Adsorption Properties and Composition of Binary Kolliphor Mixtures at the Water–Air Interface at Different Temperatures
Abstract
:1. Introduction
2. Results and Discussion
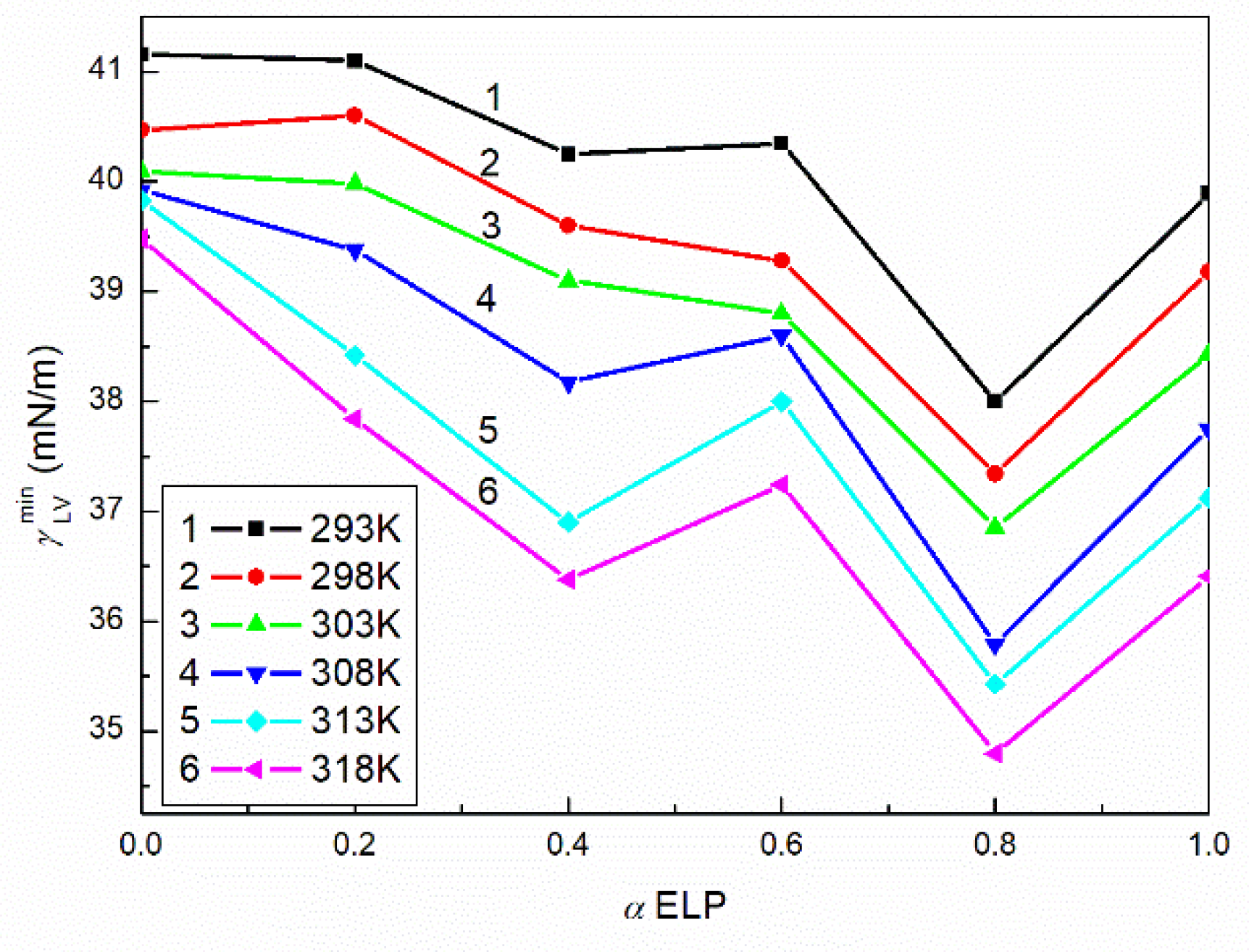
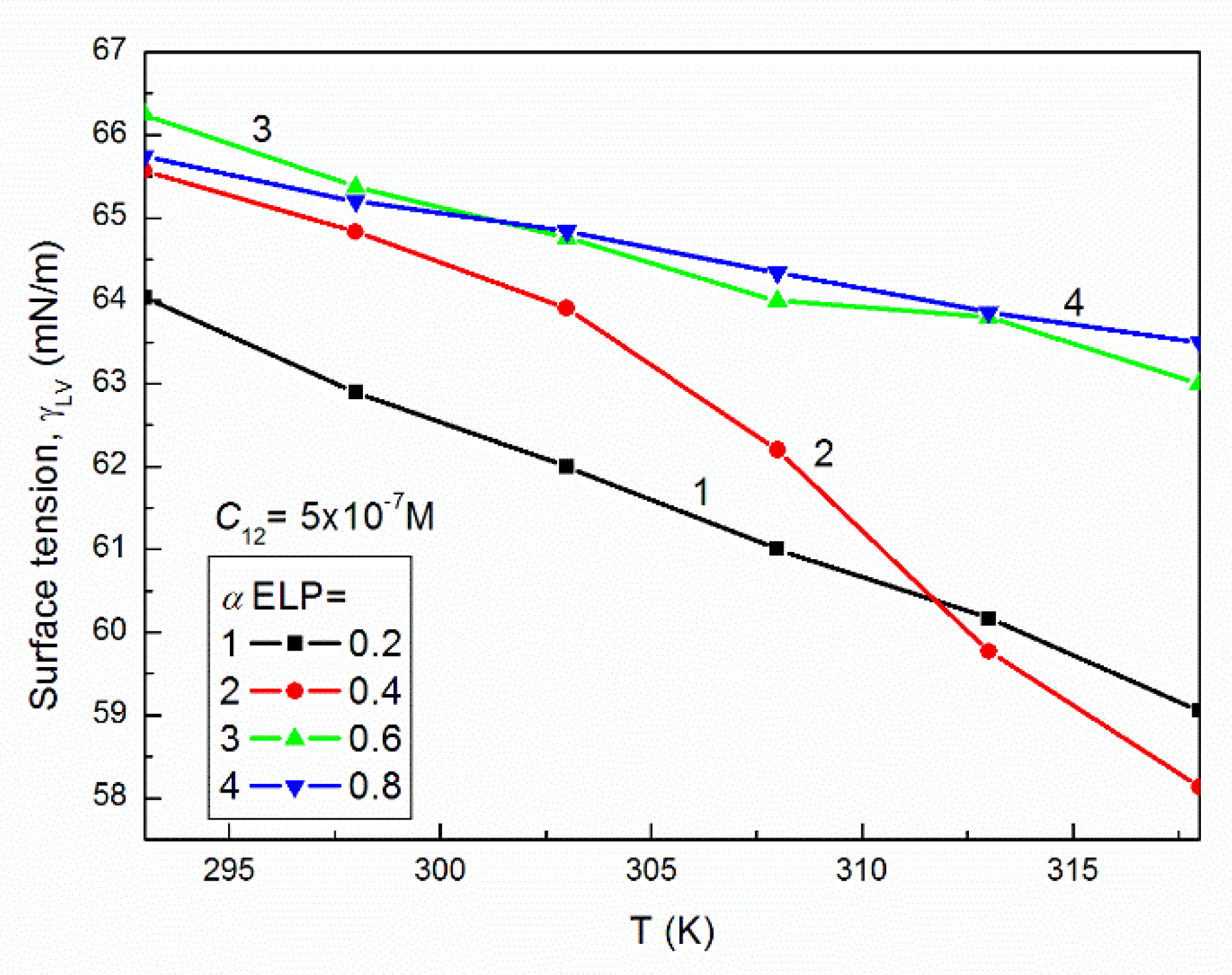
2.1. Surface Tension
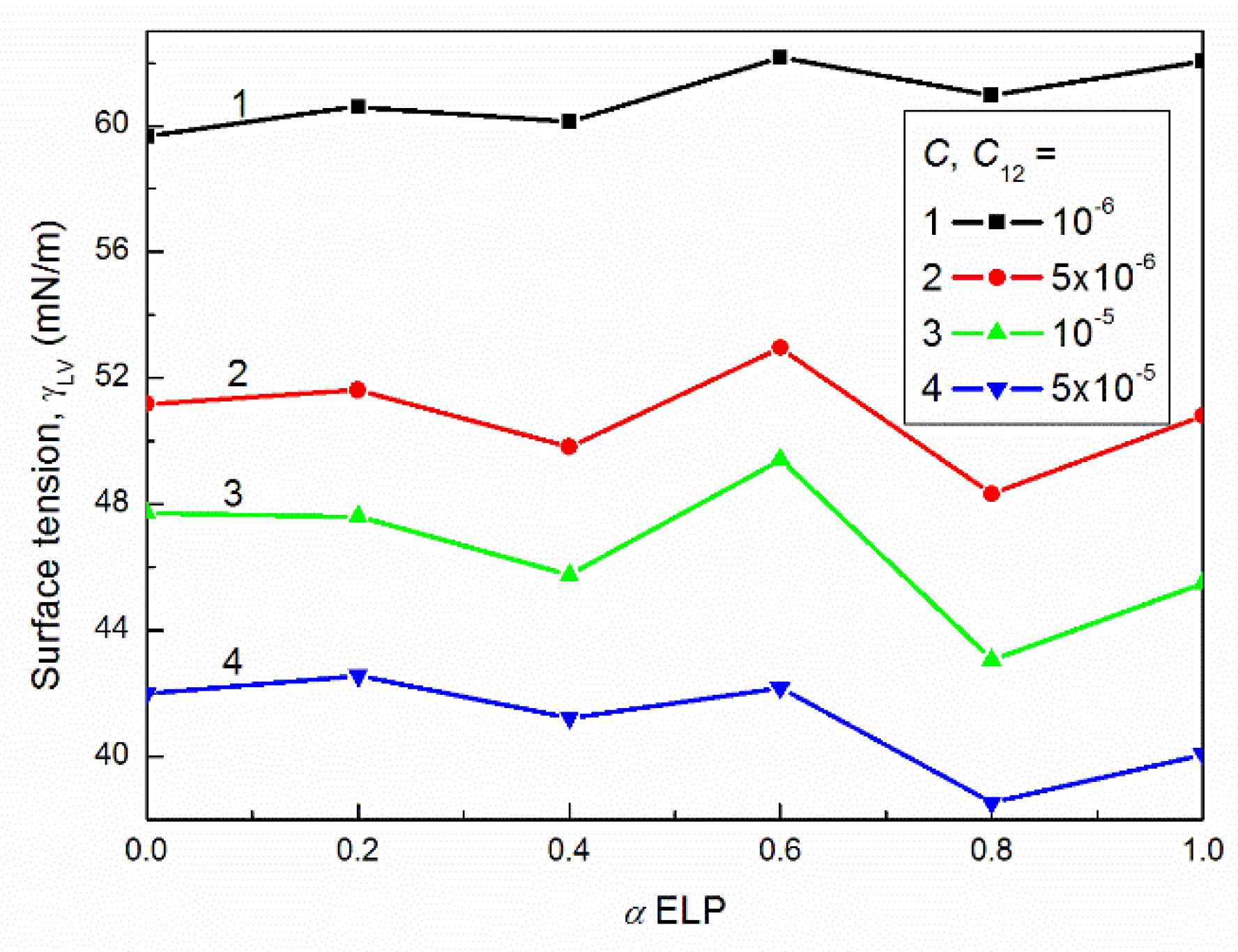
2.2. Concentration of Surfactants at the Water–Air Interface and Composition of the Mixed Monolayer
2.3. Thermodynamic Parameters of Adsorption
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Guo, J.; Xia, Y.; Liu, Y.; Liu, S.; Zhang, L.; Li, B. Microscopic adsorption behaviors of ionic surfactants on lignite surface and its effect on the wettability of lignite: A simulation and experimental study. J. Mol. Liq. 2022, 345, 117851. [Google Scholar] [CrossRef]
- Corona, R.R.B.; Sad, C.M.S.; da Silva, M.; Lopes, D.L.; Leite, J.S.D.; de F. Viegas, G.M.; Gonçalves, G.R.; Filgueiras, P.R.; de Castro, E.V.R. Adsorption of anionic surfactant in graphite oxide: A study for treatment of laundry wastewater. J. Environ. Chem. Eng. 2021, 9, 106858. [Google Scholar] [CrossRef]
- Reeve, J.R.; Thomas, J.R.; Penfold, J. Surface activity of ethoxylate surfactants with different hydrophobic architectures: The effect of layer substructure on surface tension and adsorption. Langmuir 2021, 37, 9269–9280. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed.; Wiley-Interscience: New York, NY, USA, 2004; pp. 34–178. [Google Scholar]
- Kronberg, B.; Holmberg, K.; Lindman, B. Surface Chemistry of Surfactants and Polymers; John Wiley& Sons, Ltd.: West Sussex, UK, 2014; pp. 251–269. [Google Scholar]
- Holland, P.M.; Rubingh, D.N. Mixed Surfactant Systems; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1992; Chapter 1; pp. 1–30. [Google Scholar]
- Scamehorn, J.F. An Overview of Phenomena Involving Surfactant Mixtures; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1986; pp. 1–27. [Google Scholar]
- Seo, S.H.; Kim, E.; Joo, Y.; Lee, J.; Oh, K.T.; Hwang, S.-J.; Choi, K.-Y. A mixed micellar formulation for the transdermal delivery of an indirubin analog. Pharmaceutics 2020, 12, 175. [Google Scholar] [CrossRef] [Green Version]
- Shakeel, F.; Salem-Bekhit, M.M.; Haq, N.; Alshehri, S. Nanoemulsification improves the pharmaceutical properties and bioactivities of niaouli essential oil (Melaleuca quinquenervia L.). Molecules 2021, 26, 4750. [Google Scholar] [CrossRef]
- Jadhav, S.R.; Bryant, G.; Mata, J.P.; Eldridge, D.S.; Palombo, E.A.; Harding, I.H.; Shah, R.M. Structural aspects of a self-emulsifying multifunctional amphiphilic excipient: Part II. The case of Cremophor EL. J. Mol. Liq. 2021, 344, 117881. [Google Scholar] [CrossRef]
- Parikh, K.J.; Sawant, K.K. Solubilization of vardenafil HCl in lipid-based formulations enhances its oral bioavailability in vivo: A comparative study using Tween-20 and Cremophor-EL. J. Mol. Liq. 2019, 277, 189–199. [Google Scholar] [CrossRef]
- Farsang, E.; Gaál, V.; Horváth, O.; Bárdos, E.; Horváth, K. Analysis of non-ionic surfactant Triton X-100 using hydrophilic interaction liquid chromatography and mass spectrometry. Molecules 2019, 24, 1223. [Google Scholar] [CrossRef] [Green Version]
