New Experimental Conditions for Diels–Alder and Friedel-Crafts Alquilation Reactions with Thiophene: A New Selenocyanate with Potent Activity against Cancer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis Optimization
Reaction Mechanism Discussion
2.2. Biological Evaluation
2.2.1. Cytotoxicity Activity
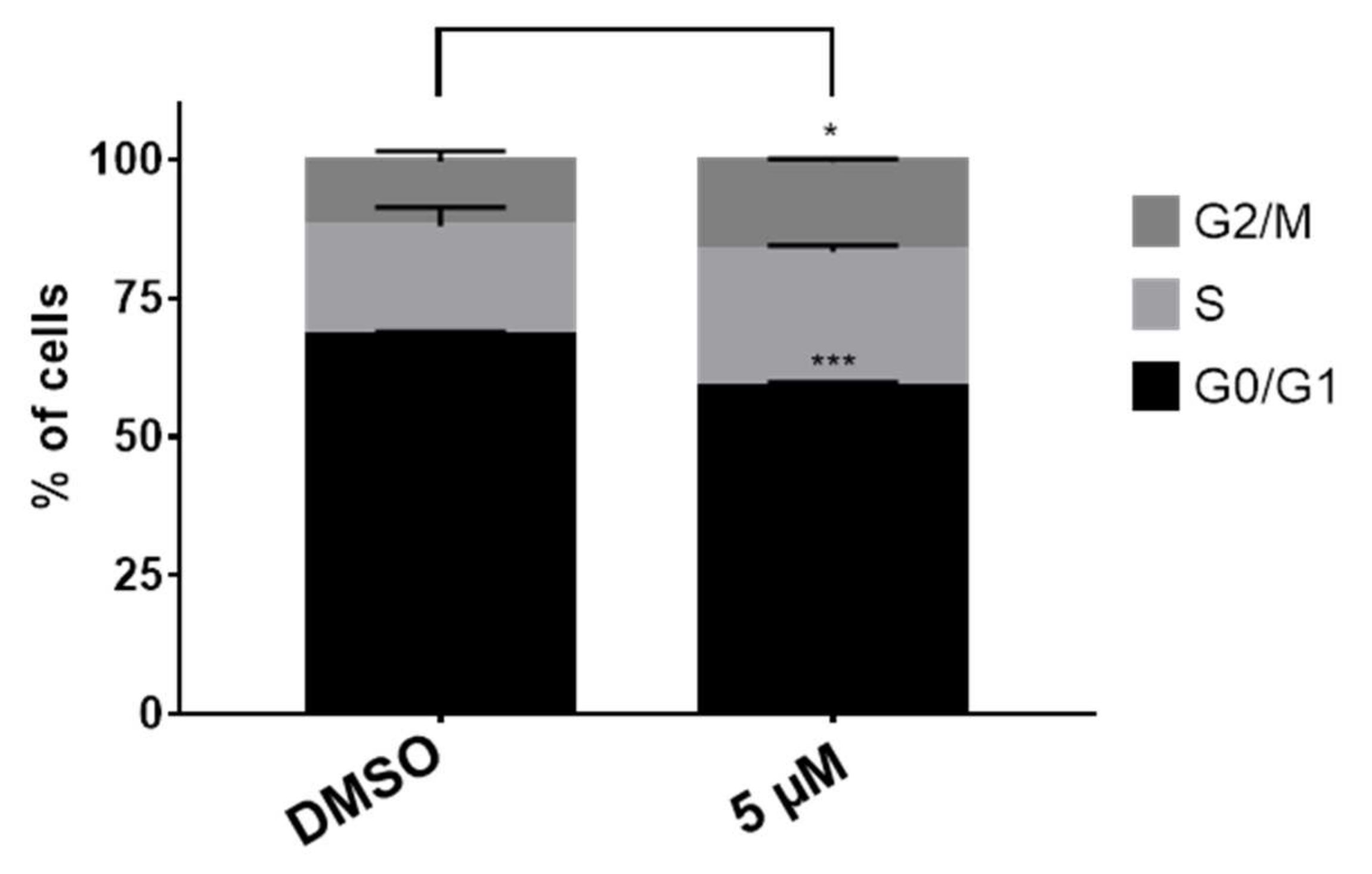
2.2.2. Compound 15 Induces an Arrest on the G2/M Phase of the Cell Cycle
3. Materials and Methods
3.1. Synthesis
3.1.1. Synthesis of 2-Bromoethyl Maleimide
3.1.2. Synthesis of N-3-Acetoxypropyl Maleimide
3.1.3. Diels–Alder Reaction of Thiophene with N-Phenylmaleimides Promoted by AlCl3
3.1.4. Synthesis of Friedel–Crafts-Type Alquilation Compounds
3.1.5. Synthesis of “Butterfly-Like” Diazatetradecenes
3.1.6. Synthesis of the Selenocyanate Derivative of Thiophene DA Adduct 15
3.2. Characterization Data
3.3. Biological Evaluation
3.3.1. NCI-60 Human Tumor Cell Line 5-Dose-Response
3.3.2. Cell Cycle Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels–Alder reaction in total synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Ramsden, C.A.; Joule, J.A.; Zhdankin, V.V. 2.3-Structure of five-membered rings with one heteroatom. In Handbook of Heterocyclic Chemistry, 3rd ed.; Katritzky, A.R., Ramsden, C.A., Joule, J.A., Zhdankin, V.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 1, pp. 87–138. [Google Scholar]
- Jursic, B.S. Suitability of furan, pyrrole and thiophene as dienes for Diels–Alder reactions viewed through their stability and reaction barriers for reactions with acetylene, ethylene and cyclopropene. An AM1 semiempirical and B3LYP hybrid density functional theory study. Theochem 1998, 454, 105–116. [Google Scholar] [CrossRef]
