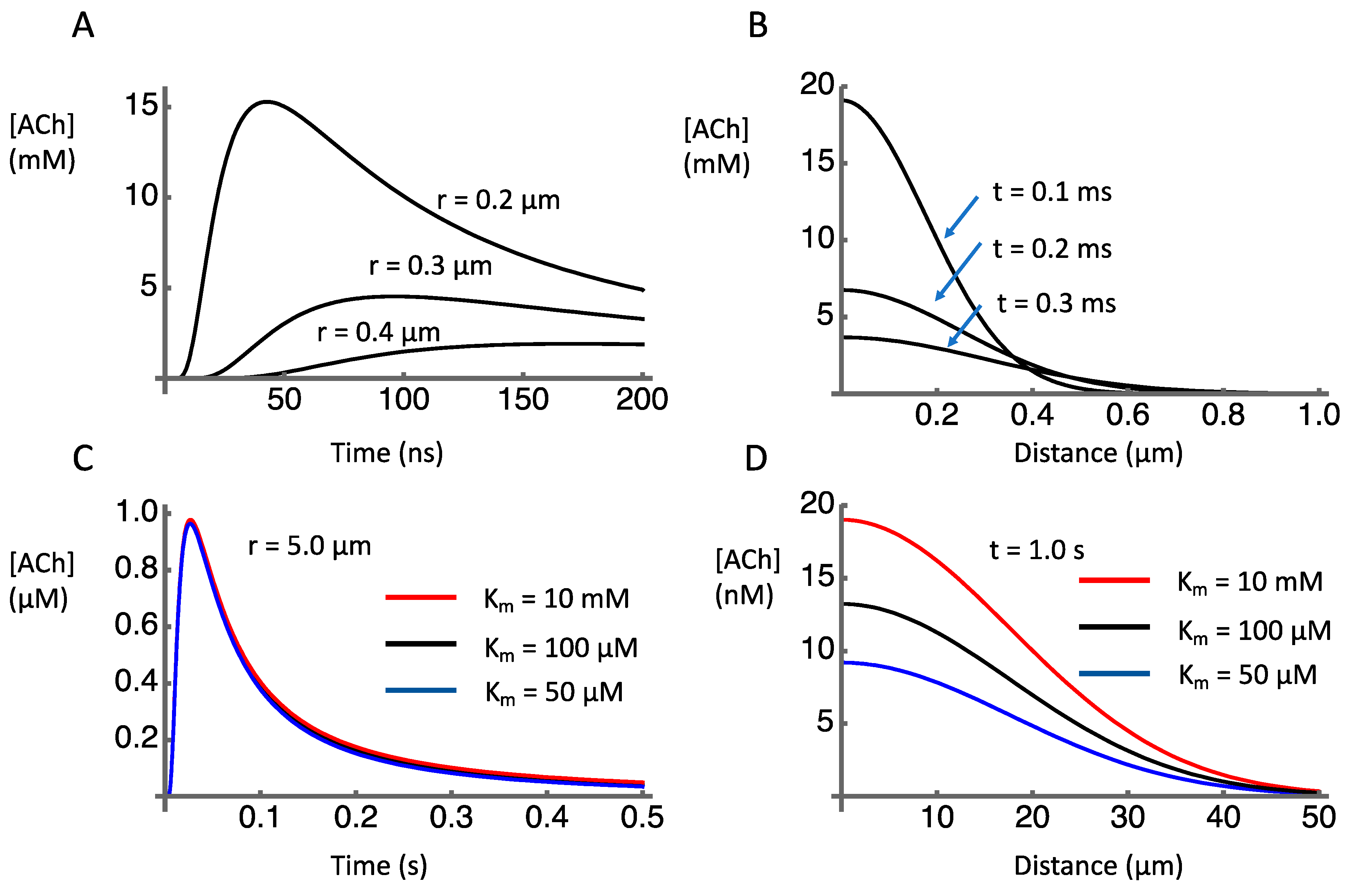
3.3. Spatial Spread of Acetylcholine: Diffusion and Hydrolysis
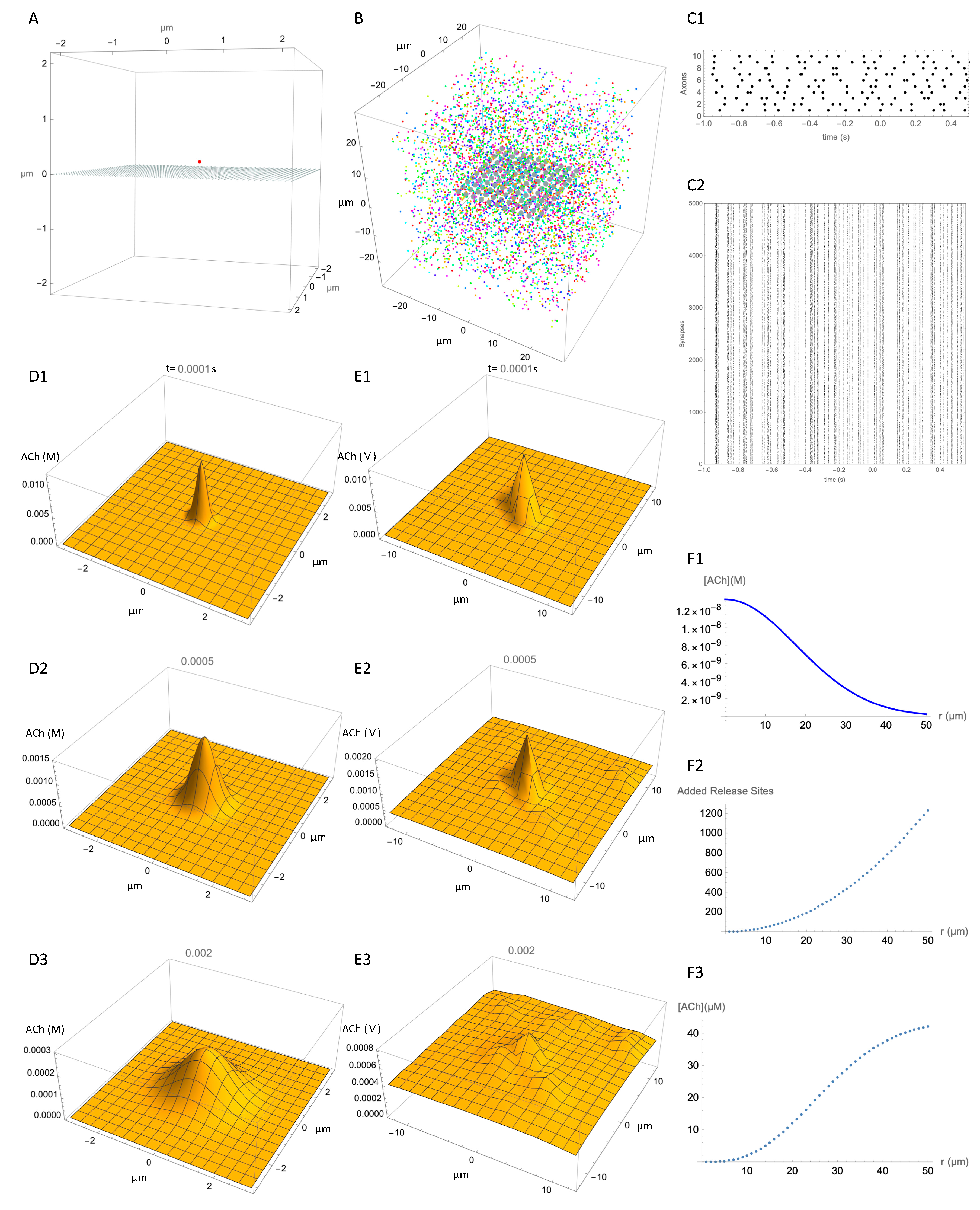
3.4. Spatial Selectivity of ACh Signaling
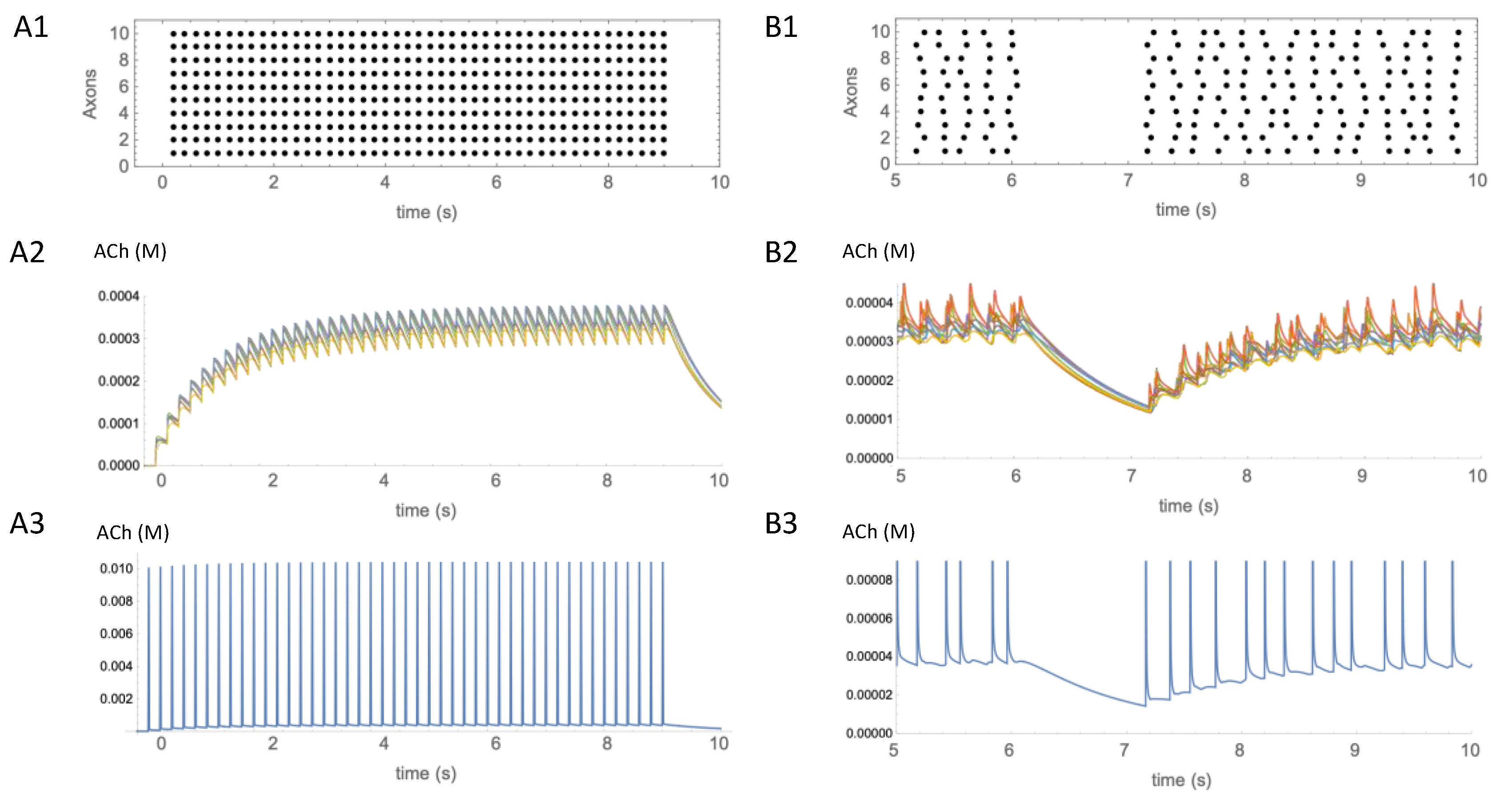
3.5. Temporal Resolution of ACh Signaling
3.6. Limitations and Tests of the Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kemp, J.M.; Powell, T.P. The synaptic organization of the caudate nucleus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1971, 262, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Bolam, J.P.; Wainer, B.H.; Smith, A.D. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience 1984, 12, 711–718. [Google Scholar] [CrossRef]
- Phelps, P.E.; Houser, C.R.; Vaughn, J.E. Immunocytochemical localization of choline acetyltransferase within the rat neostriatum: A correlated light and electron microscopic study of cholinergic neurons and synapses. J. Comp. Neurol. 1985, 238, 286–307. [Google Scholar] [CrossRef]
- Armstrong, D.M.; Saper, C.B.; Levey, A.I.; Wainer, B.H.; Terry, R.D. Distribution of cholinergic neurons in rat brain: Demonstrated by the immunocytochemical localization of choline acetyltransferase. J. Comp. Neurol. 1983, 216, 53–68. [Google Scholar] [CrossRef]
- Dautan, D.; Huerta-Ocampo, I.; Witten, I.B.; Deisseroth, K.; Bolam, J.P.; Gerdjikov, T.; Mena-Segovia, J. A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J. Neurosci. 2014, 34, 4509–4518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonnum, F.; Walaas, S.I. Localization of neurotransmitter candidates in neostriatum. In The Neostriatum; Pergamon Press: Oxford, UK, 1979; pp. 53–69. [Google Scholar]
- Difiglia, M. Synaptic organization of cholinergic neurons in the monkey neostriatum. J. Comp. Neurol. 1987, 255, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Chang, H.T.; Kitai, S.T. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J. Neurosci. 1990, 10, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Dimova, R.; Vuillet, J.; Nieoullon, A.; Kerkerian-Le Goff, L. Ultrastructural features of the choline acetyltransferase-containing neurons and relationships with nigral dopaminergic and cortical afferent pathways in the rat striatum. Neuroscience 1993, 53, 1059–1071. [Google Scholar] [CrossRef]
- Lapper, S.R.; Bolam, J.P. Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience 1992, 51, 533–545. [Google Scholar] [CrossRef]
- Meredith, G.E.; Wouterlood, F.G. Hippocampal and midline thalamic fibers and terminals in relation to the choline acetyltransferase-immunoreactive neurons in nucleus accumbens of the rat: A light and electron microscopic study. J. Comp. Neurol. 1990, 296, 204–221. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Inagaki, S.; Shimada, S.; Kito, S.; Eckenstein, F.; Tohyama, M. Neostriatal cholinergic neurons receive direct synaptic inputs from dopaminergic axons. Brain Res. 1987, 413, 179–184. [Google Scholar] [CrossRef]