- Bandivadekar, M.; Pancholi, S.; Kaul-Ghanekar, R.; Choudhari, A.; Koppikar, S. Single non-ionic surfactant based self-nanoemulsyfing drug delivery systems: Formulation, characterization, cytotoxicity and permeability enhancement study. Drug Dev. Ind. Pharm. 2013, 39, 696–703. [Google Scholar] [CrossRef]
- van Oss, C.J. Interfacial Forces in Aqueous Media; Marcel Dekker: New York, NY, USA, 1994. [Google Scholar]
- van Oss, C.J.; Constanzo, P.M. Adhesion of anionic surfactants to polymer surfaces and low-energy materials. J. Adhes. Sci. Technol. 1992, 4, 477–487. [Google Scholar] [CrossRef]
- van Oss, C.J.; Good, R.J. Surface tension and the solubility of polymers and biopolymers: The role of polar and apolar interfacial free energies. J. Macromol. Sci. Chem. 1989, 26, 1183–1203. [Google Scholar] [CrossRef]
- van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Monopolar surfaces. Adv. Coll. Interface Sci. 1987, 28, 35–64. [Google Scholar] [CrossRef]
- Fowkes, F.M. Attractive forces at interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Jańczuk, B. Properties of some nonionic fluorocarbon surfactants and their mixtures with hydrocarbon ones. Adv. Coll. Inter. Sci. 2021, 292, 102421. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Krawczyk, J.; Szymczyk, K.; Jańczuk, B. Components and parameters of liquids and some polymers surface tension at different temperature. Coll. Surf. A 2017, 529, 864–875. [Google Scholar] [CrossRef]
- Szymczyk, K.; Szaniawska, M.; Krawczyk, J. Temperature effect on the adsorption and volumetric properties of aqueous solutions of Kolliphor® ELP. Molecules 2020, 25, 743. [Google Scholar] [CrossRef] [Green Version]