- Ding, X.; Nguyen, S.T.; Williams, J.D.; Peet, N.P. Diels-Alder reactions of five-membered heterocycles containing one heteroatom. Tetrahedron Lett. 2014, 55, 7002–7006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreou, D.; Kallitsakis, M.G.; Loukopoulos, E.; Gabriel, C.; Kostakis, G.E.; Lykakis, I.N. Copper-promoted regioselective synthesis of polysubstituted pyrroles from aldehydes, amines, and nitroalkenes via 1,2-phenyl/alkyl migration. J. Org. Chem. 2018, 83, 2104–2113. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, M.; Karunakaran, J.; Mohanakrishnan, A.K. Diels-Alder reaction of 1,3-diarylbenzo[c]furans with thiophene S,S-dioxide/indenone derivatives: A facile preparation of substituted dibenzothiophene S,S-dioxides and fluorenones. Org. Lett. 2014, 16, 3068–3071. [Google Scholar] [CrossRef]
- Fringuelli, F.; Taticchi, A. High pressure Diels–Alder reaction. In The Diels–Alder Reaction, 1st ed.; Fringuelli, F., Taticchi, A., Eds.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2001; Volume 1, pp. 205–249. [Google Scholar]
- Margetic, D.; Butler, D.N.; Warrener, R.N. Sulfur-bridged molecular racks: O,S-sesquinorbornadienes, CNS>[3] and CNOS-[4]polynorbornane. Arkivoc 2002, 2002, 234–256. [Google Scholar] [CrossRef] [Green Version]
- Hiyoshizo, K.; Hitoshi, N.; Sachio, K.; Masamitsu, O.; Nakamichi, Y.; Kiyoshi, M.; Takashi, T. High pressure organic chemistry. III. Diels-Alder reaction of thiophene with maleic anhydride. Bull. Chem. Soc. Jpn. 1979, 52, 544–548. [Google Scholar] [CrossRef] [Green Version]
- Kumamoto, K.; Fukada, I.; Kotsuki, H. Diels-Alder reaction of thiophene: Dramatic effects of high-pressure/solvent-free conditions. Angew. Chem. Int. Ed. Engl. 2004, 43, 2015–2017. [Google Scholar] [CrossRef]
- Mishra, R.; Kumar, N.; Mishra, I.; Sachan, N. A review on anticancer activities of thiophene and its analogs. Mini Rev. Med. Chem. 2020, 20, 1944–1965. [Google Scholar] [CrossRef]
