The Influence of Hop Phenolic Compounds on Dry Hopping Beer Quality
Abstract
:1. Introduction
2. Results
2.1. The Determination and Mathimatical Analysis of Beer’s Sample Composition
- Y—soluble nitrogen content, mg/L;
- X1—β-glucan content, mg/L;
- X2—iso-α-acids content, mg/L;
- X3—original extract, °P;
- X4—color, °EBC.
- Y—soluble nitrogen content, mg/L;
- X1—β-glucan content, mg/L;
- X2—iso-α-acids content, mg/L;
- X3—original extract, °P;
- X4—color, °EBC.
- Y—soluble nitrogen content, mg/L;
- X1—β-glucan content, mg/L;
- X2—iso-α-acids content, mg/L;
- X3—original extract, °P;
- X4—color, °EBC.
2.2. The Determination and Mathimatical Analysis of the Beer’s Phenolic Compounds Composition
- Y—iso-α-acids content, mg/L;
- X1—isoxanthohumol content, mg/L;
- X2—catechin content, mg/L;
- X3—quercetin content, mg/L;
- X4—rutin content, mg/L.
- Y—color, °EBC;
- X1—total polyphenol content, vg/L;
- X2—isoxanthohumol content, mg/L;
- X3—catechin content, mg/L;
- X4—quercetin content, mg/L;
- X5—rutin content, mg/L;
- X6—soluble nitrogen content, mg/L;
- X7—β-glucan content, mg/L.
2.3. The Beer’s Samples Polyphenol Complex Effect on Tast Descriptors Intecity Perception
3. Discussion
4. Materials and Methods
4.1. The Research Materials
4.2. The Research Methods
4.2.1. Chemicals
4.2.2. Determination of Original Extract and Alcohol Content
4.2.3. Determination of Nitrogen Compounds
4.2.4. Determination of the Total Content of Polyphenols
4.2.5. Determination of Iso-α-Gumulon Mass Concentration
4.2.6. Determination of Catechin Mass Concentration
4.2.7. Determination of Quercetin and Rutin Mass Concentration
4.2.8. Determination of Isoxantohumol Mass Concentration
4.2.9. Determination of the Mass Concentration of β-Glucan
4.2.10. Determination of the Beer’s Color
4.2.11. Organoleptic Evaluation of Beer’s Samples by Descriptors
4.2.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kerr, E.; Caboche, C.; Pegg, C.; Phung, T.; Viejo, C.G.; Fuentes, S.; Howes, M.; Howell, K.; Schulz, B. The post-translational modification landscape of commercial beers. Sci Rep 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Kerr, E.D.; Caboche, C.H.; Schulz, B.L. Posttranslational modifications drive protein stability to control the dynamic beer brewing proteome. Mol. Cell. Proteom. 2019, 18, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Koistinen, V.M.; Tuomainen, M.; Lehtinen, P.; Peltola, P.; Auriola, S.; Jonsson, K.; Hanhineva, K. Side-stream products of malting: A neglected source of phytochemicals. NPJ Sci. Food 2020, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Knez, H.M.; Španinger, E.; Košir, I.J.; Knez, Ž.; Bren, U. Hop Compounds: Extraction Techniques, Chemical Analyses, Antioxidative, Antimicrobial, and Anticarcinogenic Effects. Nutrients 2019, 11, 257. [Google Scholar] [CrossRef] [Green Version]
- Gerhäuser, C. Beer constituents as potential cancer chemopreventive agents. Eur. J. Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef] [PubMed]
- Mikyška, A.; Hrabák, M.; Hašková, D.; Šrogl, J. The Role of Malt and Hop Polyphenols in Beer Quality, Flavour and Haze Stability. J. Inst. Brew. 2002, 108, 78–85. [Google Scholar] [CrossRef]
- Mikyška, A.; Jurková, M. Varietal specificity of polyphenols, free phenolics and antioxidant potential in hops. Kvasny Prumysl 2019, 65, 178–185. [Google Scholar] [CrossRef]