- Contant, C.; Umbriaco, D.; Garcia, S.; Watkins, K.C.; Descarries, L. Ultrastructural characterization of the acetylcholine innervation in adult rat neostriatum. Neuroscience 1996, 71, 937–947. [Google Scholar] [CrossRef]
- Takagi, H.; Somogyi, P.; Smith, A.D. Aspiny neurons and their local axons in the neostriatum of the rat: A correlated light and electron microscopic study of golgi-imprgnated material. J. Neurocytol. 1984, 13, 239–265. [Google Scholar] [CrossRef] [PubMed]
- Wainer, B.H.; Bolam, J.P.; Freund, T.F.; Henderson, Z.; Totterdell, S.; Smith, A.D. Cholinergic synapses in the rat brain: A correlated light and electron microscopic immunohistochemical study employing a monoclonal antibody against choline acetyltransferase. Brain Res 1984, 308, 69–76. [Google Scholar] [CrossRef]
- Descarries, L.; Gisiger, V.; Steriade, M. Diffuse transmission by acetylcholine in the CNS. Prog. Neurobiol. 1997, 53, 603–625. [Google Scholar] [CrossRef]
- Descarries, L.; Mechawar, N. Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog. Brain Res. 2000, 125, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Hersch, S.M.; Gutekunst, C.A.; Rees, H.D.; Heilman, C.J.; Levey, A.I. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: Light and electron microscopic immunocytochemistry using subtype-specific antibodies. J. Neurosci. 1994, 14 Pt 2, 3351–3363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, I.W.; Bolam, J.P.; Wonnacott, S. Presynaptic localisation of the nicotinic acetylcholine receptor beta2 subunit immunoreactivity in rat nigrostriatal dopaminergic neurones. J. Comp. Neurol. 2001, 439, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.K.; Zheng, W.S.; Zhang, P.; Jing, M.; Borden, P.M.; Ali, F.; Guo, K.; Feng, J.; Marvin, J.S.; Wang, Y.; et al. Nanoscopic visualization of restricted nonvolume cholinergic and monoaminergic transmission with genetically encoded sensors. Nano Lett. 2020, 20, 4073–4083. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Parikh, V.; Howe, W.M. Phasic acetylcholine release and the volume transmission hypothesis: Time to move on. Nat. Rev. Neurosci. 2009, 10, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Mamaligas, A.A. Cholinergic Interneuron Mediated Activation of G-Protein Coupled Receptors in the Dorsal Striatum; Case Western Reserve University: Cleveland, OH, USA, 2018. [Google Scholar]
- Kawaguchi, Y.; Wilson, C.J.; Augood, S.J.; Emson, P.C. Striatal interneurones: Chemical, physiological and morphological characterization. Trends Neurosci. 1995, 18, 527–535. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Wilson, C.J.; Emson, P.C. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J. Neurophysiol. 1989, 62, 1052–1068. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, K.; Liu, X.; Yan, H.; Ma, X.; Zhang, S.; Zheng, J.; Wang, L.; Wei, X. Involvement of HCN Channel in Muscarinic Inhibitory Action on Tonic Firing of Dorsolateral Striatal Cholinergic Interneurons. Front. Cell. Neurosci. 