- Li, Z.R.; Ma, T.; Guo, Y.J.; Hu, B.; Niu, S.H.; Suo, F.Z.; Du, L.N.; You, Y.H.; Kang, W.T.; Liu, S.; et al. Sanggenon O induced apoptosis of A549 cells is counterbalanced by protective autophagy. Bioorg. Chem. 2019, 87, 688–698. [Google Scholar] [CrossRef]
- Shu, Y.H.; Yuan, H.H.; Xu, M.T.; Hong, Y.T.; Gao, C.C.; Wu, Z.P.; Han, H.T.; Sun, X.; Gao, R.L.; Yang, S.F.; et al. A novel Diels-Alder adduct of mulberry leaves exerts anticancer effect through autophagy-mediated cell death. Acta Pharmacol. Sin. 2021, 42, 780–790. [Google Scholar] [CrossRef]
- McCluskey, A.; Keane, M.A.; Walkom, C.C.; Bowyer, M.C.; Sim, A.T.; Young, D.J.; Sakoff, J.A. The first two cantharidin analogues displaying PP1 selectivity. Bioorg. Med. Chem. Lett. 2002, 12, 391–393. [Google Scholar] [CrossRef]
- Fringuelli, F.; Girotti, R.; Pizzo, F.; Vaccaro, L. [AlCl3 + 2THF]: A new and efficient catalytic system for Diels-Alder cycloaddition of alpha,beta-unsaturated carbonyl compounds under solvent-free conditions. Org. Lett. 2006, 8, 2487–2489. [Google Scholar] [CrossRef] [PubMed]
- Burford, R.J.; Li, B.; Vasiliu, M.; Dixon, D.A.; Liu, S.Y. Diels-Alder reactions of 1,2-azaborines. Angew. Chem. Int. Ed. Engl. 2015, 54, 7823–7827. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nakano, R.; Hirokane, T.; Yoshida, M. Synthesis of bridgehead-functionalized triptycene quinones via Lewis acid–promoted Diels-Alder reaction of 9-acyloxyanthracenes. Tetrahedron Lett. 2019, 60, 975–978. [Google Scholar] [CrossRef]
- Harvey, S.C. Maleimide as a dienophile. JACS 1949, 71, 1121–1122. [Google Scholar] [CrossRef]
- Bouacha, S.; Nacereddine, A.K.; Djerourou, A. A theoretical study of the mechanism, stereoselectivity and Lewis acid catalyst on the Diels–Alder cycloaddition between furan and activated alkenes. Tetrahedron Lett. 2013, 54, 4030–4033. [Google Scholar] [CrossRef]
