Abstract
Clarification of the musts is carried out to remove particles that cause turbidity, oxidizable polyphenols, and eliminate excess of proteins. However, an excessive clarification of the musts can lead to the reduction of volatile compound concentrations and, as a consequence, modify the sensorial properties of the wines. Therefore, in this study, the influence of two pre-fermentation clarification techniques (static settling and flotation) on the concentrations of volatile compounds has been assessed in Albariño and Treixadura wines. Fermentations were performed at an industrial scale. Volatile compounds have been identified and quantified by gas chromatography (FID and mass spectrometry detection) and expert panelists assessed the sensory properties of the final wines. The results showed effects of the clarification techniques on the volatile composition of wines from both varieties. Flotation significantly increased the concentrations of benzyl alcohol in Treixadura wines, whereas this technique increased the concentration of 1-hexanol, octanoic acid, and furfural in Albariño wines, but without exceeding the corresponding perception thresholds. Panelists tended to score higher the wines coming from flotation, which, together with the shorter application time, makes this technique suitable for clarifying the musts of these two white varieties.
1. Introduction
The interaction of hundreds of chemical compounds produces the aroma of wines [1]. According to their origin, these compounds derive directly from grapes such as norisoprenoids and monoterpenes [2,3]; secondary metabolites released by the yeasts during fermentation (e.g., volatiles associated with the sugar and amino acid metabolism) [1,4]; and compounds related to wine storage in oak barrels or bottles [5,6]. The major groups of aroma compounds are monoterpenes, norisoprenoids, aliphatics, higher alcohols, esters, phenylpropanoids, methoxypyrazines, and volatile sulfur [2,7]. However, the character of the wine from a given grapevine variety does not relate to a single compound [1]. Therefore, the varietal character depends on the overall profile of odor-active compounds present in the grape and corresponding wine [1]. This character is extremely important for wine typicity and commercial success, as most wineries rely on this concept for marketing campaigns [8].
Grape solid particles contain some compounds, such as unsaturated and saturated fatty acids, phytosterols, nitrogen compounds, polysaccharides, etc., which are an important source of nutrients for yeasts during alcoholic fermentation [9]. The nature, size, and composition of these solids depend on the grape variety and the juice extraction process [10]. In white winemaking, the juices are usually clarified before fermentation. Among other factors, clarification of musts affects wine composition because this process removes suspended and colloidal particles that can cause instability or even health problems [11,12], but an excessive must clarification can exert negative effects, such as sluggish or unfinished fermentations and lower wine quality [13]. Therefore, it is necessary to reach a compromise between the minimum nutritional requirements for yeasts and the absence of unpleasant colors, odors, and flavors for white wine production [14]. Must stabilization and limpidity can be performed by sedimentation (natural or forced by centrifugation), filtration (macro-, micro-, or ultrafiltration), or flotation [11]. These processes are usually accompanied by a previous enzymatic pre-treatment [15], predominantly with pectinases. Another common enological operation performed to clarify musts or wines is the use of fining agents, which, alone or in combination with the techniques cited above, increase the settling efficiency, make the precipitation of suspended solids easier, minimize the browning, ensure stability, and improve the organoleptic characteristics, such as the modulation of mouthfeel perception or the reduction of off-flavors. These clarifying agents can have different origins: organic, inorganic, animal, or plant [11,16,17,18].
Albariño and Treixadura are white grapevine cultivars typical of Galicia (Northwest Spain) and the North of Portugal [19,20]. The wines produced with these cultivars have a high aromatic potential [21,22]. The volatile composition and sensory characteristics of wines made from these two cultivars have been previously reported [19,20,21,22,23,24,25]. The use of fining agents, in particular bentonite and silica gel in musts [26] and bentonite at different stages during vinification [27], has been previously studied in the Albariño variety. However, the effects of clarification techniques on volatile composition have never been determined in wines from these cultivars. Spontaneous (or natural) settling at low temperature is the most widely used pre-fermentative clarification process to remove insoluble materials from the grape must, mainly in white cultivars [28,29]. The must is left to settle for a few hours and the solid parts fall to the bottom of the tank, which is favored by lower temperatures and pectolytic enzymes [30]. However, this method is time consuming and some particles do not settle. Flotation can help to clarify grape musts more rapidly, because the solid parts adhere to the gas bubbles aided by somewhat higher temperatures, and the addition of clarifying agents that modify the must density, making the solid parts float and, therefore easier to remove [30]. In this context, the aim of the current study was to assess the potential modification of wine volatile composition and sensory profiles of monovarietal Albariño and Treixadura wines produced at an industrial scale when musts were submitted to clarification by flotation as compared to traditional static sedimentation.
2. Results
2.1. General Parameters of Wines as Affected by the Must Clarification Technique
Table 1 shows the general enological parameters for each wine obtained after the two clarification techniques applied to the musts for both Albariño and Treixadura varieties. Alcoholic content and volatile acidity were not significantly different (p < 0.05); however, pH values were significantly higher in wines obtained from musts subjected to flotation, and the total acidity showed mainly significantly lower values when the flotation was applied (Table 1). If the average behavior of each variety is considered, general parameters were not affected by the clarification technique before fermentation (Table 1). However, Treixadura wines tended to have lower alcoholic contents and total acidities when musts were clarified by flotation; however, these trends were not significant (p-values = 0.142 and 0.213, respectively, for alcohol content and total acidity).

Table 1.
General parameters of the Albariño and Treixadura wines studied (individual and average ± standard error) as a function of the must clarification treatment (S: static settling; F: flotation).
2.2. Wine Volatile Composition as Affected by the Must Clarification Technique
A total of 56 volatiles were detected in the Albariño wine samples studied, including terpenes, norisoprenoids, C6 compounds, higher and other alcohols, acetates, ethyl esters, volatile fatty acids, volatile phenols, carbonyl compounds, and sulfur compounds (Table 2). Terpenes appeared at low concentrations and the most relevant volatile within this family was linalool. Among C6 compounds, 1-hexanol was the most quantitatively important volatile in all Albariño wines. Isoamyl alcohol and methanol were the most relevant alcohols detected in the Albariño wines studied. The most relevant ester was monoethyl succinate, while octanoic acid was the most quantitatively important fatty acid in the Albariño wines studied. Finally, the most relevant volatiles among acetates, carbonyl compounds, volatile phenols, and sulfur compounds were, respectively, isoamyl acetate, acetoine, 4-vinyl-phenol and methionol (Table 2).

Table 2.
Volatile composition (average ± standard deviation, n = 3) of Albariño wines from the Rías Baixas DO after clarification of the musts by static settling (S) or flotation (F).
In the case of Treixadura wines, a total of 53 volatiles were detected in the samples analyzed (Table 3). Terpenes were detected on a single sample of Treixadura wines and were present at very low concentrations, and β-damascenone and β-ionone were not detected. Similar to Albariño wines, 1-hexanol was the C6 compound detected at greater concentrations in Treixadura wines. In addition, isoamyl alcohol, and furfuryl alcohol, and methanol in some wines obtained from static sedimentation, were, quantitatively, the most important alcohols detected in Treixadura wines. Monoethyl succinate and octanoic acid were, respectively, the ester and fatty acid compounds that appeared at greater concentrations in Treixadura wines, but below that of Albariño wines. Finally, the acetate, carbonyl compound, volatile phenol, and sulfur compound most relevant were, respectively, isoamyl and benzyl acetates, acetoine, ethyl vanillate, and methionol (Table 3).

Table 3.
Volatile composition (average ± standard deviation, n = 3) of Treixadura wines from the Ribeiro DO after clarification of the musts by static settling (S) or flotation (F).
If the average behavior of each variety is considered, the must clarification technique showed a significant effect on three volatile compounds in Albariño wines: 1-hexanol, octanoic acid, and furfural (Table 4). Flotation significantly increased the concentrations of these volatiles in Albariño wines. In addition, a trend to greater concentrations (p-value = 0.087) of hexanoic acid in wines coming from musts that underwent a clarification treatment by flotation was observed. The must clarification treatment exerted a significant effect on the concentration of benzyl alcohol in Treixadura wines (Table 4), but this was not detected in Albariño wines. Flotation significantly increased the concentration of this volatile in Treixadura wines. In addition, a trend to higher concentrations of monoethyl succinate (p-value = 0.116) and benzaldehyde (p-value = 0.125) was observed in wines from the flotation treatment (Table 4).

Table 4.
Average concentrations of volatile compounds (mean ± standard error) in Albariño and Treixadura wines after static settling or flotation clarification of the musts.
Principal Component Analysis (PCA) applied to the cumulative concentrations of the different families of volatile compounds (Figure 1) explained 67.3% of the variability within the wine samples. The first component (PC1) explained 36.9% of this variability and depended mainly on the concentrations of volatile phenols, acetates, higher alcohols, and ethyl esters, while the PC2 accounted for 30.4% of the variability within the dataset and depended mainly on the concentrations of fatty acids, C6 compounds, norisoprenoids and terpenes (Figure 1). The PC1 was able to discriminate between Albariño and Treixadura samples, but no clear discrimination between the must clarification techniques could be achieved.
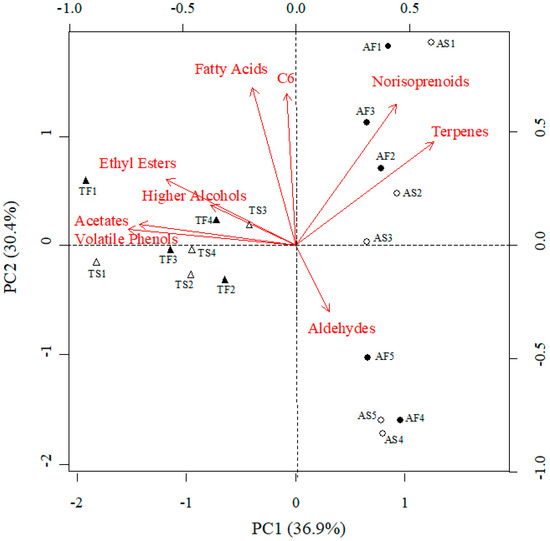
Figure 1.