- Collin, S.; Jerkovic, V.; Bröhan, M.; Callemien, D. Polyphenols and Beer Quality; Ramawat, K., Mérillon, J.M., Eds.; Natural Products; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Cheiran, K.P.; Raimundo, V.P.; Manfroi, V.; Anzanello, M.J.; Kahmann, A.; Rodrigues, E.; Frazzon, J. Simultaneous identification of low-molecular weight phenolic and nitrogen compounds in craft beers by HPLC-ESI-MS/MS. Food Chem. 2019, 286, 113–122. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Breda, C.; Barros, A. Characterization and Discrimination of Commercial Portuguese Beers Based on Phenolic Composition and Antioxidant Capacity. Foods 2021, 10, 1144. [Google Scholar] [CrossRef]
- Kielhorn, S.; Thorngate, J.H. Oral sensations associated with the flavan-3-ols (+)-catechin and (−)-epicatechin. Food Qual. Prefer. 1999, 10, 109–116. [Google Scholar] [CrossRef]
- Robichaud, J.L.; Noble, A.C. Astringency and bitterness of selected phenolics in wine. J. Sci. Food Agric. 1990, 53, 343–353. [Google Scholar] [CrossRef]
- McLaughlin, I.R.; Lederer, C.; Shellhammer, T.H. Bitterness-Modifying Properties of Hop Polyphenols Extracted from Spent Hop Material. J. Am. Soc. Brew. Chem. 2008, 66, 174–183. [Google Scholar] [CrossRef]
- Stevens, J.F.; Taylor, A.W.; Clawson, J.E.; Deinzer, M.L. Fate of xanthohumol and related prenylflavonoids from hops to beer. J. Agric. Food Chem. 1999, 47, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- Shankar, E.; Goel, A.; Gupta, K.; Gupta, S. Plant flavone apigenin: An emerging anticancer agent. Curr. Pharmacol. Rep. 2017, 3, 423–446. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, M.; Feola, A.; Ambrosio, P.; Pinto, F.; Galasso, G.; Zarrelli, A.; Di Fabio, G.; Porcelli, M.; Scacco, S.; Inchingolo, F.; et al. Antioxidant Effect of Beer Polyphenols and Their Bioavailability in Dental-Derived Stem Cells (D-dSCs) and Human Intestinal Epithelial Lines (Caco-2) cells. Stem Cells Int. 2020, 2020, 8835813. [Google Scholar] [CrossRef]
- Montanari, L.; Perretti, G.; Natella, F.; Guidi, A.; Fantozzi, P. Organic and phenolic acids in beer. LWT-Food Sci. Technol. 1999, 32, 535–539. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, W.; Lu, J.; Zhao, M. Phenolic profiles and antioxidant activities of commercial beers. Food Chem. 2010, 119, 1150–1158. [Google Scholar] [CrossRef]
- Buiatti, S. Beer composition: An overview. In Beer in Health and Disease Prevention; Academic Press: London, UK, 2009; pp. 213–225. [Google Scholar]
- Habschied, K.; Košir, I.J.; Krstanovic, V.; Kumric, G.; Mastanjevic, K. Beer Polyphenols—Bitterness, Astringency, and Off-Flavors. Beverages 2021, 7, 38. [Google Scholar] [CrossRef]
- Farooq, S.A.; Zhang, H.; Weiss, J. A comprehensive review on polarity, partitioning, and interactions of phenolic antioxidants at oil–water interface of food emulsions. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4250–4277. [Google Scholar] [CrossRef]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Dıaz, C.; Romsted, L.S. A direct correlation between the antioxidant efficiencies of caffeic acid and its alkyl esters and their concentrations in the interfacial region of olive oil emulsions. The pseudophase model interpretation of the “cut-off” effect. Food Chem. 2015, 175, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Freiría-Gándara, J.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Differential partitioning of bioantioxidants in edible oil-water and octanol-water systems: Linear free energy relationships. J. Chem. Eng. Data 2018, 63, 2999–3007. [Google Scholar] [CrossRef]
- Ambra, R.; Pastore, G.; Lucchetti, S. The Role of Bioactive Phenolic Compounds on the Impact of Beer on Health. Molecules 2021, 26, 486. [Google Scholar] [CrossRef] [PubMed]
- Keukeleire, D.D. Fundamentals of beer and hop chemistry. Quím. Nova 2000, 23, 108–112. [Google Scholar] [CrossRef]
- Tian, S.; Sun, Y.; Chen, Z.; Yang, Y.; Wang, Y. Functional Properties of Polyphenols in Grains and Effects of Physicochemical Processing on Polyphenols. J. Food Qual. 2019, 2019, 2793973. [Google Scholar] [CrossRef]
- Deng, G.-F.; Xu, X.-R.; Guo, Y.-J.; Xia, E.-Q.; Li, S.; Wu, S.; Chen, F.; Ling, W.-H.; Li, H.-B. Determination of antioxidant property and their lipophilic and hydrophilic phenolic contents in cereal grains. J. Funct. Foods 2012, 4, 906–914. [Google Scholar] [CrossRef]
- Sharma, P.; Gujral, H.S.; Singh, B. Antioxidant activity of barley as affected by extrusion cooking. Food Chem. 2012, 131, 1406–1413. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Ovais, M.; Ullah, I.; Ahmed, J.; Shahid, M. Flavonoids as Prospective Neuroprotectants and Their Therapeutic Propensity in Aging Associated Neurological Disorders. Front. Aging Neurosci. 2019, 11, 155. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Nguyen, D.H.; Zhao, B.T.; Le, D.D.; Choi, D.H.; Kim, Y.H.; Woo, M.H. A new lignan and a new alkaloid, and α-glucosidase inhibitory compounds from the grains of Echinochloa utilis Ohwi and Yabuno. Bioorg. Chem. 2017, 74, 221–227. [Google Scholar] [CrossRef]
- Liu, R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W. Phenolic compounds in cereal grains and their health benefits. Cereal Foods World 2007, 52, 105–111. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Curto, A.F.; Guido, L.F. Determination of phenolic content in different barley varieties and corresponding malts by liquid chromatography-diode array detection-electrospray ionization tandem mass spectrometry. Antioxidants 2015, 4, 563–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socha, R.; Pająk, P.; Fortuna, T.; Buksa, K. Antioxidant activity and the most abundant phenolics in commercial dark beers. Int. J. Food Prop. 2017, 20, S595–S609. [Google Scholar] [CrossRef]
- Chadwick, L.R.; Pauli, G.F.; Farnsworth, N.R. The pharmacognosy of Humulus lupulus L. (hops) with an emphasis on estrogenic properties. Phytomedicine 2006, 13, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Steiner, E.; Gastl, M.; Becker, T. Protein changes during malting and brewing with focus on haze and foam formation: A review. Eur. Food Res. Technol. 2011, 232, 191–204. [Google Scholar] [CrossRef]
- Muller, C.; Neves, L.E.; Gomez, L.; Guimaraes, M.; Ghesti, G. Processes for alcohol-free beer production: A review. Food Sci. Technol. 2020, 40, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Yassue-Cordeiro, P.H.; Zandonai, C.H.; Genesi, B.P.; Lopes, P.S.; Sanchez-Lopez, E.; Garcia, M.L.; Fernandes-Machado, N.R.C.; Severino, P.; Souto, E.B.; Ferreira da Silva, E. Development of Chitosan/Silver Sulfadiazine/Zeolite Composite Films for Wound Dressing. Pharmaceutics 2019, 11, 535. [Google Scholar] [CrossRef] [Green Version]
- Pohl, P. Determination and fractionation of metals in beer: A review. Food Addit. Contam. 2008, 25, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Almaguer, C.; Gastl, M.; