Transition-Metal-Free Synthesis of Unsymmetrical Diaryl Tellurides via SH2 Reaction of Aryl Radicals on Tellurium
Abstract
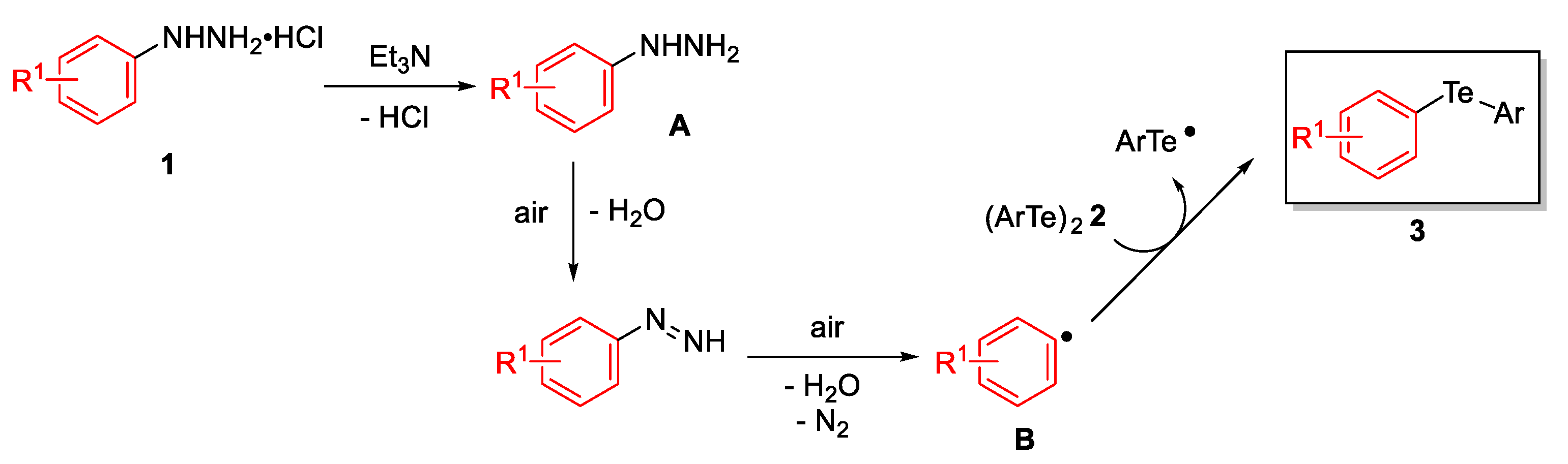
:1. Introduction
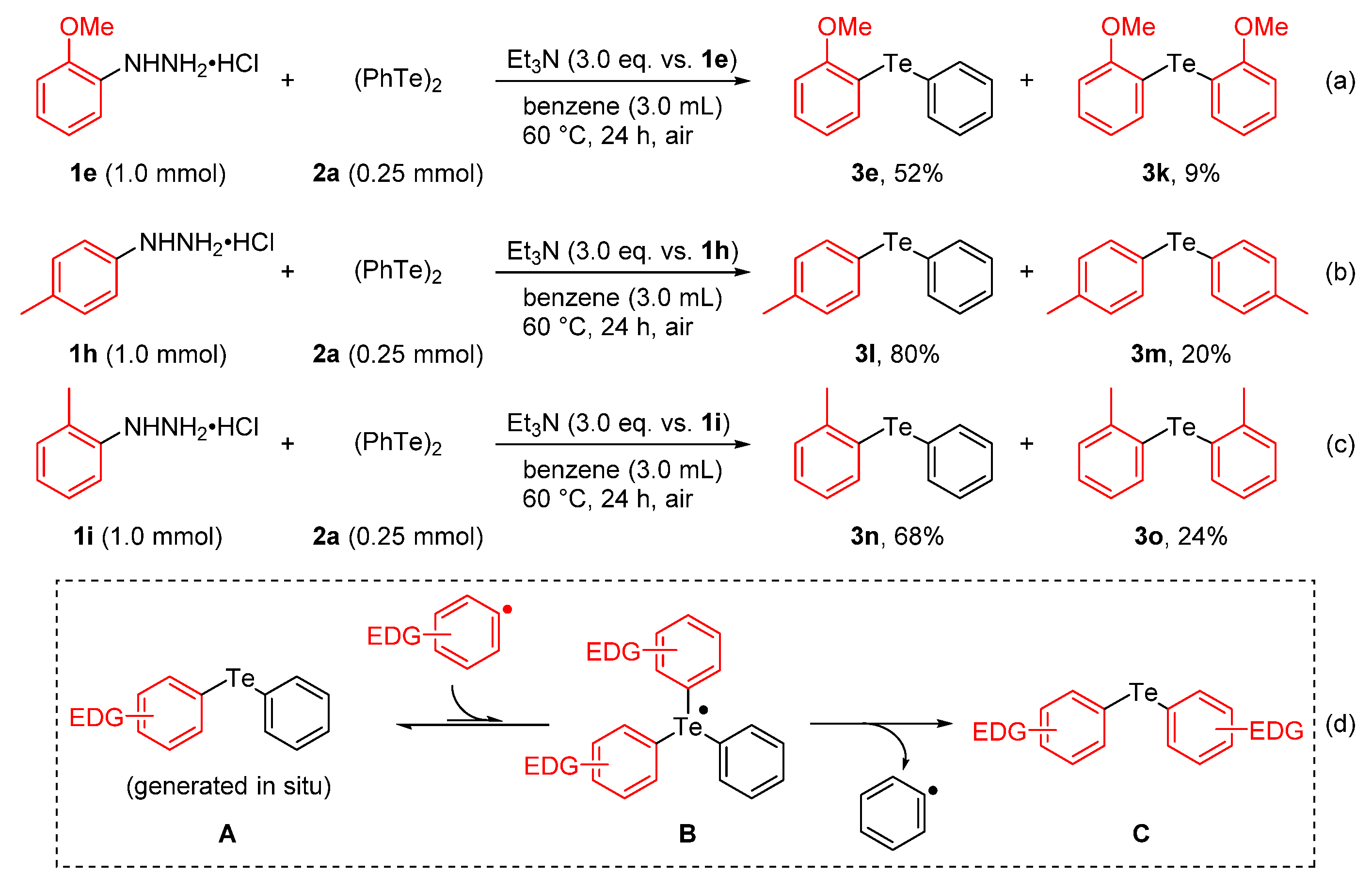
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for Synthesis of Diaryl Tellurides from Arylhydrazine Hydrochlorides and Diaryl Ditellurides under Air
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Alonso, F.; Beletskaya, I.P.; Yus, M. Transition-Metal-Catalyzed Addition of Heteroatom−Hydrogen Bonds to Alkynes. Chem. Rev. 2004, 104, 3079–3160. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.; Moberg, C. Element−Element Additions to Unsaturated Carbon−Carbon Bonds Catalyzed by Transition Metal Complexes. Chem. Rev. 2006, 106, 2320–2354. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.P.; Ananikov, V.P. Transition-Metal-Catalyzed C−S, C−Se, and C−Te Bond Formation via Cross-Coupling and Atom-Economic Addition Reactions. Chem. Rev. 2011, 111, 1596–1636. [Google Scholar] [CrossRef] [PubMed]
- Cavedon, C.; Seeberger, P.H.; Pieber, B. Photochemical Strategies for Carbon–Heteroatom Bond Formation. Eur. J. Org. Chem. 2020, 2020, 1379–1392. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.-p.; Nakamura, K.; Taniguchi, T.; Mita, S.; Kodama, S.; Kawaguchi, S.-i.; Nomoto, A.; Ogawa, A.; Mizuno, T. Synthesis of Aryl Iodides from Arylhydrazines and Iodine. ACS Omega 2018, 3, 9814–9821. [Google Scholar] [CrossRef]
- Tran, D.P.; Nomoto, A.; Mita, S.; Dong, C.-p.; Kodama, S.; Mizuno, T.; Ogawa, A. Metal- and base-free synthesis of aryl bromides from arylhydrazines. Tetrahedron Lett. 2020, 61, 151959. [Google Scholar] [CrossRef]
- Kobiki, Y.; Kawaguchi, S.-i.; Ohe, T.; Ogawa, A. Photoinduced synthesis of unsymmetrical diaryl selenides from triarylbismuthines and diaryl diselenides. Beilstein J. Org. Chem. 2013, 9, 1141–1147. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, T.; Naka, T.; Imoto, M.; Takeda, M.; Nakai, T.; Mihara, M.; Mizuno, T.; Nomoto, A.; Ogawa, A. Transition-Metal-Free and Oxidant-Free Cross-Coupling of Arylhydrazines with Disulfides: Base-Promoted Synthesis of Unsymmetrical Aryl Sulfides. J. Org. Chem. 2017, 82, 6647–6655. [Google Scholar] [CrossRef]
- Taniguchi, T.; Murata, A.; Takeda, M.; Mizuno, T.; Nomoto, A.; Ogawa, A. Atom-Economical Synthesis of Unsymmetrical Diaryl Selenides from Arylhydrazines and Diaryl Diselenides. Eur. J. Org. Chem. 2017, 2017, 4928–4934. [Google Scholar] [CrossRef]