Bi-plot of the first two principal components (PC) for families of volatile compounds related to wine aroma of Albariño (circles) and Treixadura (triangles) wines coming from musts that underwent different clarification treatments: static clarification (open symbols) or flotation (full symbols). The label of each wine is provided close to its symbol (A stands for Albariño, T stands for Treixadura, S stands for Static settling, and F stands for Flotation).
2.3. Wine Sensorial Properties as Affected by the Must Clarification Treatment
The clarification treatment did not have a significant effect on the sensory perception of either Albariño or Treixadura wines (Figure 2). The only descriptor that showed a significant difference between treatments was the positive intensity in the mouth of the Treixadura wines (Figure 2b). The panelists tended to give higher marks of overall quality to the wines from the flotation treatment (Figure 2c), despite this difference was not statistically significant.
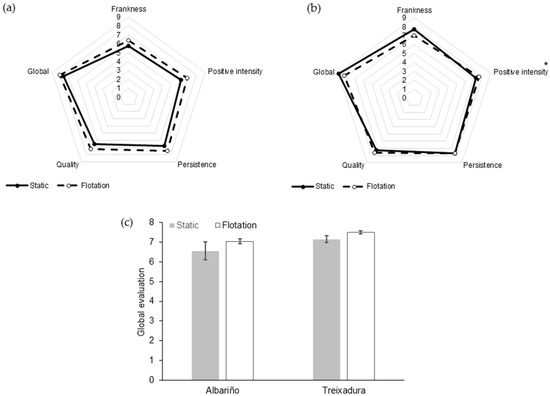
Figure 2.
Sensory profile of Albariño (a) and Treixadura (b) wines as a function of the clarification treatment of the musts. Panel (c) displays the global mark given to the wines. The asterisk indicate significant differences (p-Value < 0.05) between treatments for a given descriptor.
3. Discussion
Pre-fermentative clarification removes insoluble materials from the grape must, and added with fining agents or alone, can allow obtainment of fruity wines, reduce browning, and remove compounds that produce unwanted flavors [31]. However, it can have negative impacts on the composition of resulting wines depending on the technique employed [11,28,29].
The current study confirmed that clarification techniques based on physical processes, such as static settling and flotation, do not significantly affect the average general parameters of these white wines (Table 1), independently of the grapevine variety. This confirms previous research comparing static sedimentation vs. vacuum filtration [28,32], vs. turbidity adjusted by addition of part of the sediment [31], or vs. flotation [33]. Total and volatile acidities were lower in both wine varieties obtained from flotation, but not significantly different, and this behavior was also detected by Ma et al. [34] comparing the centrifugation and the membrane filtration in musts of Italian Riesling icewines vs. the wine control without any treatment. Conversely, Karagiannis and Lanaridis [35], in wines from three Greek varieties, and Albertin et al. [36], in Chardonnay wines, quantified higher volatile acidities when the musts showed lower turbidity, whereas the individual wines analyzed in this work showed lower volatile acidity, although only significantly different between clarification techniques in samples A1, A4, and T1 (Table 1). Except for T3 and T4 wines, all samples showed significantly different values according to the clarification technique, and wines with higher pH values showed simultaneous lower total acidity in both varieties (Table 1), in agreement with Karagiannis and Lanaridis [35]. Debina wines made from musts clarified by flotation using nitrogen (with and without pectolytic enzyme treatment) had lower total acidity than wines from must clarified by sedimentation [33].
Several studies reported alterations on the concentrations of wine volatile compounds depending on the clarification technique, either physical [28,37] or by the application of fining agents [26,34,38]. This could result in a loss of typicity, and alternatives have been proposed, such as the use of plant proteins [17] or the aforementioned physical methods. According to the data showed in Table 2 and Table 3, the concentrations of most of the volatile compounds significantly varied due to the clarification technique tested in this work. Moio et al. [38] showed that different clarification techniques (spontaneous settling or filtration with or without added pectic enzymes) did not affect the concentration of free terpenols in Falanghina musts and wines, but the glycosylated precursors decreased. The two clarification treatments analyzed in the current study affected the concentrations of those compounds responsible for the varietal aroma (terpenes and norisoprenoids) in individual Albariño or Treixadura wines. In general, the concentrations of these varietal aroma compounds decreased in wines obtained from the flotation technique, but these concentrations are so low in both varieties that they never exceed their corresponding perception thresholds, so that these changes will not be perceived at the sensory level. This suggests that using these techniques, instead of pectolytic enzymes or fining agents, such as bentonite, which are known for altering strongly wine varietal aroma [26,34], can provide a satisfactory elimination of those particles causing turbidity, while maintaining the wine typicity. This is extremely relevant for terpenic varieties such as Albariño, which usually presents high concentrations of linalool [20,23]. In contrast, Treixadura wines do not have a marked terpenic character [21,24], and the effect that fining agents could have on their varietal character would be less significant.
Contents of C6 alcohols were, in general, significantly higher in wines obtained from musts clarified by flotation for both varieties. Several authors reported that the highest mass of grape solids in the must causes an increase in this family of volatile compounds [31,34,35], but remained unchanged in Malvar white wine whose must was clarified by cold settling or by tangential-flow membrane filtration [39]. These alcohols are formed through the action of lypoxygenases from linoleic and linolenic acids during grape processing and, perhaps, enzymatic treatment after clarification favored the liberation of these precursors [40].
The other families of volatile compounds showed an undefined behavior neither linked to the clarification technique, nor to the variety, and the synthesis of some molecules may depend on the must turbidity, initial assimilable nitrogen content, lipid concentration, etc. [41], even from the same clarification technique, whereas contradictory results may be obtained according to the variety [35,37]. It must also be taken into account that the grapes of each variety come from different areas in each Denomination of Origin (DO), with different climates, different degrees of ripeness, etc., and that each winery wants a different type of wine. Furthermore, the different yeasts used in winemaking can modulate the release of volatile compounds differently depending on whether they are Saccharomyces cerevisiae yeasts [42], non-Saccharomyces [43], or a combination of both [44]. Although each winery used the same yeast for the alcoholic fermentation of the must clarified by both technologies, grape solid particles can affect yeast performances that drive alcoholic fermentation, promoting yeast cell growth, fermentation kinetics, and nitrogen assimilation, with a corresponding impact on wine aroma [41].
However, if we consider the average behavior of each wine variety, the current study proved that the effects of clarification by flotation on the volatiles are minimal in Albariño and Treixadura wines. In general, flotation only increased significantly the concentrations of 1-hexanol, octanoic acid and furfural in Albariño wines, whereas it increased the concentration of benzyl alcohol in Treixadura wines. In the case of Albariño wines, the volatiles that increased in concentration following the flotation technique provide herbaceous (1-hexanol), rancid (octanoic acid), and toasted (furfural) notes to wine aroma. The increase in concentrations of C6 compounds, such as 1-hexanol, could be negative from the sensorial point of view, because these compounds contribute negative nuances when they are present in high concentrations [45]. However, the concentrations in which they have been detected in the samples of the current study were lower than their corresponding odor thresholds [46,47]. In contrast, octanoic acid reached its odor threshold (10 mg/L) in the wines from the flotation technique and can have an incidence on the aroma of the resulting wine. A lower must turbidity allows increasing the concentrations of short-chain (C6, C8, C10) fatty acids, likely released as intermediate metabolites of the long-chain fatty acid synthesis [35]. Furfural content was higher in wines obtained from static settling in both varieties, mainly in Albariño wines, in agreement with the lower concentrations in Italian Riesling icewines obtained from musts clarified by membrane filtration or centrifugation in comparison with must without any treatment [34].
In the case of Treixadura wines, clarification of musts by flotation significantly increased the concentration of benzyl alcohol. This compound is a varietal alcohol and can exist as free or as glycoside in Albariño and Treixadura grapes [48] and, therefore, can be released from bound forms through the action of the enzyme clarification [49], being predominant in Treixadura variety [20]. Benzyl alcohol gives a pleasant aroma to blackberry and fruity [46]; however, the concentrations observed in the wines, from 31 to 206 μg/L in Albariño wines and between 21 and 131 μg/L in Treixadura wines, were below its odor threshold (200 mg/L, [50]) and, consequently, it would not cause a significant change on wine aroma.
Finally, it must be highlighted that the nonvolatile matrix exerts a powerful impact on wine aroma perception, similar to that of the volatile composition [51,52], and, since the wines of the current study did not present significant differences in alcohol content, the marks given by the panelists were similar between clarification techniques. However, panelists tended to give higher scores to wines coming from the flotation technique in the case of both varieties, although these scores were not significantly different. In the current study, the mouthfeel attributes of the wines from the flotation technique tended to a greater quality and this could have positively affected their global scores, as previously reported for Cabernet Sauvignon wines [53].