- Zdziennicka, A.; Szymczyk, K.; Krawczyk, J.; Jańczuk, B. Activity and thermodynamic parameters of some surfactants adsorption at the water–air interface. Fluid Phase Equilib. 2012, 318, 25–33. [Google Scholar] [CrossRef]
- Beyer, K. Phase structures, water binding, and molecular dynamics in liquid crystalline and frozen states of the system Triton X-100-D2O: A deuteron and carbon NMR study. J. Colloid Interface Sci. 1982, 86, 73–89. [Google Scholar] [CrossRef]
- Szymczyk, K.; Szaniawska, M.; Terpiłowski, K. Determination of acoustical parameters of aqueous solution of Kolliphors binary mixtures using density, speed of sound, viscosity and surface tension measurements. J. Surfact. Deterg. 2019, 22, 1163–1174. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Krawczyk, J.; Szymczyk, K.; Jańczuk, B. Macroscopic and microscopic properties of some surfactants and biosurfactants. Int. J. Mol. Sci. 2018, 19, 1934. [Google Scholar] [CrossRef] [Green Version]
- Frank, C.; Frielinghaus, H.; Allgaier, J.; Prast, H. Nonionic surfactants with linear and branched hydrocarbon tails: compositional analysis, phase behavior, and film properties in bicontinuous microemulsions. Langmuir 2007, 23, 6526–6535. [Google Scholar] [CrossRef] [PubMed]
- Adkins, S.S.; Chen, X.; Nguyen, Q.P.; Sanders, A.W.; Johnston, K.P. Effect of branching on the interfacial properties of nonionic hydrocarbon surfactants at the air-water and carbon dioxide-water interfaces. J. Colloid Interface Sci. 2010, 346, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Khatua, P.K.; Bhattacharya, S.C. Aggregation of nonionic surfactant Igepal in aqueous solution: Fluorescence and light scattering studies. Int. J. Mol. Sci. 2003, 4, 562–571. [Google Scholar] [CrossRef] [Green Version]
