Effect of Yellowing Duration on the Chemical Profile of Yellow Tea and the Associations with Sensory Traits
Abstract
1. Introduction
2. Results
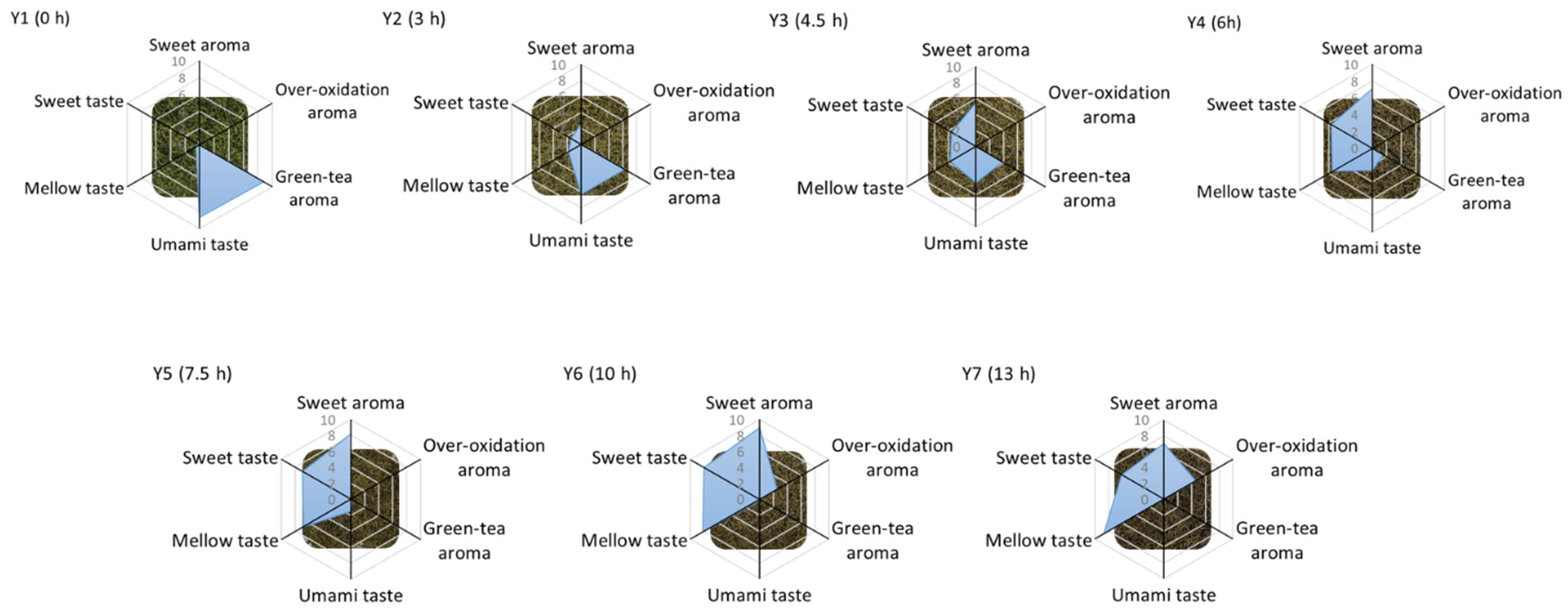
2.1. Sensory Analysis of Yellow Tea
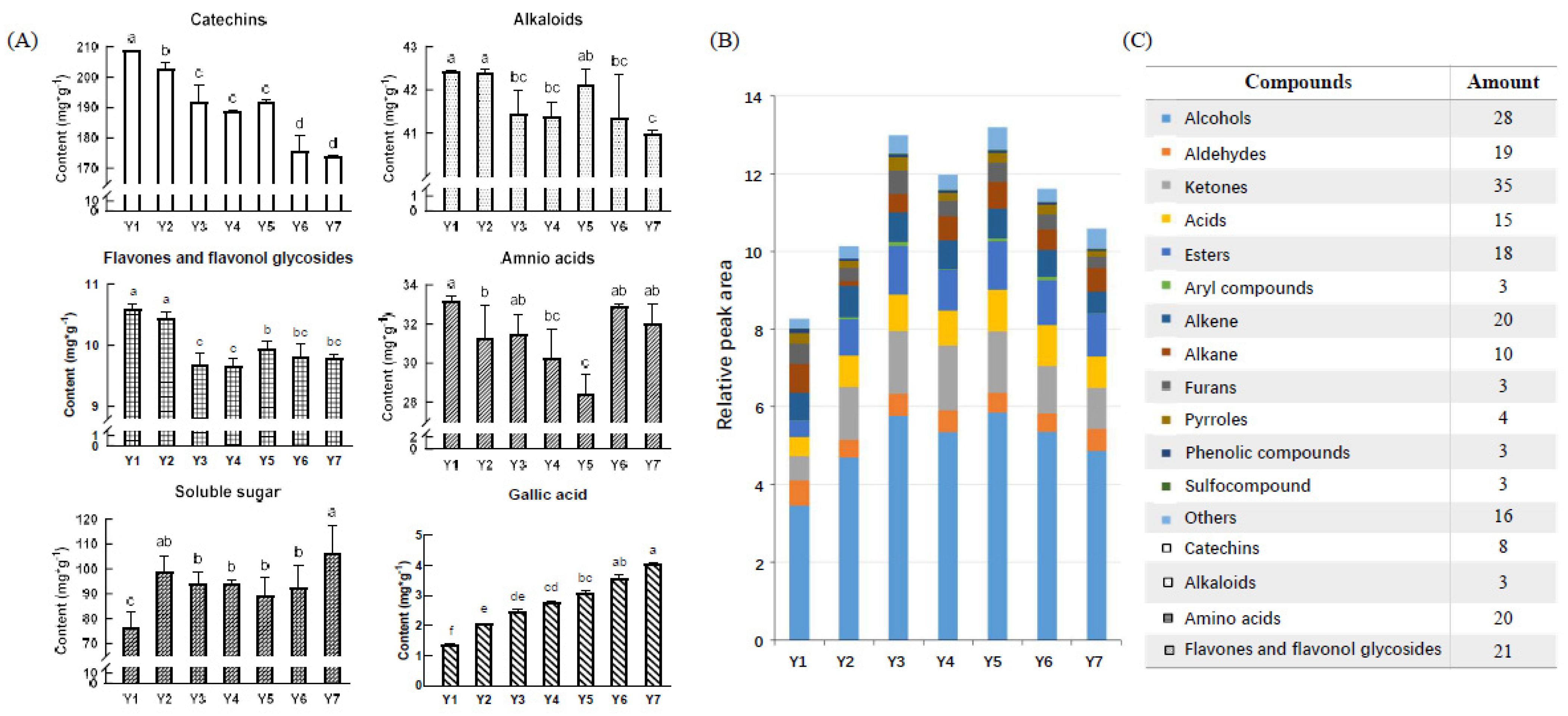
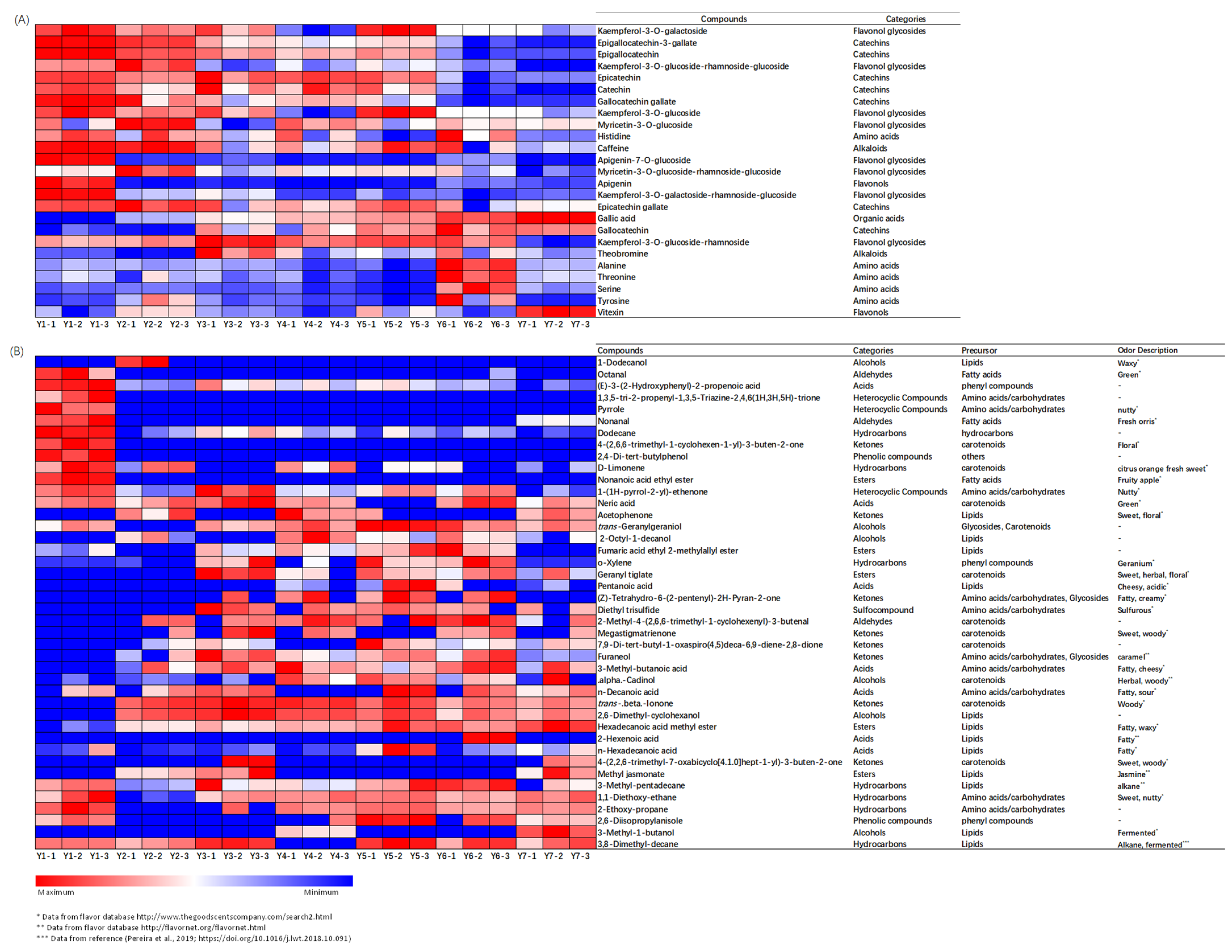
