Antioxidant and Antitumor Activities of Newly Synthesized Hesperetin Derivatives
Abstract
:1. Introduction
2. Results
2.1. Chemistry
2.2. Antioxidant Activities Research In Vitro
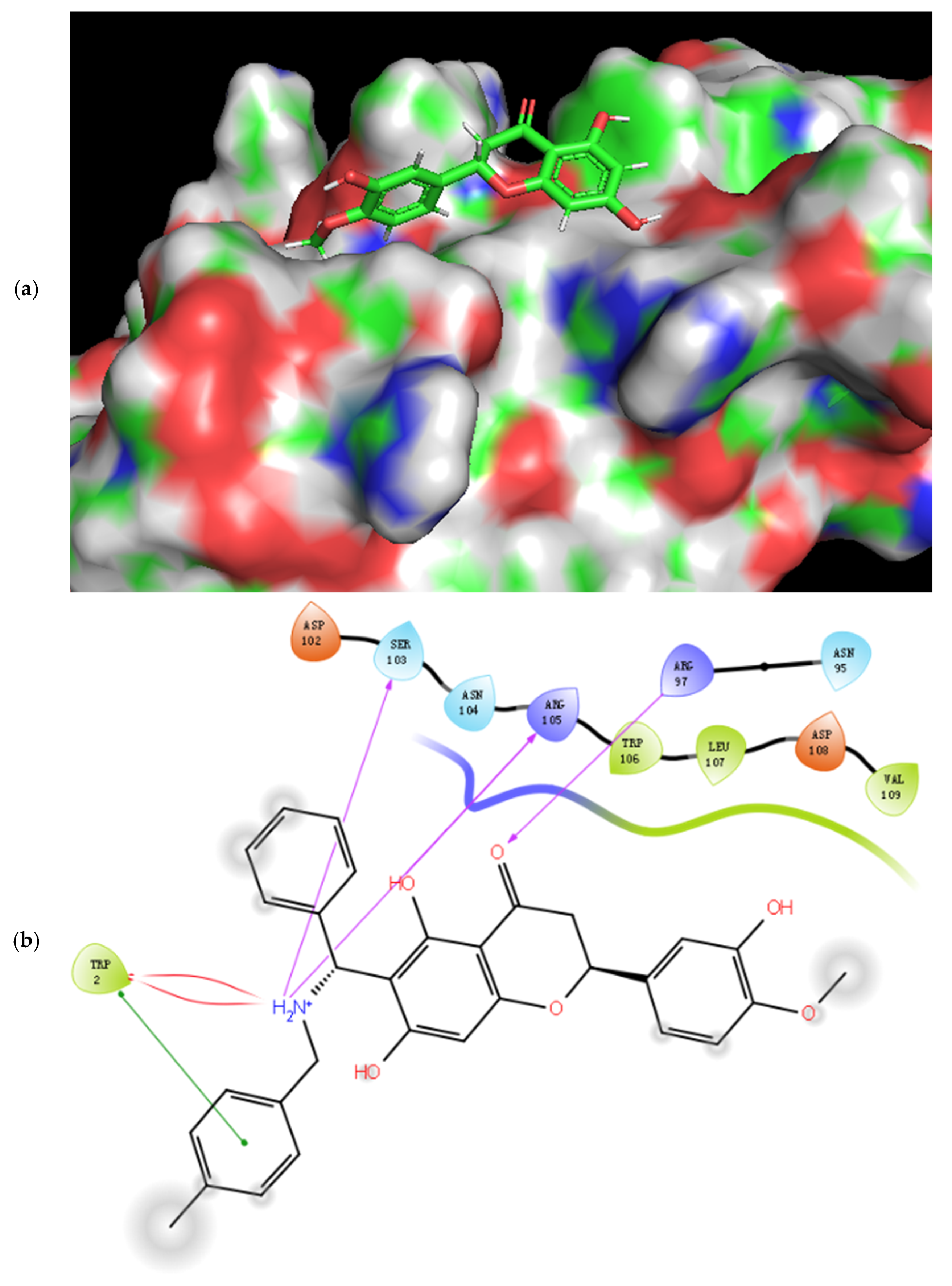
2.3. Molecular Docking
2.4. Antitumor Activity Research In Vitro
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. Synthesis of Hesperetin (2)
4.1.2. Synthesis of Hesperetin Derivatives (3a–3k)
4.1.3. Structure Characterization of Hesperetin and Hesperetin Derivatives (3a–3k)
4.2. Antitumor Activities
4.2.1. Cell Culture
4.2.2. MTT Assay
4.2.3. Molecular Docking
4.3. Free-Radical-Scavenging Capacity
4.3.1. DPPH Antioxidant Activity Assay
4.3.2. ABTS Antioxidant Activity Assay
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ferreira de Oliveira, J.M.P.; Santos, C.; Fernandes, E. Therapeutic potential of hesperidin and its aglycone hesperetin: Cell cycle regulation and apoptosis induction in cancer models. Phytomedicine 2020, 73, 152887. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lim, S.-B. Semi-Continuous Subcritical Water Extraction of Flavonoids from Citrus unshiu Peel: Their Antioxidant and Enzyme Inhibitory Activities. Antioxidants 2020, 9, 360. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.E.; Gálvez, J.; Cruz, T.; Ocete, M.A.; Zarzuelo, A. Anti-Inflammatory Activity of Diosmin and Hesperidin in Rat Colitis Induced by TNBS. Planta Med. 1999, 65, 651–653. [Google Scholar] [CrossRef]
- Wilmsen, P.K.; Spada, D.S.; Salvador, M. Antioxidant Activity of the Flavonoid Hesperidin in Chemical and Biological Systems. J. Agric. Food Chem. 2005, 53, 4757–4761. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- López-Pérez, M.; Ballester, A.-R.; González-Candelas, L. Identification and functional analysis of Penicillium digitatum genes putatively involved in virulence towards citrus fruit. Mol. Plant Pathol. 2015, 16, 262–275. [Google Scholar] [CrossRef] [Green Version]
- Tempesti, T.C.; Alvarez, M.G.; de Araújo, M.F.; Catunda Júnior, F.E.A.; de Carvalho, M.G.; Durantini, E.N. Antifungal activity of a novel quercetin derivative bearing a trifluoromethyl group on Candida albicans. Med. Chem. Res. 2012, 21, 2217–2222. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, M.J.; Ha, E.; Chung, J.H. Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells, SNU-C4. Phytomedicine 2008, 15, 147–151. [Google Scholar] [CrossRef]