- Hung, V.T.; Tran, C.C.; Yamamoto, Y.; Kodama, S.; Nomoto, A.; Ogawa, A. Clarification on the Reactivity of Diaryl Diselenides toward Hexacyclohexyldilead under Light. Molecules 2021, 26, 6265. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sato, F.; Kodama, S.; Nomoto, A.; Ogawa, A. Reaction of arylhydrazines with diaryl ditellurides in the air: Insight into bimolecular homolytic substitution on tellurium via Aryl–Te bond cleavage. Heteroat. Chem. 2018, 29, e21471. [Google Scholar] [CrossRef]
- Chivers, T.; Laitinen, R.S. Tellurium: A maverick among the chalcogens. Chem. Soc. Rev. 2015, 44, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Minoura, M.; Sagami, T.; Akiba, K.; Modrakowski, C.; Sudau, A.; Seppelt, K.; Wallenhauer, S. Hexaaryltellurium, the First Neutral Compounds Comprising Hexaarylated Elements. Angew. Chem. Int. Ed. 1996, 35, 2660–2662. [Google Scholar] [CrossRef]
- Yamago, S.; Iida, K.; Yoshida, J.-i. Organotellurium Compounds as Novel Initiators for Controlled/Living Radical Polymerizations. Synthesis of Functionalized Polystyrenes and End-Group Modifications. J. Am. Chem. Soc. 2002, 124, 2874–2875. [Google Scholar] [CrossRef]
- Yamago, S. Precision Polymer Synthesis by Degenerative Transfer Controlled/Living Radical Polymerization Using Organotellurium, Organostibine, and Organobismuthine Chain-Transfer Agents. Chem. Rev. 2009, 109, 5051–5068. [Google Scholar] [CrossRef]
- Oba, M.; Okada, Y.; Nishiyama, K.; Ando, W. Aerobic Photooxidation of Phosphite Esters Using Diorganotelluride Catalysts. Org. Lett. 2009, 11, 1879–1881. [Google Scholar] [CrossRef]
- Okada, Y.; Oba, M.; Arai, A.; Tanaka, K.; Nishiyama, K.; Ando, W. Diorganotelluride-Catalyzed Oxidation of Silanes to Silanols under Atmospheric Oxygen. Inorg. Chem. 2010, 49, 383–385. [Google Scholar] [CrossRef]
- Oba, M.; Okada, Y.; Endo, M.; Tanaka, K.; Nishiyama, K.; Shimada, S.; Ando, W. Formation of Diaryl Telluroxides and Tellurones by Photosensitized Oxygenation of Diaryl Tellurides. Inorg. Chem. 2010, 49, 10680–10686. [Google Scholar] [CrossRef]
- Han, L.B.; Ishihara, K.; Kambe, N.; Ogawa, A.; Ryu, I.; Sonoda, N. Carbotelluration of alkynes. J. Am. Chem. Soc. 1992, 114, 7591–7592. [Google Scholar] [CrossRef]
- Fujiwara, S.-i.; Shimizu, Y.; Shin-ike, T.; Kambe, N. Photoinduced Group Transfer Radical Addition of Carbamotelluroates to Acetylenes. Org. Lett. 2001, 3, 2085–2088. [Google Scholar] [CrossRef]