4. Materials and Methods
4.1. Wine Samples
Albariño (5) and Treixadura (4) wines used in the current study were elaborated at industrial scale by several wineries from the Rías Baixas and Ribeiro Denomination of Origin (DO), respectively, employing their standard winemaking protocols, except for the clarification process of the musts, which is the factor studied in the current work. After grape pressing, the must was divided into two batches. In half of the samples Novoclair speed (Lamothe-Abiet, Bordeaux, France) pectolytic enzyme was added at 2 g/hL to clarification at 12 °C following the traditional static clarification process, while in the other half Rapidase flotation (DSM Food Specialties, Seclin, France) enzyme (2 mL/hL) was added and then submitted to a clarification by flotation at 14–18 °C by using an Enolmix equipment (Enartis, La Rioja, Spain) with food-grade nitrogen as a gas. In the first case, the process lasts for 12–60 h or 24–48 h, while in the second case the process takes 3–12 h or 4–12 h for Albariño and Treixadura juices, respectively. Then, musts from the same winery were fermented at 16–20 °C in both cases under the same enological conditions (yeast, temperature, etc.). Each winery used the same yeast to ferment the clarified musts using both technologies, but the yeasts differed between wineries: Zymaflore V1 (Laffort, Bordeaux, France), Excellence FW (Lamothe-Abiet, Bordeaux, France), Lalvin QA-23 (Lallemand Inc., Montréal, Canada), Fermivin LVCB (DSM Food Specialties, Seclin, France), and Viniferm Elegancia (Agrovin, Ciudad Real, Spain). Wine samples were collected in 0.75 L bottles directly from each tank and were kept refrigerated (4 °C) until analysis.
4.2. Analytical Methods
Basic parameters of wines (including alcohol content and pH, among others) were determined according to the official methods [54]. Analytical determinations in the wines were carried out in triplicate five months after bottling.
Methanol and higher alcohols were determined in triplicate. As internal standard, 1 mL of 4-methyl-2-pentanol (1 g/L) was added to 5 mL of wine. Then, 2 mL of this mixture were injected directly into a Hewlett Packard 5890 gas chromatograph equipped with a flame ionization detector (FID) using an HP-Innowax capillary column (60 m × 0.25 mm i.d.; film thickness 0.25 μm) according to the method described by Bertrand and Ribéreau-Gayon [55].
The remainder of the volatile compounds were extracted as described by Armada et al. [15]. Briefly, a wine sample of 100 mL containing 2 mL of 3-octanol (20 mg/L) and 2 mL of 3,4-dimethyl-phenol (100 mg/L) as internal standards was extracted three times (10, 5, and 5 mL) with dichloromethane (Merck, Darmstadt, Germany). Then, the organic extract was dried with sodium sulfate and concentrated to 0.5 mL under nitrogen, and 3 mL was injected in triplicate in splitless mode (purge time, 30 s; purge rate 70) in a Hewlett Packard HP 5890-I gas chromatograph coupled to a Hewlett Packard 5970 mass spectrometer (Agilent Technologies, Palo Alto, CA, USA). Spectra were recorded in electron impact mode (ionization energy, 70 eV; source temperature, 250 °C), using an HP-Innowax column (60 × 0.25 mm i.d.; film thickness 0.25 μm). The carrier gas was helium (18 psi). The temperature program was isothermal at 45 °C for 1 min, then 3 °C/min to 230 °C with a final isotherm of 25 min. The acquisition was made in scanning mode (mass range, 30–300 amu; 1.9 spectra/s).
Volatile compounds were identified by comparing their mass spectra (MS Chemstation Wiley 7N library) and their retention times with those of the commercial standards from Merck (Darmstadt, Germany), Fluka (Buchs, Switzerland) or Sigma-Aldrich (Steinheim, Germany). For obtaining the calibration curves, five known amounts of the analytes were subjected to the same liquid–liquid extraction as that for the wine samples, and the quantification was carried out by the interpolation of relative peak areas with respect to the response of internal standards. Sulfur compounds (methionol and thiazole) for which pure compounds were not available were referred as a function of the normalized area (as %) respect to the internal standard (3-octanol). Each wine sample was analyzed in triplicate.
4.3. Sensory Evaluation
The wine sensory assessment was carried out approximately 15 days after the performance of the gas chromatographic determinations. The panel consisted of nine professional enologists (25–50 years of age, 22% females and 78% males), most of them from the wineries that supplied the samples. All wines were tasted in the same session, but the sessions were not replicated due to the availability of the tasting panel. The wines were served in standard tasting glasses coded with three random numbers and covered with a watch-glass to minimize the loss of volatile compounds. Testing temperature was 10 °C and room temperature was 20–22 °C.
A scorecard including general descriptors for odor and mouthfeel was given to the judges. These descriptors included the frankness and positive aromatic intensity for the olfactory stage; and frankness, positive intensity and persistence for the mouthfeel stage. Moreover, the judges were asked to give a global score for the aroma and the taste of the wines. The descriptors were scored on a scale from 0 to 9. In addition, judges were asked to provide a mark for the overall quality of the wine.
4.4. Statistical Analysis
Significant differences between clarification techniques (pre-fermentation) for the average concentrations of each volatile compound were assessed using paired t tests. Normality and homoscedasticity assumptions were checked with Shapiro–Wilk and Bartlett tests, respectively. Principal component analysis (PCA) was applied to discriminate among the sums of families of volatile compounds in the samples according to the clarification technique. Statistical analysis was conducted using the R environment v. 3.6.2 (The R Foundation, Vienna, Austria) [56].
5. Conclusions
The current study provided a preliminary assessment, at an industrial scale, of the effect of two clarification techniques on the volatile composition of Albariño and Treixadura wines. Most volatile compounds in the analyzed wines showed similar concentrations independently of the clarification method employed. However, flotation increased the concentrations of 1-hexanol, octanoic acid and furfural in Albariño wines, and that of benzyl alcohol in Treixadura wines. Sensory evaluation showed a slight trend to high scores of wines from the flotation technique in both Albariño and Treixadura varieties. Therefore, the current study suggests that must clarification through flotation has advantages over the static settling: it saves time and, consequently, decreases the costs, does not change the chemical basic parameters (alcoholic content, pH, etc.), does not reduce the concentration of relevant volatile compounds, and experts tend to value more the global quality of wines coming from the flotation technique; however, further research is needed to evaluate the combination of these physical methods with fining agents and assess their effects on wine flavor chemistry.
Author Contributions
Conceptualization, I.V.-P. and E.F.; methodology, I.V.-P. and E.F.; validation, I.V.-P., J.M.M.-A. and E.F.; formal analysis, I.V.-P. and J.M.M.-A.; investigation, I.V.-P. and E.F.; resources, I.V.-P. and E.F.; data curation, E.F.; writing—original draft preparation, J.M.M.-A. and E.F.; writing—review and editing, J.M.M.-A. and E.F.; project administration, E.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors express their gratitude to the wineries that collaborated in the experimental part of this research. We also thank the enologists that participated in the sensory evaluation of the wines.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Sample Availability
Samples of the compounds are available from the authors.
References
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Ebeler, S.E.; Thorngate, J.H. Wine chemistry and flavor: Looking into the crystal glass. J. Agric. Food Chem. 2009, 57, 8098–8108. [Google Scholar] [CrossRef] [PubMed]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Austr. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Review of quality factors on wine ageing in oak barrels. Trends Food Sci. Technol. 2006, 17, 438–447. [Google Scholar] [CrossRef]
- Maza, M.A.; Martínez, J.M.; Cebrián, G.; Sánchez-Gimeno, A.C.; Camargo, A.; Álvarez, J.; Raso, J. Evolution of polyphenolic compounds and sensory properties of wines obtained from Grenache grapes treated by pulsed electric fields during aging in bottles and oak barrels. Foods 2020, 9, 542. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Austr. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Martinho, V.J.P.D. Contributions from Literature for Understanding Wine Marketing. Sustainability 2021, 13, 7468. [Google Scholar] [CrossRef]
- Casalta, E.; Salmon, J.M.; Picou, C.; Sablayrolles, J.M. Grape solids: Lipid composition and role during alcoholic fermentation under enological conditions. Am. J. Enol. Vitic. 2019, 70, 147–154. [Google Scholar] [CrossRef]
- Casalta, E.; Vernhet, A.; Sablayrolles, J.M.; Tesnière, C.; Salmon, J.M. Review: Characterization and role of grape solids during alcoholic fermentation under enological conditions. Am. J. Enol. Vitic. 2016, 67, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Mierczynska-Vasilev, A.; Smith, P.A. Current state of knowledge and challenges in wine clarification. Austr. J. Grape Wine Res. 2015, 21, 615–626. [Google Scholar] [CrossRef]
- Doulia, D.S.; Anagnos, E.K.; Liapis, K.S.; Klimentzos, D.A. Effect of clarification process on the removal of pesticide residues in white wine. Food Control 2017, 72, 134–144. [Google Scholar] [CrossRef]
- Ayestarán, B.M.; Ancín, M.C.; García, A.M.; González, A.; Garrido, J.J. Influence of prefermentation clarification on nitrogenous contents of musts and wines. J. Agric. Food Chem. 1995, 43, 476–482. [Google Scholar] [CrossRef]
- Sablayrolles, J.M. Control of alcoholic fermentation in winemaking: Current situation and prospect. Food Res. Int. 2009, 42, 418–424. [Google Scholar] [CrossRef]
- Armada, L.; Fernández, E.; Falqué, E. Influence of several enzymatic treatments on aromatic composition of white wines. LWT-Food Sci. Technol. 2010, 43, 1517–1525. [Google Scholar] [CrossRef]
- Parish, K.J.; Herbst-Johnstone, M.; Bouda, F.; Klaere, S.; Fedrizzi, B. Pre-fermentation fining effects on the aroma chemistry of Marlborough Sauvignon blanc press fractions. Food Chem. 2016, 208, 326–335. [Google Scholar] [CrossRef]