- Fringuelli, F.; Minuti, L.; Pizzo, F.; Taticchi, A. Reactivity and selectivity of Lewis-acid-catalyzed Diels-Alder reactions of 2-cyclohexenones. Acta Chem. Scand. 1993, 47, 255–263. [Google Scholar] [CrossRef]
- An, Y.-L.; Shao, Z.-Y.; Cheng, J.; Zhao, S.-Y. Highly efficient aluminum trichloride catalyzed michael addition of indoles and pyrroles to maleimides. Synthesis 2013, 45, 2719–2726. [Google Scholar] [CrossRef]
- Mandal, A.; Sahoo, H.; Dana, S.; Baidya, M. Ruthenium(II)-catalyzed hydroarylation of haleimides using carboxylic acids as a traceless directing group. Org. Lett. 2017, 19, 4138–4141. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, S.; De, U.; Mishra, N.K.; Park, J.; Sharma, S.; Kwak, J.H.; Han, S.; Kim, H.S.; Kim, I.S. Synthesis of succinimide-containing chromones, naphthoquinones, and xanthones under Rh(III) catalysis: Evaluation of anticancer activity. J. Org. Chem. 2016, 81, 12416–12425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Han, S.; Tang, M.; Ackermann, L.; Li, J. C-H alkylations of (hetero)arenes by maleimides and maleate esters through cobalt(III) catalysis. Org. Lett. 2017, 19, 3315–3318. [Google Scholar] [CrossRef]
- Turk, S.D.; Cobb, R.L. Chapter 2-Formation of five-membered cyclic sulfones. In 1,4-Cycloaddition Reactions. The Diels-Alder Reaction in Heterocyclic Syntheses; Hamer, J.A.N., Ed.; Elsevier: Amsterdam, The Netherlands, 1967; Volume 8, pp. 13–45. [Google Scholar]
- Desai, D.; Salli, U.; Vrana, K.E.; Amin, S. SelSA, selenium analogs of SAHA as potent histone deacetylase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 2044–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karelia, N.; Desai, D.; Hengst, J.A.; Amin, S.; Rudrabhatla, S.V.; Yun, J. Selenium-containing analogs of SAHA induce cytotoxicity in lung cancer cells. Bioorg. Med. Chem. Lett. 2010, 20, 6816–6819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plano, D.; Karelia, D.N.; Pandey, M.K.; Spallholz, J.E.; Amin, S.; Sharma, A.K. Design, synthesis, and biological evaluation of novel selenium (Se-NSAID) molecules as anticancer agents. J. Med. Chem. 2016, 59, 1946–1959. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef]