- Petragnani, N.; Stefani, H.A. Advances in organic tellurium chemistry. Tetrahedron 2005, 61, 1613–1679. [Google Scholar] [CrossRef]
- Petragnani, N.; Stefani, H.A. Tellurium in Organic Synthesis, Second Updated and Enlarged; Academic Press: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Stefani, H.A.; Pena, J.M.; Manarin, F.; Ando, R.A.; Leal, D.M.; Petragnani, N. Negishi cross-coupling of organotellurium compounds: Synthesis of biaryls, aryl-, and diaryl acetylenes. Tetrahedron Lett. 2011, 52, 4398–4401. [Google Scholar] [CrossRef]
- Zhang, S.; Kolluru, L.; Vedula, S.K.; Whippie, D.; Jin, J. Carbon-carbon bond forming reactions via Pd-catalyzed detellurative homocoupling of diorganyl tellurides. Tetrahedron Lett. 2017, 58, 3594–3597. [Google Scholar] [CrossRef]
- Uemura, S.; Fukuzawa, S.-i. Ni(II)- and Co(II)-phosphine complex catalyzed carbon–carbon bond formation between organic tellurides and grignard reagents. Tetrahedron Lett. 1982, 23, 1181–1184. [Google Scholar] [CrossRef]
- Nomoto, A.; Ogawa, A. Organic Selenium and Tellurium Compounds; Wiley-VCH: Weinheim, Germany, 2012; Volume 3, p. 623. [Google Scholar]
- Wang, L.; Wang, M.; Huang, F. A Simple Copper Salt-Catalyzed Synthesis of Unsymmetrical Diaryl Selenides and Tellurides from Arylboronic Acids with Diphenyl Diselenide and Ditelluride. Synlett 2005, 13, 2007–2010. [Google Scholar] [CrossRef]
- Kumar, S.; Engman, L. Microwave-Assisted Copper-Catalyzed Preparation of Diaryl Chalcogenides. J. Org. Chem. 2006, 71, 5400–5403. [Google Scholar] [CrossRef]
- Taniguchi, N. Convenient Synthesis of Unsymmetrical Organochalcogenides Using Organoboronic Acids with Dichalcogenides via Cleavage of the S−S, Se−Se, or Te−Te Bond by a Copper Catalyst. J. Org. Chem. 2007, 72, 1241–1245. [Google Scholar] [CrossRef]
- Alves, D.; Pena, J.M.; Vieira, A.S.; Botteselle, G.V.; Guadagnin, R.C.; Stefani, H.A. Copper Catalyzed Cross-Coupling Reactions of Diaryl Ditellurides with Potassium Aryltrifluoroborate Salts. J. Braz. Chem. Soc. 2009, 20, 988–992. [Google Scholar] [CrossRef] [Green Version]
- Kundu, D.; Ahammed, S.; Ranu, B.C. Microwave-assisted reaction of aryl diazonium fluoroborate and diaryl dichalcogenides in dimethyl carbonate: A general procedure for the synthesis of unsymmetrical diaryl chalcogenides. Green Chem. 2012, 14, 2024–2030. [Google Scholar] [CrossRef]