- Marangon, M.; Vincenzi, S.; Curioni, A. Wine fining agents with plant proteins. Molecules 2019, 24, 2186. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, W.; Seidel, L.; Zorn, H.; Will, F.; Gand, M. Haze formation and the challenges for peptidases in wine protein fining. J. Agric. Food Chem. 2021, 69, 14402–14414. [Google Scholar] [CrossRef]
- Falqué, E.; Fernández, E.; Dubourdieu, D. Volatile composition of Loureira, Dona Branca, and Treixadura wines. J. Agric. Food Chem. 2002, 50, 538–543. [Google Scholar] [CrossRef]
- Vilanova, M.; Escudero, A.; Graña, M.; Cacho, J. Volatile composition and sensory properties of North West Spain white wines. Food Res. Int. 2013, 54, 562–568. [Google Scholar] [CrossRef]
- Falqué, E.; Fernández, E.; Dubourdieu, D. Differentiation of white wines by their aromatic index. Talanta 2001, 54, 271–281. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Masa, A.; Oliveira, J.M. Correlation between volatile composition and sensory properties in Spanish Albariño wines. Microchem. J. 2010, 95, 240–246. [Google Scholar] [CrossRef]
- Falqué, E.; Darriet, P.; Fernández, E.; Dubourdieu, D. Volatile profile and differentiation between Albariño wines from different origins. Int. J. Food Sci. Technol. 2008, 43, 464–475. [Google Scholar] [CrossRef]
- Bouzas-Cid, Y.; Falqué, E.; Orriols, I.; Mirás-Avalos, J.M. Effects of irrigation over three years on the amino acid composition of Treixadura (Vitis vinifera L.) musts and wines, and on the aromatic composition and sensory profiles of its wines. Food Chem. 2018, 240, 707–716. [Google Scholar] [CrossRef]
- Vázquez-Pateiro, I.; Arias-González, U.; Mirás-Avalos, J.M.; Falqué, E. Evolution of the aroma of Treixadura wines during bottle aging. Foods 2020, 9, 1419. [Google Scholar] [CrossRef]
- Armada, L.; Falqué, E. Repercussion of the clarification treatment agents before the alcoholic fermentation on volatile composition of white wines. Eur. Food Res. Technol. 2007, 225, 553–558. [Google Scholar] [CrossRef]
- Lira, E.; Rodríguez-Bencomo, J.J.; Salazar, F.N.; Orriols, I.; Fornos, D.; López, F. Impact of bentonite additions during vinification on protein stability and volatile compounds of Albariño wines. J. Agric. Food Chem. 2015, 63, 3004–3011. [Google Scholar] [CrossRef]
- Ancín, C.; Ayestarán, B.; Corroza, M.; Garrido, J.; González, A. Influence of prefermentation clarification on the higher alcohol contents of wines. Food Chem. 1996, 55, 241–249. [Google Scholar] [CrossRef]
- Burin, V.M.; Caliari, V.; Bordignon-Luiz, M.T. Nitrogen compounds in must and volatile profile of white wine: Influence of clarification process before alcoholic fermentation. Food Chem. 2016, 202, 417–425. [Google Scholar] [CrossRef]
- Togores, J.H. Tratado de Enología; Mundi-Prensa Libros: Madrid, Spain, 2018; Volume 1, pp. 891–898. [Google Scholar]
- Kechagia, D.; Paraskevopoulos, Y.; Symeou, E.; Galiotou-Panayotou, M.; Kotseridis, Y. Influence of prefermentative treatments to the major volatile compounds of Assyrtiko wines. J. Agric. Food Chem. 2008, 56, 4555–4563. [Google Scholar] [CrossRef]
- Puig-Deu, M.; López-Tamames, E.; Buxaderas, S.; Torres-Boronat, M.C. Influence of must racking and fining procedures on the composition of white wine. Vitis 1996, 35, 141–145. [Google Scholar]
- Sindou, E.; Vaimakis, V.; Vaimakis, T.; Roussis, I.G. Effect of juice clarification by flotation on the quality of white wine and orange juice and drink. Czech J. Food Sci. 2008, 26, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.Z.; Gong, P.F.; Lu, R.R.; Zhang, B.; Morata, A.; Han, S.Y. Effect of different clarification treatments on the volatile composition and aromatic attributes of ‘Italian Riesling’ icewine. Molecules 2020, 25, 2657. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, S.; Lanaridis, P. Insoluble grape material present in must affects the overall fermentation aroma of dry white wines made from three grape cultivars cultivated in Greece. J. Food Sci. 2002, 67, 369–374. [Google Scholar] [CrossRef]
- Albertin, W.; Miot-Sertier, C.; Bely, M.; Marullo, P.; Coulon, J.; Moine, V.; Colonna-Ceccaldi, B.; Masneuf-Pomarede, I. Oenological prefermentation practices strongly impact yeast population dynamics and alcoholic fermentation kinetics in Chardonnay grape must. Int. J. Food Microbiol. 2014, 178, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, M.; Güell, C.; López, F. Industrial wine making: Comparison of must clarification treatments. J. Agric. Food Chem. 1998, 46, 1523–1528. [Google Scholar] [CrossRef]