2016, 10, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, P.; Zhang, Y.; Xu, Z.C. Involvement of I(h) in dopamine modulation of tonic firing in striatal cholinergic interneurons. J. Neurosci. 2007, 27, 3148–3156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, C.J. The mechanism of intrinsic amplification of hyperpolarizations and spontaneous bursting in striatal cholinergic interneurons. Neuron 2005, 45, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M.; Rajkowski, J.; Evarts, E. Tonically discharging putamen neurons exhibit set-dependent responses. Proc. Natl. Acad. Sci. USA 1984, 81, 4998–5001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aosaki, T.; Graybiel, A.M.; Kimura, M. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science 1994, 265, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Aosaki, T.; Kimura, M.; Graybiel, A.M. Temporal and spatial characteristics of tonically active neurons of the primate’s striatum. J. Neurophysiol. 1995, 73, 1234–1252. [Google Scholar] [CrossRef] [PubMed]
- Shimo, Y.; Hikosaka, O. Role of tonically active neurons in primate caudate in reward-oriented saccadic eye movement. J. Neurosci. 2001, 21, 7804–7814. [Google Scholar] [CrossRef]
- Morris, G.; Arkadir, D.; Nevet, A.; Vaadia, E.; Bergman, H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron 2004, 43, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Apicella, P. Leading tonically active neurons of the striatum from reward detection to context recognition. Trends Neurosci. 2007, 30, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Apicella, P. The role of the intrinsic cholinergic system of the striatum: What have we learned from TAN recordings in behaving animals? Neuroscience 2017, 360, 81–94. [Google Scholar] [CrossRef]
- Ravel, S.; Sardo, P.; Legallet, E.; Apicella, P. Influence of spatial information on responses of tonically active neurons in the monkey striatum. J. Neurophysiol. 2006, 95, 2975–2986. [Google Scholar] [CrossRef]
- Lee, I.H.; Seitz, A.R.; Assad, J.A. Activity of tonically active neurons in the monkey putamen during initiation and withholding of movement. J. Neurophysiol. 2006, 95, 2391–2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Finkelstein, J.; Choi, J.Y.; Witten, I.B. Linking cholinergic interneurons, synaptic plasticity, and behavior during the extinction of a cocaine-context association. Neuron 2016, 90, 1071–1085. [Google Scholar] [CrossRef] [Green Version]
- Stalnaker, T.A.; Berg, B.; Aujla, N.; Schoenbaum, G. Cholinergic interneurons use orbitofrontal input to track beliefs about current state. J. Neurosci. 2016, 36, 6242–6257. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.D.; Baker, P.M.; Ragozzino, M.E. The parafascicular thalamic nucleus concomitantly influences behavioral flexibility and dorsomedial striatal acetylcholine output in rats. J. Neurosci. 2010, 30, 14390–14398. [Google Scholar] [CrossRef] [Green Version]
- Bradfield, L.A.; Bertran-Gonzalez, J.; Chieng, B.; Balleine, B.W. The thalamostriatal pathway and cholinergic control of goal-directed action: Interlacing new with existing learning in the striatum. Neuron 2013, 79, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Aoki, S.; Liu, A.W.; Zucca, A.; Zucca, S.; Wickens, J.R. Role of striatal cholinergic interneurons in set-shifting in the rat. J. Neurosci. 2015, 35, 9424–9431. [Google Scholar] [CrossRef] [Green Version]
- Aoki, S.; Liu, A.W.; Akamine, Y.; Zucca, A.; Zucca, S.; Wickens, J.R. Cholinergic interneurons in the rat striatum modulate substitution of habits. Eur. J. Neurosci. 2018, 47, 1194–1205. [Google Scholar] [CrossRef] [Green Version]
- Aoki, S.; Liu, A.W.; Zucca, A.; Zucca, S.; Wickens, J.R. New variations for strategy set-shifting in the rat. JOVE 2017, 119, e55005. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.D.; Wilson, C.J. Spontaneous activity of neostriatal cholinergic interneurons in vitro. J. Neurosci. 1999, 19, 5586–5596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravel, S.; Legallet, E.; Apicella, P. Responses of tonically active neurons in the monkey striatum discriminate between motivationally opposing stimuli. J. Neurosci. 2003, 23, 8489–8497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apicella, P.; Deffains, M.; Ravel, S.; Legallet, E. Tonically active neurons in the striatum differentiate between delivery and omission of expected reward in a probabilistic task context. Eur. J. Neurosci. 2009, 30, 515–526. [Google Scholar] [CrossRef]
- Apicella, P.; Scarnati, E.; Schultz, W. Tonically discharging neurons of monkey striatum respond to preparatory and rewarding stimuli. Exp. Brain Res. 1991, 84, 672–675. [Google Scholar] [CrossRef]
- Apicella, P.; Legallet, E.; Trouche, E. Responses of tonically discharging neurons in monkey striatum to visual stimuli presented under passive conditions and during task performance. Neurosci. Lett. 1996, 203, 147–150. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Reynolds, J.N.J.; Cragg, S.J. Pauses in Cholinergic Interneuron Activity Are Driven by Excitatory Input and Delayed Rectification, with Dopamine Modulation. Neuron 2018, 98, 918–925.e3. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.F.; Cragg, S.J. Pauses in Striatal Cholinergic Interneurons: What is Revealed by Their Common Themes and Variations? Front. Syst. Neurosci. 2017, 11, 80. [Google Scholar] [CrossRef]
- Goldberg, J.A.; Reynolds, J.N. Spontaneous firing and evoked pauses in the tonically active cholinergic interneurons of the striatum. Neuroscience 2011, 198, 27–43. [Google Scholar] [CrossRef]
- Reynolds, J.N.; Hyland, B.I.; Wickens, J.R. Modulation of an afterhyperpolarization by the substantia nigra induces pauses in the tonic firing of striatal cholinergic interneurons. J. Neurosci. 2004, 24, 9870–9877. [Google Scholar] [CrossRef] [Green Version]
- Schulz, J.M.; Oswald, M.J.; Reynolds, J.N. Visual-induced excitation leads to firing pauses in striatal cholinergic interneurons. J. Neurosci. 2011, 31, 11133–11143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oswald, M.J.; Oorschot, D.E.; Schulz, J.M.; Lipski, J.; Reynolds, J.N. IH current generates the afterhyperpolarisation following activation of subthreshold cortical synaptic inputs to striatal cholinergic interneurons. J. Physiol. 2009, 587 Pt 24, 5879–5897. [Google Scholar] [CrossRef] [PubMed]
- Doig, N.M.; Magill, P.J.; Apicella, P.; Bolam, J.P.; Sharott, A. Cortical and thalamic excitation mediate the multiphasic responses of striatal cholinergic interneurons to motivationally salient stimuli. J. Neurosci. 2014, 34, 3101–3117. [Google Scholar] [CrossRef] [Green Version]
- Groves, P.M. Synaptic endings and their postsynaptic targets in neostriatum: Synaptic specializations revealed from analysis of serial sections. Proc. Natl. Acad. Sci. USA 1980, 77, 6926–6929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingham, C.A.; Hood, S.H.; Taggart, P.; Arbuthnott, G.W. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J. Neurosci. 1998, 18, 4732–4743. [Google Scholar] [CrossRef] [PubMed]
- Groves, P.M.; Linder, J.C.; Young, S.J. 5-hydroxydopamine-labeled dopaminergic axons: Three-dimensional reconstructions of axons, synapses and postsynaptic targets in rat neostriatum. Neuroscience 1994, 58, 593–604. [Google Scholar] [CrossRef]
- Clark, P.J.; Evans, F.C. Generalization of a nearest neighbor measure of dispersion for use in K dimensions. Ecology 1979, 60, 316–317. [Google Scholar] [CrossRef]
- Whittaker, V.P.; Sheridan, M.N. The Morphology and Acetylcholine Content of Isolated Cerebral Cortical Synaptic Vesicles. J. Neurochem. 1965, 12, 363–372. [Google Scholar] [CrossRef]
- Wilson, W.S.; Schulz, R.A.; Cooper, J.R. The isolation of cholinergic synaptic vesicles from bovine superior cervical ganglion and estimation of their acetylcholine content. J. Neurochem. 1973, 20, 659–667. [Google Scholar] [CrossRef]
- Whittaker, V.P.; Michaelson, I.A.; Kirkland, R.J. The separation of synaptic vesicles from nerve-ending particles (‘synaptosomes’). Biochem. J. 1964, 90, 293–303. [Google Scholar] [CrossRef] [Green Version]
- Westerink, B.H.; Damsma, G.; Rollema, H.; De Vries, J.B.; Horn, A.S. Scope and limitations of in vivo brain dialysis: A comparison of its application to various neurotransmitter systems. Life Sci. 1987, 41, 1763–1776. [Google Scholar] [CrossRef]
- Westerink, B.H.; Hofsteede, H.M.; Damsma, G.; de Vries, J.B. The significance of extracellular calcium for the release of dopamine, acetylcholine and amino acids in conscious rats, evaluated by brain microdialysis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1988, 337, 373–378. [Google Scholar] [CrossRef]
- Damsma, G.; Westerink, B.H.; de Boer, P.; de Vries, J.B.; Horn, A.S. Basal acetylcholine release in freely moving rats detected by on-line trans-striatal dialysis: Pharmacological aspects. Life Sci. 1988, 43, 1161–1168. [Google Scholar] [CrossRef]
- Chefer, V.I.; Thompson, A.C.; Zapata, A.; Shippenberg, T.S. Overview of brain microdialysis. Curr. Protoc. Neurosci. 2009, 47. [Google Scholar] [CrossRef] [Green Version]
- Benveniste, H.; Huttemeier, P.C. Microdialysis—Theory and application. Prog. Neurobiol. 1990, 35, 195–215. [Google Scholar] [CrossRef]
- Nicholson, C.; Phillips, J.M. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J. Physiol. 1981, 321, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Hayakawa, T.; Kashima, Y.; Suzuki, T.; Fujimoto, K.; Oohata, H. Determination of acetylcholine release in the striatum of anesthetized rats using in vivo microdialysis and a radioimmunoassay. J. Neurochem. 1991, 57, 882–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Boer, P.; Westerink, B.H.; Horn, A.S. The effect of acetylcholinesterase inhibition on the release of acetylcholine from the striatum in vivo: Interaction with autoreceptor responses. Neurosci. Lett. 1990, 116, 357–360. [Google Scholar] [CrossRef]
- de Boer, P.; Westerink, B.H. GABAergic modulation of striatal cholinergic interneurons: An in vivo microdialysis study. J. Neurochem. 1994, 62, 70–75. [Google Scholar] [CrossRef]
- Nicholson, C. Interaction between diffusion and Michaelis-Menten uptake of dopamine after iontophoresis in striatum. Biophys. J. 1995, 68, 1699–1715. [Google Scholar] [CrossRef] [Green Version]
- Land, B.R.; Salpeter, E.E.; Salpeter, M.M. Kinetic parameters for acetylcholine interaction in intact neuromuscular junction. Proc. Natl. Acad. Sci. USA 1981, 78, 7200–7204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, L.; Nicholson, C. Diffusion of albumins in rat cortical slices and relevance to volume transmission. Neuroscience 1996, 75, 839–847. [Google Scholar] [CrossRef]
- Rothenberg, M.A.; Nachmansohn, D. Studies on cholinesterase; purification of the enzyme from electric tissue by fractional ammonium sulfate precipitation. J. Biol. Chem. 1947, 168, 223–231. [Google Scholar] [CrossRef]
- Wilson, I.B.; Harrison, M.A. Turnover number of acetyl-cholinesterase. J. Biol. Chem. 1961, 236, 2292–2295. [Google Scholar] [CrossRef]
- Rosenberry, T.L. Acetylcholinesterase. Adv. Enzymol. Relat. Areas Mol. Biol. 1975, 43, 103–218. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, G.; Tavitian, B.; Pappata, S.; Crouzel, C.; Jobert, A.; Doignon, I.; Di Giamberardino, L. Quantitative measurement of cerebral acetylcholinesterase using. J. Cereb. Blood Flow Metab. 2001, 21, 114–131. [Google Scholar] [CrossRef] [Green Version]
- Wilson, I.B.; Cabib, E. Acetylcholinesterase: Enthalpies and Entropies of Activation. J. Am. Chem. Soc. 1956, 78, 202–217. [Google Scholar] [CrossRef]
- Lodge, A.M. Kinetic Evaluation of the Inhibition and Reactivation of Human Acetylcholinesterase; University of Iowa: Iowa City, IA, USA, 2013. [Google Scholar]