- FitzGerald, P.A.; Davey, T.W.; Warr, G.G. Micellar structure in gemini nonionic surfactants from small-angle neutron scattering. Langmuir 2005, 21, 7121–7128. [Google Scholar] [CrossRef]
- Jańczuk, B.; Zdziennicka, A.; Szymczyk, K.; González-Martin, M.L. Prediction of aqueous solution surface tension of some surfactant mixtures and composition of their monolayers at the solution-air interface. Colloids Interfaces 2022, 5, 53. [Google Scholar] [CrossRef]
- Fainerman, V.B.; Miller, R.; Aksenenko, E.V. Simple model for prediction of surface tension of mixed surfactant solutions. Adv. Colloid Interface Sci. 2002, 96, 339–359. [Google Scholar] [CrossRef]
- Fainerman, V.B.; Miller, R. Simple method to estimate surface tension of mixed surfactant solutions. J. Phys. Chem. B 2001, 105, 11432–11438. [Google Scholar] [CrossRef]
- Rosen, J.M.; Hua, X.Y. Surface concentrations and molecular interactions in binary mixtures of surfactants. J. Colloid Interface Sci. 1982, 86, 164–172. [Google Scholar] [CrossRef]
- Rubingh, D.N. Solution Chemistry of Surfactants; Mittal, K.L., Ed.; Plenum Press: New York, NY, USA, 1979; Volume 3, pp. 337–354. [Google Scholar]
- Adamson, W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; Wiley Interscience: New York, NY, USA, 1997. [Google Scholar]
- De Boer, J.H. The Dynamic Character of Adsorption; Oxford University: Oxford, UK, 1953. [Google Scholar]
- Szymczyk, K. Composition of multicomponent surfactant systems at the water–air interface. J. Surfact. Deterg. 2012, 15, 647–656. [Google Scholar] [CrossRef]

| [kJ/molK] | ||||||