- Moio, L.; Ugliano, M.; Gambuti, A.; Genovese, A.; Piombino, P. Influence of clarification treatment on concentrations of selected free varietal aroma compounds and glycoconjugates in Falanghina (Vitis vinifera L.) must and wine. Am. J. Enol. Vitic. 2004, 55, 7–12. [Google Scholar]
- Prodanov, M.; Aznar, M.; Cabellos, J.M.; Vacas, V.; López, F.; Hernández, M.T.; Estrella, M.I. Tangential-flow membrane clarification of Malvar (Vitis vinifera L.) wine: Incidence on chemical composition and sensorial expression. OENO One 2019, 53, 41–755. [Google Scholar] [CrossRef]
- Varela, F.; Calderón, F.; González, M.C.; Colomo, B.; Suárez, J.A. Effect of clarification on the fatty acid composition of grape must and the fermentation kinetics of white wines. Eur. Food Res. Technol. 1999, 209, 439–444. [Google Scholar] [CrossRef]
- Guittin, C.; Maçna, F.; Sánchez, I.; Poitou, X.; Sablayrolles, J.M.; Mouret, J.R.; Farines, V. Impact of high lipid contents on the production of fermentative aromas during white wine fermentation. Appl. Microbiol. Biotechnol. 2021, 105, 6435–6449. [Google Scholar] [CrossRef]
- Blanco, P.; Mirás-Avalos, J.M.; Suárez, V.; Orriols, I. Inoculation of Treixadura musts with autochthonous Saccharomyces cerevisiae strains: Fermentative performance and influence on the wine characteristics. Food Sci. Technol. Int. 2013, 19, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, M.; Fragasso, M.; Pico, J.; Panighel, A.; Castellarin, S.D.; Flamini, R.; Grieco, F. Influence of Non-Saccharomyces on Wine Chemistry: A Focus on Aroma-Related Compounds. Molecules 2021, 26, 644. [Google Scholar] [CrossRef] [PubMed]
- Berbegal, C.; Khomenko, I.; Russo, P.; Spano, G.; Fragasso, M.; Biasioli, F.; Capozzi, V. PTR-ToF-MS for the Online Monitoring of Alcoholic Fermentation in Wine: Assessment of VOCs Variability Associated with Different Combinations of Saccharomyces/Non-Saccharomyces as a Case-Study. Fermentation 2020, 6, 55. [Google Scholar] [CrossRef]
- Rocha, S.; Coutinho, P.; Barros, A.; Coimbra, M.A.; Delgadillo, I.; Dias Cardoso, A. Aroma potential of two Bairrada white grape varieties: Maria Gomes and Bical. J. Agric. Food Chem. 2000, 48, 4802–4807. [Google Scholar] [CrossRef] [PubMed]
- Meilgaard, M. Flavor chemistry of beer. II: Flavor and threshold of 239 aroma volatiles. Tech. Quart. Master. Brew. Assoc. Am. 1975, 12, 151–168. [Google Scholar]
- Simpson, R.F. Some important aroma composition of components of white wine. Food Technol. 1979, 31, 516–522. [Google Scholar]
- Lois, L.C.; Diéguez, S.C.; Gil de la Peña, M.G.; Gómez, E.F. SPE-GC Determination of aromatic compounds in two varieties of white grape during ripening. Chromatographia 2001, 53, S350–S355. [Google Scholar] [CrossRef]
- Ridge, M.; Sommer, S.; Dycus, D.A. Addressing Enzymatic Clarification Challenges of Muscat Grape Juice. Fermentation 2021, 7, 198. [Google Scholar] [CrossRef]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas chromatography-Olfactometry and chemical quantitative study of the aroma of six Premium Quality Spanish aged red wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef]
- Robinson, A.L.; Ebeler, S.E.; Heymann, H.; Boss, P.K.; Solomon, P.S.; Trengove, R.D. Interactions between wine volatile compounds and grape and wine matrix components influence aroma compound headspace partitioning. J. Agric. Food Chem. 2009, 57, 10313–10322. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.P.; Campo, E.; Culleré, L.; Fernández-Zurbano, P.; Valentin, D.; Ferreira, V. Effects of the nonvolatile matriz on the aroma perception of wine. J. Agric. Food Chem. 2010, 58, 5574–5585. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xing, R.R.; Li, Z.; Yang, D.M.; Pan, Q.H. Evolution of volatile compounds, aroma attributes, and sensory perception in bottle-aged red wines and their correlation. Eur. Food Res. Technol. 2016, 242, 1937–1948. [Google Scholar] [CrossRef]
- Office International de la Vigne et du Vin (OIV). Compendium of International Methods of Wine and Must Analysis; Office International de la Vigne et du Vin: Paris, France, 2021; Available online: https://www.oiv.int/public/medias/7787/oiv-compendium-of-international-methods-of-analysis-vol1-en.pdf (accessed on 17 October 2021).
- Bertrand, A.; Ribéreau-Gayon, P. Determination of volatile components of wine by gas-phase chromatography. Ann. Falsif. Expert. Chim. 1970, 63, 148–156. [Google Scholar]
- R Core Team. R: A Language for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).