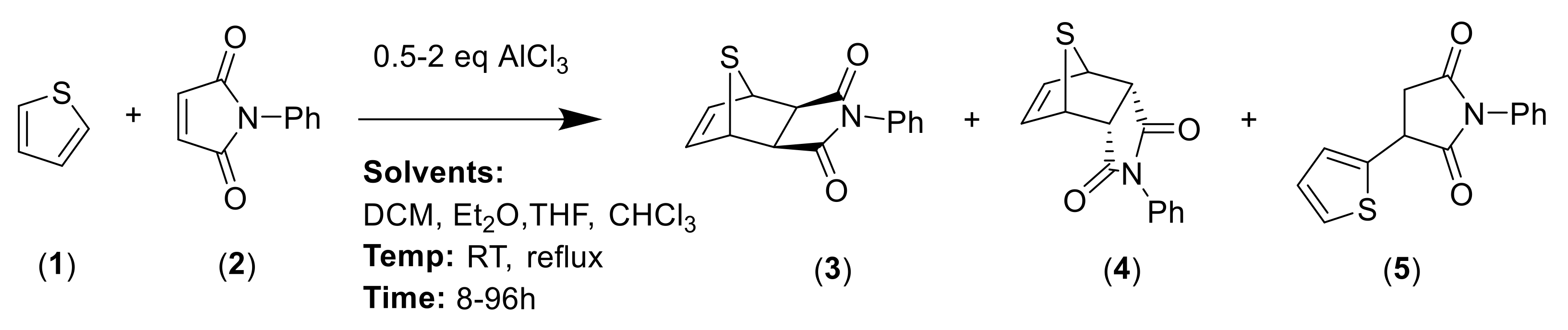

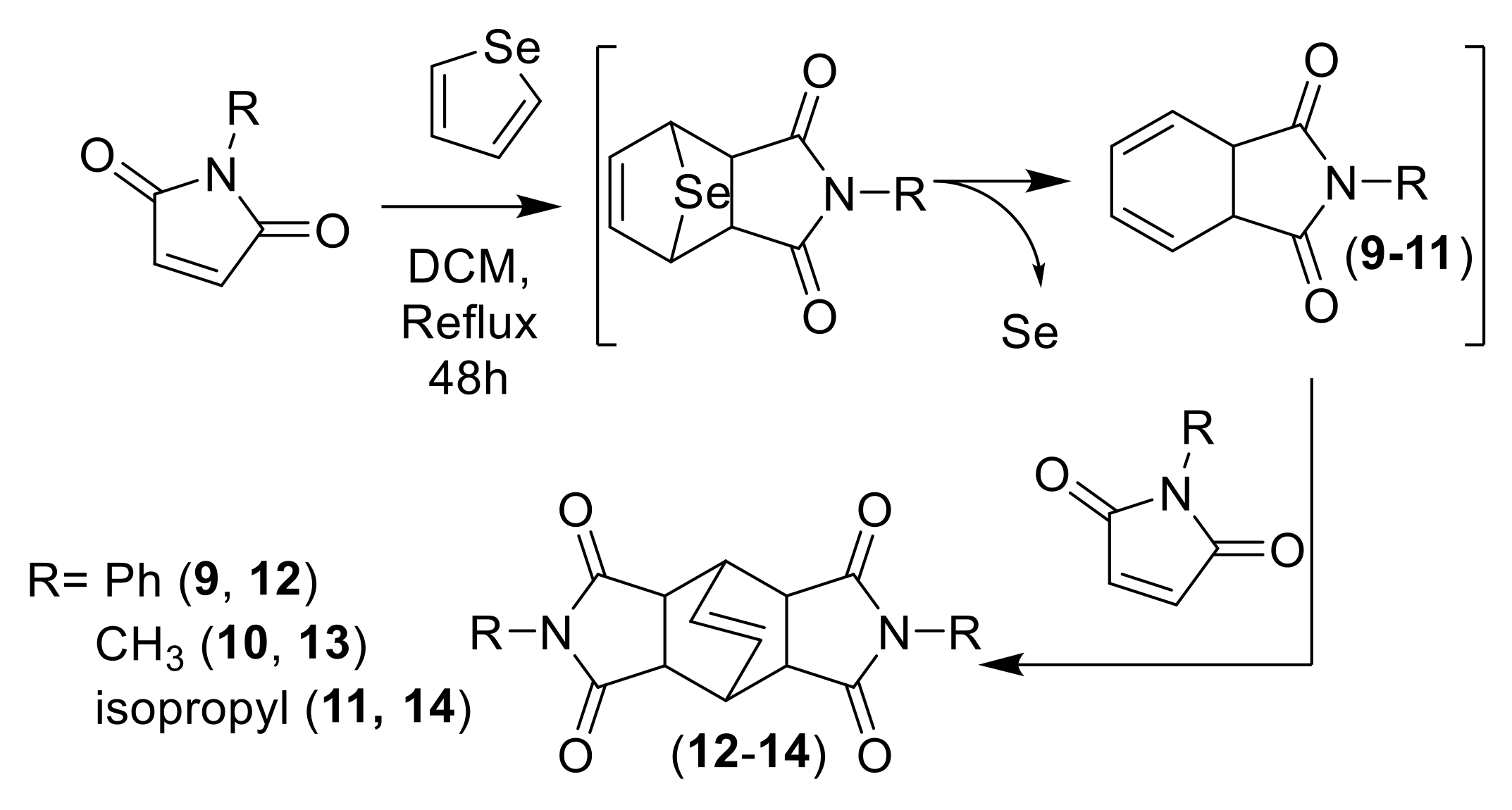
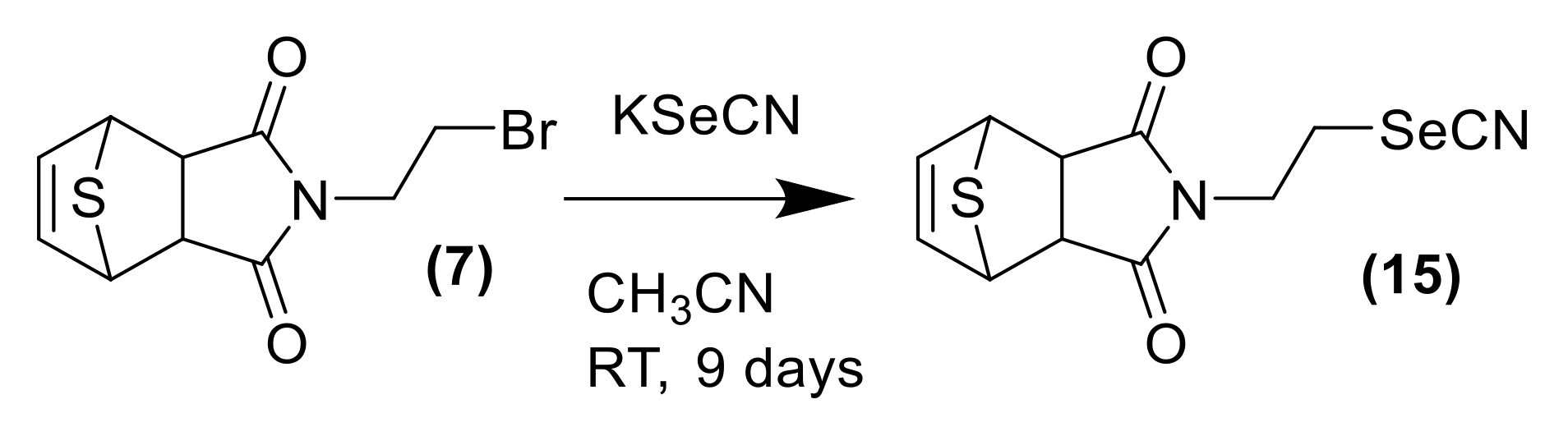

| Entry | Solvent | (1):(2) | eq AlCl3 | Temp | Time (h) | Yield (%) 1 (3) | Yield (%) 1 (4) | (3):(4) | Yield (%) 1 (5) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | DCM | 1:1 | 0.5 | RT | 24 | 4.4 | 1.1 | 78:22 | - |
| 2 | DCM | 1:1 | 1 | RT | 24 | 10.5 | 2.4 | 82:18 | 2.0 |
| 3 | DCM | 1:1 | 2 | RT | 24 | 11.8 | 0.9 | 94:6 | 21.7 |
| 4 | DCM | 5:1 | 0.5 | RT | 24 | 11.0 | 2.7 | 80:20 | 2.7 |
| 5 | DCM | 5:1 | 1 | RT | 24 | 25.9 | 7.4 | 77:23 | 4.6 |
| 6 | DCM | 5:1 | 2 | RT | 24 | - | - | - | 31.0 |
| 7 | DCM | 5:1 | 1 | RT | 48 | 33.4 | 6.8 | 83:17 | 4.8 |
| 8 | DCM | 5:1 | 1 | RT | 72 | 37.3 | 3.9 | 90:10 | 11.6 |
| 9 | DCM | 5:1 | 1 | RT | 96 | 47.2 | 4.9 | 91:9 | 8.8 |
| 10 | DCM | 5:1 | 1 | Reflux | 8 | 15.4 | 2.1 | 87:13 | 2.1 |
| 11 | DCM | 5:1 | 1 | Reflux | 24 | 38.8 | 3.0 | 93:7 | 6.7 |
| 12 | DCM | 5:1 | 1 | Reflux | 48 | 8.2 | 0.6 | 93:7 | - |
| 13 | THF | 5:1 | 1 | RT | 24 | - | - | - | - |
| 14 | Et2O | 5:1 | 1 | RT | 24 | - | - | - | - |
| 15 | CHCl3 | 5:1 | 1 | RT | 24 | 8 | 3.2 | 72:28 | 12.1 |
| 16 | DCM | 1:1 | 0.5 | RT | 24 | 4.4 | 1.1 | 78:22 | - |
| 17 | DCM | 1:1 | 1 | RT | 24 | 10.5 | 2.4 | 82:18 | 2.0 |
| 18 | DCM | 1:1 | 2 | RT | 24 | 11.8 | 0.9 | 94:6 | 21.7 |
| Entry | Lewis Acid | Yield (%) 1 3 | Yield (%) 1 4 | (3):(4) | Yield (%) 1 5 |
|---|---|---|---|---|---|
| 5 | AlCl3 | 25.9 | 7.4 | 77:23 | 4.6 |
| 16 | Me2AlCl | 0.05 | 0.2 | 66:34 | 0 |
| 17 | FeCl3 | 0 | 0 | - | 0 |
| 18 | SnCl4 | 0 | 0 | - | 0 |
| 19 | TiCl4 | 0.019 | 0.002 | 81:19 | 0 |