- Quinn, D.M. Acetylcholinesterase: Enzyme structure, reaction dynamics and virtual transition states. Chem. Rev. 1987, 87, 955–979. [Google Scholar] [CrossRef]
- Dunant, Y.; Gisiger, V. Ultrafast and Slow Cholinergic Transmission. Different Involvement of Acetylcholinesterase Molecular Forms. Molecules 2017, 22, 1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H. Dopamine Clearance in the Rat Striatum. Unpublished Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2002. [Google Scholar]
- Cachope, R.; Mateo, Y.; Mathur, B.N.; Irving, J.; Wang, H.L.; Morales, M.; Lovinger, D.M.; Cheer, J.F. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: Setting the tone for reward processing. Cell Rep. 2012, 2, 33–41. [Google Scholar] [CrossRef] [Green Version]
- English, D.F.; Ibanez-Sandoval, O.; Stark, E.; Tecuapetla, F.; Buzsaki, G.; Deisseroth, K.; Tepper, J.M.; Koos, T. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat. Neurosci. 2011, 15, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, J.A.; Ding, J.B.; Surmeier, D.J. Muscarinic modulation of striatal function and circuitry. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 208, pp. 223–241. [Google Scholar] [CrossRef]
- Kosillo, P.; Zhang, Y.F.; Threlfell, S.; Cragg, S.J. Cortical control of striatal dopamine transmission via striatal cholinergic interneurons. Cereb. Cortex 2016, 26, 4160–4169. [Google Scholar] [CrossRef] [PubMed]
- Threlfell, S.; Lalic, T.; Platt, N.J.; Jennings, K.A.; Deisseroth, K.; Cragg, S.J. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 2012, 75, 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harpsoe, K.; Hald, H.; Timmermann, D.B.; Jensen, M.L.; Dyhring, T.; Nielsen, E.O.; Peters, D.; Balle, T.; Gajhede, M.; Kastrup, J.S.; et al. Molecular determinants of subtype-selective efficacies of cytisine and the novel compound NS3861 at heteromeric nicotinic acetylcholine receptors. J. Biol. Chem. 2013, 288, 2559–2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharott, A.; Doig, N.M.; Mallet, N.; Magill, P.J. Relationships between the firing of identified striatal interneurons and spontaneous and driven cortical activities in vivo. J. Neurosci. 2012, 32, 13221–13236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucca, S.; Zucca, A.; Nakano, T.; Aoki, S.; Wickens, J. Pauses in cholinergic interneuron firing exert an inhibitory control on striatal output in vivo. eLife 2018, 7, e32510. [Google Scholar] [CrossRef] [PubMed]
- Briggs, C.A.; McKenna, D.G. Activation and inhibition of the human alpha7 nicotinic acetylcholine receptor by agonists. Neuropharmacology 1998, 37, 1095–1102. [Google Scholar] [CrossRef]
- Dobbertin, A.; Hrabovska, A.; Dembele, K.; Camp, S.; Taylor, P.; Krejci, E.; Bernard, V. Targeting of acetylcholinesterase in neurons in vivo: A dual processing function for the proline-rich membrane anchor subunit and the attachment domain on the catalytic subunit. J. Neurosci. 2009, 29, 4519–4530. [Google Scholar] [CrossRef] [Green Version]
- Farar, V.; Mohr, F.; Legrand, M.; Lamotte d’Incamps, B.; Cendelin, J.; Leroy, J.; Abitbol, M.; Bernard, V.; Baud, F.; Fournet, V.; et al. Near-complete adaptation of the PRiMA knockout to the lack of central acetylcholinesterase. J. Neurochem. 2012, 122, 1065–1080. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Zhang, P.; Wang, G.; Feng, J.; Mesik, L.; Zeng, J.; Jiang, H.; Wang, S.; Looby, J.C.; Guagliardo, N.A.; et al. A genetically encoded fluorescent acetylcholine indicator for in vitro and in vivo studies. Nat. Biotechnol. 2018, 36, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Marcott, P.F.; Mamaligas, A.A.; Ford, C.P. Phasic dopamine release drives rapid activation of striatal D2-receptors. Neuron 2014, 84, 164–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Disney, A.A.; Higley, M.J. Diverse Spatiotemporal Scales of Cholinergic Signaling in the Neocortex. J. Neurosci. 2020, 40, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Faust, T.W.; Assous, M.; Tepper, J.M.; Koos, T. Neostriatal GABAergic interneurons mediate cholinergic inhibition of spiny projection neurons. J. Neurosci. 2016, 36, 9505–9511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graybiel, A.M.; Baughman, R.W.; Eckenstein, F. Cholinergic neuropil of the striatum observes striosomal boundaries. Nature 1986, 323, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Borden, P.M.; Zhang, P.; Shivange, A.V.; Marvin, J.S.; Cichon, J.; Dan, C.; Podgorski, K.; Figueiredo, A.; Novak, O.; Tanimoto, M.; et al. A fast genetically encoded fluorescent sensor for faithful in vivo acetylcholine detection in mice, fish, worms and flies. bioRxiv 2018. [Google Scholar] [CrossRef]
| Parameter | Symbol | Value | Units | Sources |
|---|---|---|---|---|
| Diffusion coefficient | d | 4.0 × 10−10 | m2 s−1 | [73] |
| Tortuosity | λ | 1.6 | [74] | |
| Volume fraction | α | 0.2 | [68] | |
| Molecules/vesicle | M | 1000 | See text | |
| Turnover number | Kcat | 1.23 × 104 | s−1 | [75,76,77] |
| AChE concentration | Et | 300 | nM | [78] |
| Rate constant | Vm | 36.9 | µM s−1 | Calculated (Et × Kcat) |
| Binding constant | Km | 100 | µM | [79,80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nosaka, D.; Wickens, J.R. Striatal Cholinergic Signaling in Time and Space. Molecules 2022, 27, 1202. https://doi.org/10.3390/molecules27041202
Nosaka D, Wickens JR. Striatal Cholinergic Signaling in Time and Space. Molecules. 2022; 27(4):1202. https://doi.org/10.3390/molecules27041202
Chicago/Turabian StyleNosaka, Dvyne, and Jeffery R. Wickens. 2022. "Striatal Cholinergic Signaling in Time and Space" Molecules 27, no. 4: 1202. https://doi.org/10.3390/molecules27041202
APA StyleNosaka, D., & Wickens, J. R. (2022). Striatal Cholinergic Signaling in Time and Space. Molecules, 27(4), 1202. https://doi.org/10.3390/molecules27041202