2.2. Chemical Changes of Flavor Compounds under Yellowing
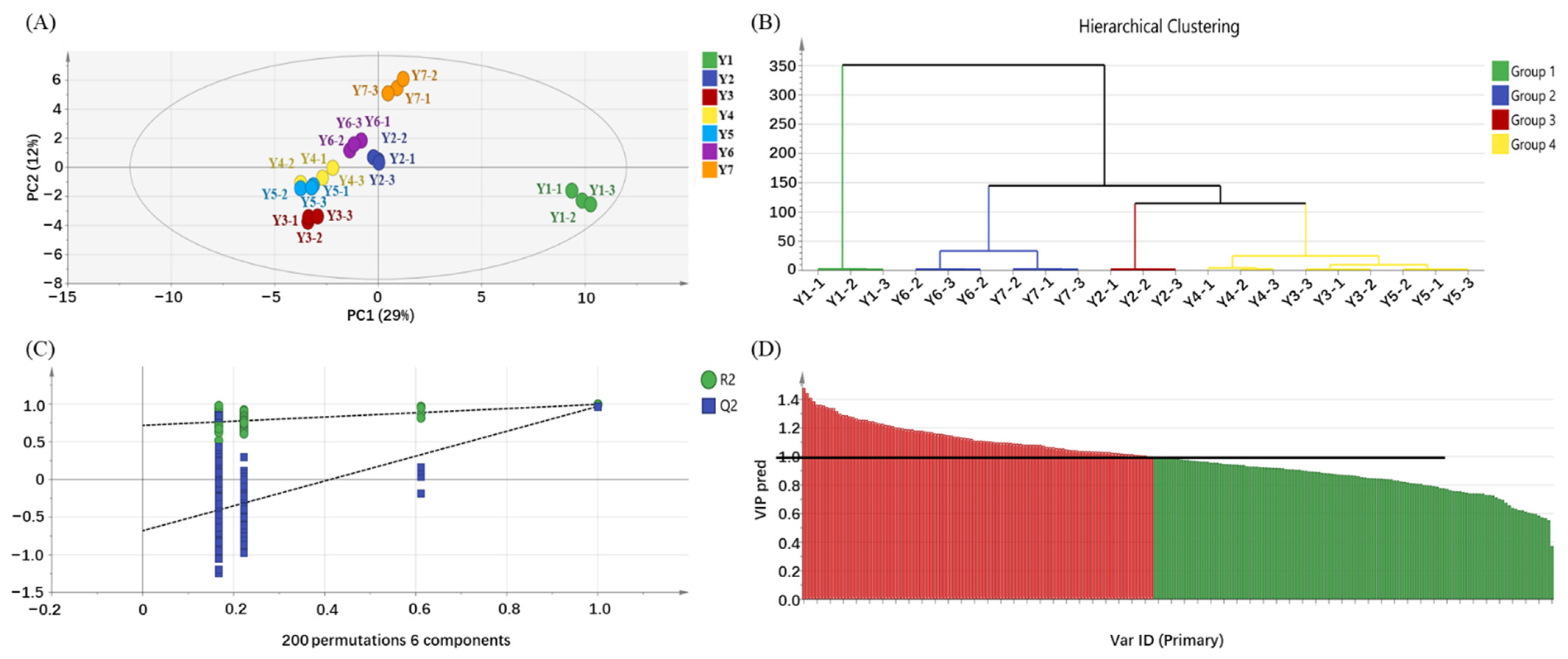
2.3. OPLS-DA of Flavor Compounds in the Yellowing Process
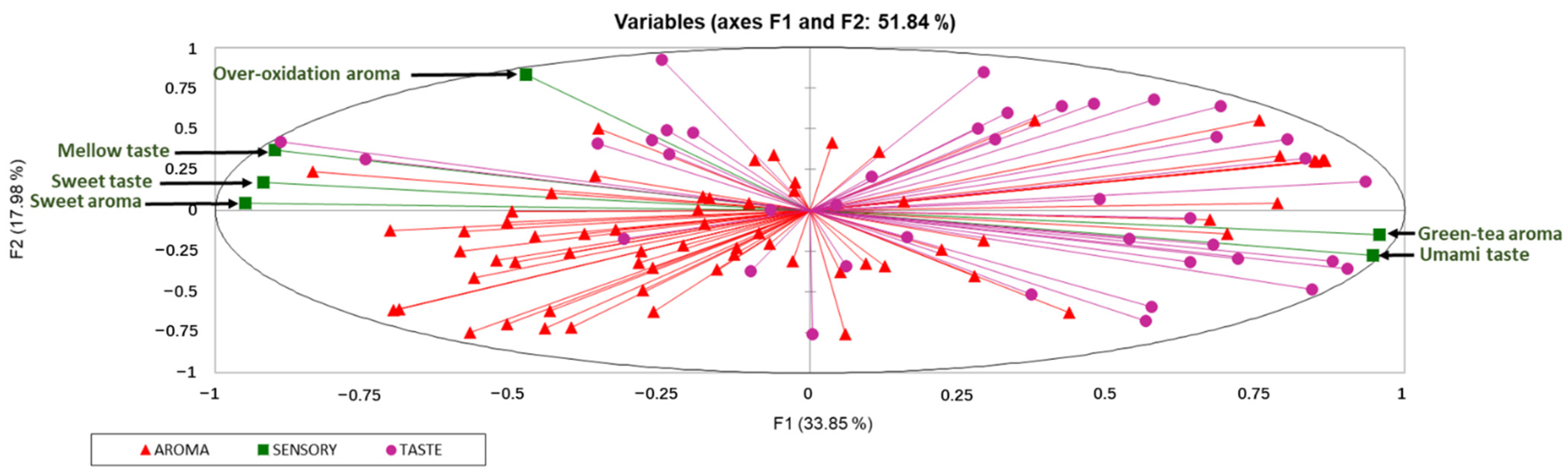
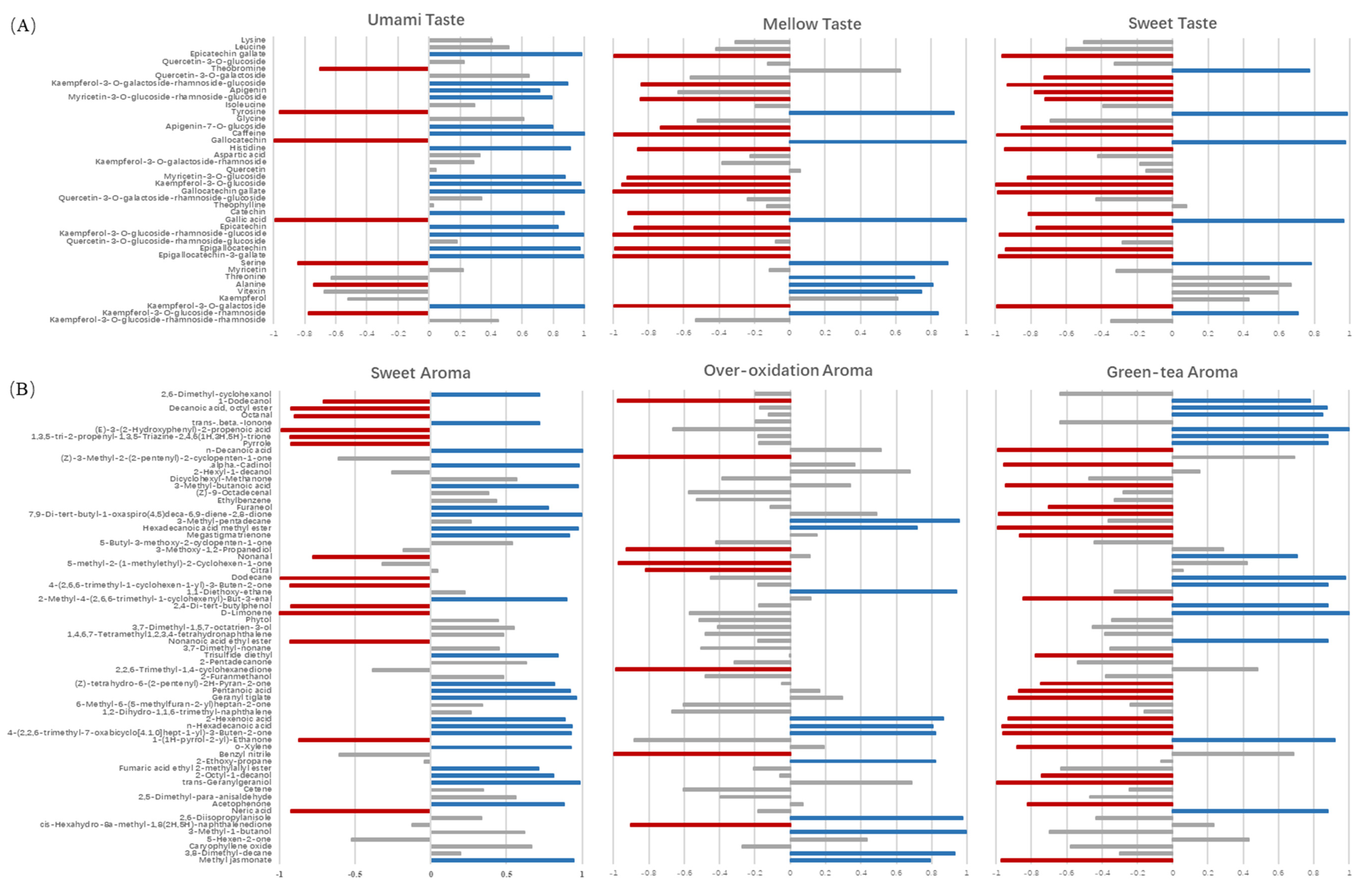
2.4. Association between Key Chemical Contributors and Sensory Traits
3. Discussion
4. Materials and Methods
4.1. Preparation of Yellow Tea Samples
4.2. Reagents
4.3. Sensory Evaluation
4.4. Determination of Non-Volatile Compounds
4.5. Determination of Volatiles
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xu, J.; Wang, M.; Zhao, J.; Wang, Y.-H.; Tang, Q.; Khan, I.A. Yellow tea (Camellia sinensis L.), a promising Chinese tea: Processing, chemical constituents and health benefits. Food Res. Int. 2018, 107, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.Y.; Tang, G.Z.; Gong, S.Y.; Zong, B.Z.; Guo, H.W.; Li, C.L. Typical Yellow Tea Taste Characteristic and Its Related Taste-Chemical Compositions. Sci. Agric. Sin. 2020, 53, 371–382. Available online: https://www.chinaagrisci.com/CN/10.3864/j.issn.0578-1752.2020.02.011 (accessed on 25 January 2022).
- Wang, Z.-H.; Yue, C.-N.; Tong, H.-R. Analysis of taste characteristics and identification of key chemical components of fifteen Chinese yellow tea samples. J. Food Sci. Technol. 2021, 58, 1378–1388. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, M.; Dong, Z.; Zhu, Y.; Shi, J.; Ma, W.; Lin, Z.; Lv, H. Volatile components and key odorants of Chinese yellow tea (Camellia sinensis L.). LWT 2021, 146, 111512. [Google Scholar] [CrossRef]
- Wei, Y.; Fang, S.; Jin, G.; Ni, T.; Hou, Z.; Li, T.; Deng, W.; Ning, J. Effects of two yellowing process on colour, taste and nonvolatile compounds of bud yellow tea. Int. J. Food Sci. Technol. 2020, 55, 2931–2941. [Google Scholar] [CrossRef]
- Wei, Y.; Li, T.; Xu, S.; Ni, T.; Deng, W.-W.; Ning, J. The profile of dynamic changes in yellow tea quality and chemical composition during yellowing process. LWT 2020, 139, 110792. [Google Scholar] [CrossRef]
- Ye, M.J.; Zhou, W.L.; Xu, J.F. Preliminary study on the determination and distribution of theaflavins content in different types of tea. Farm Prod. Proc. 2015, 2, 49–53. [Google Scholar] [CrossRef]
- Kujawska, M.; Ewertowska, M.; Ignatowicz, E.; Adamska, T.; Szaefer, H.; Gramza-Michałowska, A.; Korczak, J.; Jodynis-Liebert, J. Evaluation of Safety and Antioxidant Activity of Yellow Tea (Camellia sinensis L.) Extract for Application in Food. J. Med. Food 2016, 19, 330–336. [Google Scholar] [CrossRef]
- Fan, F.Y.; Yang, X.L.; Gong, S.Y.; Guo, H.W.; Li, C.L.; Qian, H.; Hu, J.P. The effect of technological factors on Yellow tea qual-ity and taste-chemical constituents in the yellowing process. J. Tea Sci. 2019, 39, 63–73. Available online: http://www.tea-science.com/CN/10.13305/j.cnki.jts.2019.01.007 (accessed on 25 January 2022).
- Gibson, M.; Newsham, P. Taste, Flavor and Aroma. In Food Science and the Culinary Arts; Elsevier: Amsterdam, The Netherlands, 2018; pp. 35–52. [Google Scholar] [CrossRef]
- Wang, H.; Hua, J.; Jiang, Y.; Yang, Y.; Wang, J.; Yuan, H. Influence of fixation methods on the chestnut-like aroma of green tea and dynamics of key aroma substances. Food Res. Int. 2020, 136, 109479. [Google Scholar] [CrossRef]
- Ye, N.X.; Guang, Y.; Zheng, N.H.; Yang, J.F.; Wang, Z.K.; Liang, X.X. Analysis of the aroma of refined baked green tea using SPME/GC-MS. J. Fujian Agric. For. Univ. 2006, 2, 165–168. [Google Scholar] [CrossRef]
- Xia, J.; Guo, Z.; Fang, S.; Gu, J.; Liang, X. Effect of Drying Methods on Volatile Compounds of Burdock (Arctium lappa L.) Root Tea as Revealed by Gas Chromatography Mass Spectrometry-Based Metabolomics. Foods 2021, 10, 868. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.-Y.; Shi, M.; Nie, Y.; Zhao, Y.; Ye, J.-H.; Liang, Y.-R. Differential behaviors of tea catechins under thermal processing: Formation of non-enzymatic oligomers. Food Chem. 2016, 196, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Q.-Q.; Granato, D.; Xu, Y.-Q.; Ho, C.-T. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Zhu, Y.-M.; Dong, J.-J.; Jin, J.; Liu, J.-H.; Zheng, X.-Q.; Lu, J.-L.; Liang, Y.-R.; Ye, J.-H. Roasting process shaping the chemical profile of roasted green tea and the association with aroma features. Food Chem. 2021, 353, 129428. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-T.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea aroma formation from six model manufacturing processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef]
- Bosse, R.; Wirth, M.; Weiss, J.; Gibis, M. Effect of storage temperature on volatile marker compounds in cured loins fermented with Staphylococcus carnosus by brine injection. Eur. Food Res. Technol. 2021, 247, 233–244. [Google Scholar] [CrossRef]
- Vollmer, D.M.; Lafontaine, S.R.; Shellhammer, T.H. Aroma Extract Dilution Analysis of Beers Dry-Hopped with Cascade, Chinook, and Centennial. J. Am. Soc. Brew. Chem. 2018, 76, 190–198. [Google Scholar] [CrossRef]
- Zhu, L.-X.; Zhang, M.-M.; Liu, Z.; Shi, Y.; Duan, C.-Q. Levels of Furaneol in Msalais Wines: A Comprehensive Overview of Multiple Stages and Pathways of Its Formation during Msalais Winemaking. Molecules 2019, 24, 3104. [Google Scholar] [CrossRef]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, Q.; Zhuang, J.; Feng, T.; Ho, C.-T.; Song, S. Characterization of Aroma-Active Compounds in Four Yeast Extracts Using Instrumental and Sensory Techniques. J. Agric. Food Chem. 2020, 68, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, F.; Dimauro, C.; Bozzi, R.; Aquilani, C.; Franci, O.; Calamai, L.; Pezzati, A.; Pugliese, C. Evolution of volatile compounds and physical, chemical and sensory characteristics of Toscano PDO ham from fresh to dry-cured product. Z. Lebensm.-Unters. Forsch. 2019, 246, 409–424. [Google Scholar] [CrossRef]
- Wang, C.; Lv, S.; Wu, Y.; Gao, X.; Li, J.; Zhang, W.; Meng, Q. Oolong tea made from tea plants from different locations in Yunnan and Fujian, China showed similar aroma but different taste characteristics. SpringerPlus 2016, 5, 576. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Kubota, K.; Kobayashi, A.; Juan, I.-M. Analysis of Glycosidically Bound Aroma Precursors in Tea Leaves. 3. Change in the Glycoside Content of Tea Leaves during the Oolong Tea Manufacturing Process. J. Agric. Food Chem. 2001, 49, 5391–5396. [Google Scholar] [CrossRef] [PubMed]
- Achir, N.; Pénicaud, C.; Bechoff, A.; Boulanger, R.; Dornier, M.; Dhuique-Mayer, C. Use of multi-response modelling to in-vestigate mechanisms of β-carotene degradation in dried orange-fleshed sweet potato during storage: From carotenoids to aroma compounds. Food Bioprocess Technol. 2014, 7, 1656–1669. [Google Scholar] [CrossRef]
- Cheng, S.-S.; Lin, C.-Y.; Yang, C.-K.; Chen, Y.-J.; Chung, M.-J.; Chang, S.-T. Chemical Polymorphism and Composition of Leaf Essential Oils of Cinnamomum kanehirae Using Gas Chromatography/Mass Spectrometry, Cluster Analysis, and Principal Component Analysis. J. Wood Chem. Technol. 2015, 35, 207–219. [Google Scholar] [CrossRef]
- Slaghenaufi, D.; Perello, M.-C.; Marchand, S.; de Revel, G. Quantification of megastigmatrienone, a potential contributor to tobacco aroma in spirits. Food Chem. 2016, 203, 41–48. [Google Scholar] [CrossRef]
- Karunagoda, R. Analysis of prenyllipids in the chloroplasts of the liverwort Heteroscyphus planus. Ceylon J. Sci. 2011, 39, 121. [Google Scholar] [CrossRef][Green Version]
- Zhao, L.; Deng, S.P.; Liu, W. Food Sensory Analysis of Vocabulary, 1st ed.; China Light Industry Press: Beijing, China, 2015. [Google Scholar]
- Lee, J.; Chambers, D.H. A Lexicon for Flavor Descriptive Analysis of Green Tea. J. Sens. Stud. 2007, 22, 256–272. [Google Scholar] [CrossRef]
- Di Serio, M.G.; Di Giacinto, L.; Di Loreto, G.; Giansante, L.; Pellegrino, M.; Vito, R.; Perri, E. Chemical and sensory characteristics of Italian virgin olive oils from Grossa di Gerace cv. Eur. J. Lipid Sci. Technol. 2015, 118, 288–298. [Google Scholar] [CrossRef]
- Li, C.; Zong, B.; Guo, H.; Luo, Z.; He, P.; Gong, S.; Fan, F. Discrimination of white teas produced from fresh leaves with different maturity by near-infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117697. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.-T.; Song, C.-J.; Xu, H.-R.; Ye, J.-H. Dynamic changes in flavonol glycosides during production of green, yellow, white, oolong and black teas from Camellia sinensis L. (cv. Fudingdabaicha). Int. J. Food Sci. Technol. 2018, 54, 490–498. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, Y.; Dai, L.-L.; Zhang, D.-L.; Li, J.-H.; Yuan, W.-X.; Li, Y.-L.; Zhou, H.-J. A High-Performance Liquid Chromatographic Method for Simultaneous Determination of 21 Free Amino Acids in Tea. Food Anal. Methods 2012, 6, 69–75. [Google Scholar] [CrossRef]
- Haldar, D.; Sen, D.; Gayen, K. Development of Spectrophotometric Method for the Analysis of Multi-component Carbohydrate Mixture of Different Moieties. Appl. Biochem. Biotechnol. 2017, 181, 1416–1434. [Google Scholar] [CrossRef]
- Liu, W.Q.; Zhang, Y.B.; Gong, S.Y.; Chen, P.; Fan, F.Y.; Yang, M.X.; Gu, Z.Q. Effects of different fixatives on fidelity and slow-release of Huangjingui tea essential oil aroma. J. Tea Sci. 2016, 36, 372–378. Available online: http://www.tea-science.com/CN/10.13305/j.cnki.jts.2016.04.005 (accessed on 25 January 2022).
- Ma, C.; Li, J.; Chen, W.; Wang, W.; Qi, D.; Pang, S.; Miao, A. Study of the aroma formation and transformation during the manufacturing process of oolong tea by solid-phase micro-extraction and gas chromatography–mass spectrometry combined with chemometrics. Food Res. Int. 2018, 108, 413–422. [Google Scholar] [CrossRef]
- Zhou, S.J.; Huang, C.S.; Li, C.L.; Gong, S.Y.; Guo, H.W.; Tong, Y.L.; Fan, F.Y. Aroma compositions and comparison of their related components of Longjing tea with different aroma types. J. Zhejiang Univ. 2021, 47, 203–211. Available online: http://www.zjujournals.com/agr/CN/Y2021/V47/I2/203 (accessed on 25 January 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, F.-Y.; Zhou, S.-J.; Qian, H.; Zong, B.-Z.; Huang, C.-S.; Zhu, R.-L.; Guo, H.-W.; Gong, S.-Y. Effect of Yellowing Duration on the Chemical Profile of Yellow Tea and the Associations with Sensory Traits. Molecules 2022, 27, 940. https://doi.org/10.3390/molecules27030940
Fan F-Y, Zhou S-J, Qian H, Zong B-Z, Huang C-S, Zhu R-L, Guo H-W, Gong S-Y. Effect of Yellowing Duration on the Chemical Profile of Yellow Tea and the Associations with Sensory Traits. Molecules. 2022; 27(3):940. https://doi.org/10.3390/molecules27030940
Chicago/Turabian StyleFan, Fang-Yuan, Sen-Jie Zhou, Hong Qian, Bang-Zheng Zong, Chuang-Sheng Huang, Ruo-Lan Zhu, Hao-Wei Guo, and Shu-Ying Gong. 2022. "Effect of Yellowing Duration on the Chemical Profile of Yellow Tea and the Associations with Sensory Traits" Molecules 27, no. 3: 940. https://doi.org/10.3390/molecules27030940
APA StyleFan, F.-Y., Zhou, S.-J., Qian, H., Zong, B.-Z., Huang, C.-S., Zhu, R.-L., Guo, H.-W., & Gong, S.-Y. (2022). Effect of Yellowing Duration on the Chemical Profile of Yellow Tea and the Associations with Sensory Traits. Molecules, 27(3), 940. https://doi.org/10.3390/molecules27030940