- Jelica, V.; Katarina, N.; John, B.O.M. Rational Drug Design of Antineoplastic Agents Using 3D-QSAR, Cheminformatic, and Virtual Screening Approaches. Curr. Med. Chem. 2019, 26, 3874–3889. [Google Scholar]
- Elshazly, S.M.; Mahmoud, A.A.A. Antifibrotic activity of hesperidin against dimethylnitrosamine-induced liver fibrosis in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 559–567. [Google Scholar] [CrossRef]
- Yeh, C.-C.; Kao, S.-J.; Lin, C.-C.; Wang, S.-D.; Liu, C.-J.; Kao, S.-T. The immunomodulation of endotoxin-induced acute lung injury by hesperidin in vivo and in vitro. Life Sci. 2007, 80, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Nectoux, A.M.; Abe, C.; Huang, S.-W.; Ohno, N.; Tabata, J.; Miyata, Y.; Tanaka, K.; Tanaka, T.; Yamamura, H.; Matsui, T. Absorption and Metabolic Behavior of Hesperidin (Rutinosylated Hesperetin) after Single Oral Administration to Sprague-Dawley Rats. J. Agric. Food Chem. 2019, 67, 9812–9819. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Jokura, H.; Hashizume, K.; Ominami, H.; Shibuya, Y.; Suzuki, A.; Hase, T.; Shimotoyodome, A. Hesperidin metabolite hesperetin-7-O-glucuronide, but not hesperetin-3′-O-glucuronide, exerts hypotensive, vasodilatory, and anti-inflammatory activities. Food Funct. 2013, 4, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Ling, X.; Chen, Y.; Wu, X.; Zhao, Z.; Wang, W.; Wang, S.; Lai, G.; Yu, Z. Hesperetin reverses P-glycoprotein-mediated cisplatin resistance in DDP-resistant human lung cancer cells via modulation of the nuclear factor-κB signaling pathway. Int. J. Mol. Med. 2020, 45, 1213–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Li, J.; Hu, X.; Ma, J.; Dong, W. Hesperetin inhibits Eca-109 cell proliferation and invasion by suppressing the PI3K/AKT signaling pathway and synergistically enhances the anti-tumor effect of 5-fluorouracil on esophageal cancer in vitro and in vivo. RSC Adv. 2018, 8, 24434–24443. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Zhang, J.; Wang, J.; Li, J.; Liao, F.; Dong, W. Hesperetin induces apoptosis of esophageal cancer cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species. Tumor Biol. 2016, 37, 3451–3459. [Google Scholar] [CrossRef]