| 20 | BF3·H20 | 0 | 0 | - | 0 |
| Dienophile | Adduct | Endo:exo a | Yield (%) exo a | Yield (%) endo a | Isolated Yield (%) exo |
|---|---|---|---|---|---|
 | No | n.d. | n.d. | n.d. | n.d. |
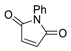 |  | 9:91 | 50 | 4.9 | 33 |
 | 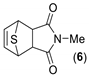 | 31:69 | 32 | 15 | 25 |
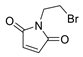 | 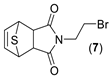 | 10:90 | 42 | 3.8 | 36 |
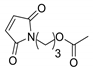 |  | 19:81 | 10 | 2.4 | 7.1 |
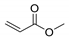 | No | n.d. | n.d. | n.d. | n.d. |
 | No | n.d. | n.d. | n.d. | n.d. |
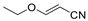 | No | n.d. | n.d. | n.d. | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Martín, G.; Plano, D.; Sanmartín, C. New Experimental Conditions for Diels–Alder and Friedel-Crafts Alquilation Reactions with Thiophene: A New Selenocyanate with Potent Activity against Cancer. Molecules 2022, 27, 982. https://doi.org/10.3390/molecules27030982
Calvo-Martín G, Plano D, Sanmartín C. New Experimental Conditions for Diels–Alder and Friedel-Crafts Alquilation Reactions with Thiophene: A New Selenocyanate with Potent Activity against Cancer. Molecules. 2022; 27(3):982. https://doi.org/10.3390/molecules27030982
Chicago/Turabian StyleCalvo-Martín, Gorka, Daniel Plano, and Carmen Sanmartín. 2022. "New Experimental Conditions for Diels–Alder and Friedel-Crafts Alquilation Reactions with Thiophene: A New Selenocyanate with Potent Activity against Cancer" Molecules 27, no. 3: 982. https://doi.org/10.3390/molecules27030982
APA StyleCalvo-Martín, G., Plano, D., & Sanmartín, C. (2022). New Experimental Conditions for Diels–Alder and Friedel-Crafts Alquilation Reactions with Thiophene: A New Selenocyanate with Potent Activity against Cancer. Molecules, 27(3), 982. https://doi.org/10.3390/molecules27030982