- Kundu, D.; Mukherjee, N.; Ranu, B.C. A general and green procedure for the synthesis of organochalcogenides by CuFe2O4 nanoparticle catalysed coupling of organoboronic acids and dichalcogenides in PEG-400. RSC Adv. 2013, 3, 117–125. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S. A convenient and efficient copper-catalyzed synthesis of unsymmetrical and symmetrical diaryl chalcogenides from arylboronic acids in ethanol at room temperature. Tetrahedron 2014, 70, 1763–1772. [Google Scholar] [CrossRef]
- Roy, S.; Chatterjee, T.; Islam, S.M. Solvent selective phenyl selenylation and phenyl tellurylation of aryl boronic acids catalyzed by Cu(II) grafted functionalized polystyrene. Tetrahedron Lett. 2015, 56, 779–783. [Google Scholar] [CrossRef]
- Panja, S.; Maity, P.; Kundu, D.; Ranu, B.C. Iron(0) nanoparticles mediated direct conversion of aryl/heteroaryl amines to chalcogenides via in situ diazotization. Tetrahedron Lett. 2017, 58, 3441–3445. [Google Scholar] [CrossRef]
- Goldani, B.; Sacramento, M.d.; Lenardão, E.J.; Schumacher, R.F.; Barcellos, T.; Alves, D. Synthesis of symmetrical and unsymmetrical tellurides via silver catalysis. New. J. Chem. 2018, 42, 15603–15609. [Google Scholar] [CrossRef]
- Koguchi, S.; Shibuya, Y.; Igarashi, Y.; Takemura, H. C–Te Cross-Coupling of Diaryl Ditellurides with Arylboronic Acids by Using Copper(I) Thiophene-2-carboxylate under Mild Conditions. Synlett 2019, 30, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Deng, J.; Chen, J.; Cao, F.; Hou, Y.; Yang, Y.; Deng, X.; Yang, J.; Wu, L.; Shao, X.; et al. Dechalcogenization of Aryl Dichalcogenides to Synthesize Aryl Chalcogenides via Copper Catalysis. ACS Catal. 2020, 10, 2707–2712. [Google Scholar] [CrossRef]
- Ren, K.; Wang, M.; Wang, L. Lewis acid InBr3-catalyzed arylation of diorgano diselenides and ditellurides with arylboronic acids. Org. Biomol. Chem. 2009, 7, 4858–4861. [Google Scholar] [CrossRef]
- Arnauld, T.; Barton, D.H.R.; Normant, J.-F. New and Facile Synthesis of Symmetrical and Unsymmetrical Diaryl Chalcogenides Using Trivalent Organobismuth Derivatives. J. Org. Chem. 1999, 64, 3722–3725. [Google Scholar] [CrossRef]
- Engman, L.; Stern, D. Bis(phenylethynyl) telluride as a tellurium(II) equivalent. Synthesis of symmetrical and unsymmetrical diaryl tellurides. Organometallics 1993, 12, 1445–1448. [Google Scholar] [CrossRef]
- Sun, N.; Zheng, K.; Sun, P.; Chen, Y.; Jin, L.; Hu, B.; Shen, Z.; Hu, X. Trichloroisocyanuric Acid-Promoted Synthesis of Arylselenides and Aryltellurides from Diorganyl Dichalcogenides and Arylboronic Acids at Ambient Temperature. Adv. Synth. Catal. 2021, 363, 3577–3584. [Google Scholar] [CrossRef]
- Schiesser, C.H.; Smart, B.A. An Ab Initio Study of Some Free-Radical Homolytic Substitution Reactions at Sulfur, Selenium, and Tellurium. Tetrahedron 1995, 51, 6051–6060. [Google Scholar] [CrossRef]
- Ryu, I.; Okuda, T.; Nagahara, K.; Kambe, N.; Komatsu, M.; Sonoda, N. Intramolecular Homolytic Substitution Behavior of Acyl Radicals at Sulfur: New Carbonylative Access to γ-Thiolactones. J. Org. Chem. 1997, 62, 7550–7551. [Google Scholar] [CrossRef]
- Schiesser, C.H.; Wild, L.M. Intramolecular Homolytic Substitution Chemistry: An Ab Initio Study of 1,n-Chalcogenyl Group Transfer and Cyclization Reactions in Some ω-Chalcogenylalkyl Radicals. J. Org. Chem. 1997, 62, 7550–7551. [Google Scholar] [CrossRef]
- Horvat, S.M.; Schiesser, C.H. An ab initio and DFT Study of homolytic substitution reactions of acyl radicals at sulfur, selenium, and tellurium. New J. Chem. 2010, 34, 1692–1699. [Google Scholar] [CrossRef]
- Horvat, S.M.; Schiesser, C.H. An ab initio and DFT Study of homolytic substitution reactions by oxyacyl radicals at sulfur, selenium, and tellurium. Tetrahedron 2012, 68, 10482–10488. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Petrov, P.A. Aromatic substitution and dealkylation by alkanetellurolate anions. Tetrahedron Lett. 1992, 33, 6515–6518. [Google Scholar] [CrossRef]
- Musalova, M.V.; Musalov, M.V.; Potapov, V.A.; Amosova, S.V. Selective synthesis of allenyl aryl tellurides from diaryl ditellurides and propargyl bromide. Russ. J. Org. Chem. 2012, 48, 1569–1570. [Google Scholar] [CrossRef]
- Haller, W.S.; Irgolic, K.J. Diaryl Ditellurides from grignard reagents and elemental tellurium. J. Organomet. Chem. 1972, 38, 97–103. [Google Scholar] [CrossRef]

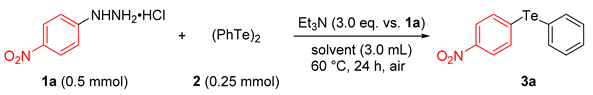
| Entry | Solvent | Yield of 3a (%) a |
|---|---|---|
| 1 | MeOH | 52 |
| 2 b | MeOH | 54 |
| 3 | iPrOH | 58 |
| 4 | CH3CN | 57 |
| 5 | AcOEt | 61 |
| 6 | 1,4-Dioxane | 54 |
| 7 | Toluene | 57 |
| 8 | Benzene | 65 |
| 9 c | Benzene | 72 |
| 10 d | Benzene | 74 (66) |
| 11 e | Benzene | 67 |
| 12 f | Benzene | 56 |
| 13 d,g | Benzene | 27 |
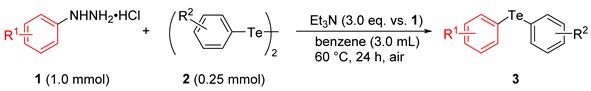
| Entry | Arylhydrazine•HCl 1 | Ditelluride 2 | Product 3 | Yield (%) a | |
|---|---|---|---|---|---|
| 1 | 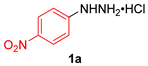 | 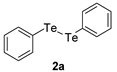 | 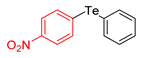 | 3a | 74 (66) |
| 2 |  | 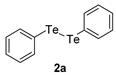 | 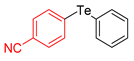 | 3b | (42) |
| 3 | 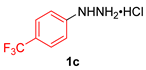 | 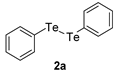 | 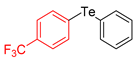 | 3c | 37 |
| 4 | 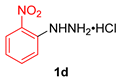 | 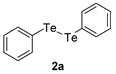 |  | 3d | 52 (41) |
| 5 | 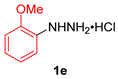 | 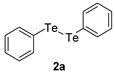 | 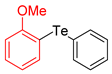 | 3e | 52 b (40) |
| 6 | 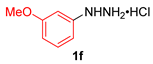 | 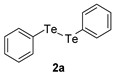 | 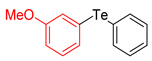 | 3f | 42 (36) |
| 7 |  | 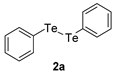 | 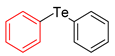 | 3g | 97 (87) |
| 8 |  | 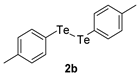 | 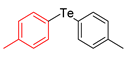 | 3h | 100 (75) |
| 9 | 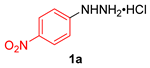 | 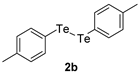 | 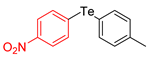 | 3i | 82 (75) |
| 10 | 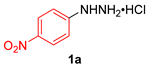 | 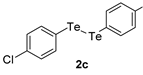 | 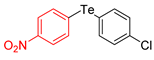 | 3j | 77 (71) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, Y.; Sato, F.; Chen, Q.; Kodama, S.; Nomoto, A.; Ogawa, A. Transition-Metal-Free Synthesis of Unsymmetrical Diaryl Tellurides via SH2 Reaction of Aryl Radicals on Tellurium. Molecules 2022, 27, 809. https://doi.org/10.3390/molecules27030809
Yamamoto Y, Sato F, Chen Q, Kodama S, Nomoto A, Ogawa A. Transition-Metal-Free Synthesis of Unsymmetrical Diaryl Tellurides via SH2 Reaction of Aryl Radicals on Tellurium. Molecules. 2022; 27(3):809. https://doi.org/10.3390/molecules27030809
Chicago/Turabian StyleYamamoto, Yuki, Fumiya Sato, Qiqi Chen, Shintaro Kodama, Akihiro Nomoto, and Akiya Ogawa. 2022. "Transition-Metal-Free Synthesis of Unsymmetrical Diaryl Tellurides via SH2 Reaction of Aryl Radicals on Tellurium" Molecules 27, no. 3: 809. https://doi.org/10.3390/molecules27030809
APA StyleYamamoto, Y., Sato, F., Chen, Q., Kodama, S., Nomoto, A., & Ogawa, A. (2022). Transition-Metal-Free Synthesis of Unsymmetrical Diaryl Tellurides via SH2 Reaction of Aryl Radicals on Tellurium. Molecules, 27(3), 809. https://doi.org/10.3390/molecules27030809