- Li, R.; Cai, L.; Xie, X.-f.; Peng, L.; Li, J. 7,3′-dimethoxy hesperetin induces apoptosis of fibroblast-like synoviocytes in rats with adjuvant arthritis through caspase 3 activation. Phytother. Res. 2010, 24, 1850–1856. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Jung, K.-Y.; Park, J.; Han, Y.-S.; Lee, Y.H.; Shin, S.Y.; Lim, Y. Synthesis and biological evaluation of hesperetin derivatives as agents inducing apoptosis. Bioorganic Med. Chem. 2017, 25, 397–407. [Google Scholar] [CrossRef]
- Roman, G. Mannich bases in medicinal chemistry and drug design. Eur. J. Med. Chem. 2015, 89, 743–816. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Frederick, M.O.; Aversa, R.J. The Continuing Saga of the Marine Polyether Biotoxins. Angew. Chem. Int. Ed. 2008, 47, 7182–7225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Hu, T.-S.; Zhu, J.; Wu, H.; Yao, Z.-J. Application of a Regioselective Mannich Reaction on Naringenin and its Use in Fluorescent Labeling. Synlett 2006, 2006, 1225–1229. [Google Scholar]
- Zhou, X.; Qin, D.; Xiang, B.; Xi, J. Cyclodextrin-based liquid-phase pulsed discharge extraction of flavonoids from tangerine (Citrus reticulata) pericarp: Optimization, Antioxidant activity and storage stability. Sep. Purif. Technol. 2021, 278, 119603. [Google Scholar] [CrossRef]
- Al-Saman, M.A.; Abdella, A.; Mazrou, K.E.; Tayel, A.A.; Irmak, S. Antimicrobial and Antioxidant activities of different extracts of the peel of kumquat (Citrus japonica Thunb). J. Food Meas. Charact. 2019, 13, 3221–3229. [Google Scholar] [CrossRef]
- Jaganjac, M.; Sredoja Tisma, V.; Zarkovic, N. Short Overview of Some Assays for the Measurement of Antioxidant Activity of Natural Products and Their Relevance in Dermatology. Molecules 2021, 26, 5301. [Google Scholar] [CrossRef]
- Ling, W.; Dai, T.; Zhang, J.; Liang, Y.; Yin, W.; Zhong, B.; Zhang, J. Evaluation of Pomelo Seed Extracts as Natural Antioxidant, Antibacterial, Herbicidal Agents, and Their Functional Components. Chem. Biodivers. 2021, 18, e2100679. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Zapien, D.; Ruiz, F.X.; Poirson, J.; Mitschler, A.; Ramirez, J.; Forster, A.; Cousido-Siah, A.; Masson, M.; Pol, S.V.; Podjarny, A.; et al. Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53. Nature 2016, 529, 541–545. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.-G.; Hu, Q.-P.; Liu, Y. Antioxidant and DNA-Protective Activities of Chlorogenic Acid Isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]

| Samples | DPPH IC50 μM | ABTS IC50 μM |
|---|---|---|
| 2 | 70 ± 1 | 276 ± 5 |
| 3a | 16 ± 1 | 105 ± 2 |
| 3b | 16 ± 1 | 104 ± 2 |
| 3c | 22 ± 1 | 114 ± 2 |
| 3d | 26 ± 0. | 161 ± 3 |
| 3e | 1.7 ± 0 | 45 ± 1 |
| 3f | 1.2 ± 0 | 24 ± 0 |
| 3g | 11 ± 0 | 64 ± 0 |
| 3h | 10 ± 0 | 70 ± 1 |
| 3i | 10 ± 0 | 62 ± 0 |
| 3j | 26 ± 0 | 224 ± 3 |
| 3k | 1.2 ± 0 | 27 ± 0 |
| Vc | 59 ± 1 | 236 ± 1 |
| Compounds | MCF-7 | HepG2 | HeLa |
|---|---|---|---|
| 2 | 26 ± 6 | 54 ± 2 | 20 ± 1 |
| 3a | 30 ± 2 | 52 ± 1 | 31 ± 7 |
| 3b | 25 ± 2 | 46 ± 2 | 33 ± 7 |
| 3c | 28 ± 2 | 30 ± 5 | 26 ± 2 |
| 3d | 15 ± 7 | 44 ± 1 | 25 ± 2 |
| 3e | 68 ± 2 | 63 ± 0 | 70 ± 1 |
| 3f | 77 ± 1 | 69 ± 1 | 74 ± 0 |
| 3g | 52 ± 1 | 48 ± 2 | 45 ± 2 |
| 3h | 23 ± 1 | 41 ± 5 | 22 ± 2 |
| 3i | 36 ± 7 | 53 ± 1 | 53 ± 2 |
| 3j | 19 ± 2 | 39 ± 7 | 32 ± 4 |
| 3k | 56 ± 1 | 63 ± 2 | 61 ± 3 |
| Cisplatin | 78 ± 1 | 86 ± 1 | 82 ± 1 |
| Compounds | MCF-7 | HepG2 | HeLa |
|---|---|---|---|
| 3a | >50 | 50 ± 2 | >50 |
| 3e | 15 ± 2 | 43 ± 2 | 19 ± 1 |
| 3f | 5.3 ± 0 | 8.8 ± 1 | 8.6 ± 1 |
| 3g | 48 ± 3 | >50 | >50 |
| 3i | >50 | 34 ± 0 | 46 ± 1 |
| 3k | 48 ± 1 | 11 ± 2 | 17 ± 6 |
| 2 | >50 | >50 | >50 |
| Cisplatin | 3.5 ± 0 | 1.1 ± 0 | 1.4 ± 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, G.; Shen, J.; Chen, Z.; Lin, Z.; Long, L.; Wu, J.; Long, C.; Huang, S.; Lian, P.; Luo, G. Antioxidant and Antitumor Activities of Newly Synthesized Hesperetin Derivatives. Molecules 2022, 27, 879. https://doi.org/10.3390/molecules27030879
Zhong G, Shen J, Chen Z, Lin Z, Long L, Wu J, Long C, Huang S, Lian P, Luo G. Antioxidant and Antitumor Activities of Newly Synthesized Hesperetin Derivatives. Molecules. 2022; 27(3):879. https://doi.org/10.3390/molecules27030879
Chicago/Turabian StyleZhong, Guanlin, Jiayi Shen, Zhengwang Chen, Zunxian Lin, Lipeng Long, Jiaying Wu, Chenhuan Long, Siyu Huang, Ping Lian, and Guotian Luo. 2022. "Antioxidant and Antitumor Activities of Newly Synthesized Hesperetin Derivatives" Molecules 27, no. 3: 879. https://doi.org/10.3390/molecules27030879
APA StyleZhong, G., Shen, J., Chen, Z., Lin, Z., Long, L., Wu, J., Long, C., Huang, S., Lian, P., & Luo, G. (2022). Antioxidant and Antitumor Activities of Newly Synthesized Hesperetin Derivatives. Molecules, 27(3), 879. https://doi.org/10.3390/molecules27030879