| RH40 | = 0.2 | = 0.4 | = 0.6 | = 0.8 | ELP | |
| −0.172 | −0.178 | −0.191 | −0.146 | −0.159 | −0.161 | |
| [kJ/mol] | ||||||
| T [K] | RH40 | = 0.2 | = 0.4 | = 0.6 | = 0.8 | ELP |
| 293 | 3.344 | 5.034 | 8.984 | −3.262 | −0.197 | 0.857 |
| 298 | 3.403 | 5.123 | 9.140 | −2.983 | −0.201 | 0.871 |
| 303 | 3.473 | 5.221 | 9.305 | −3.034 | −0.196 | 0.896 |
| 308 | 3.292 | 5.069 | 9.101 | −3.084 | −0.200 | 0.910 |
| 313 | 3.352 | 5.037 | 8.977 | −3.125 | −0.195 | 0.845 |
| 318 | 3.411 | 5.116 | 9.123 | −3.166 | −0.199 | 0.869 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szaniawska, M.; Szymczyk, K.; Zdziennicka, A.; Jańczuk, B. Adsorption Properties and Composition of Binary Kolliphor Mixtures at the Water–Air Interface at Different Temperatures. Molecules 2022, 27, 877. https://doi.org/10.3390/molecules27030877
Szaniawska M, Szymczyk K, Zdziennicka A, Jańczuk B. Adsorption Properties and Composition of Binary Kolliphor Mixtures at the Water–Air Interface at Different Temperatures. Molecules. 2022; 27(3):877. https://doi.org/10.3390/molecules27030877
Chicago/Turabian StyleSzaniawska, Magdalena, Katarzyna Szymczyk, Anna Zdziennicka, and Bronisław Jańczuk. 2022. "Adsorption Properties and Composition of Binary Kolliphor Mixtures at the Water–Air Interface at Different Temperatures" Molecules 27, no. 3: 877. https://doi.org/10.3390/molecules27030877
APA StyleSzaniawska, M., Szymczyk, K., Zdziennicka, A., & Jańczuk, B. (2022). Adsorption Properties and Composition of Binary Kolliphor Mixtures at the Water–Air Interface at Different Temperatures. Molecules, 27(3), 877. https://doi.org/10.3390/molecules27030877








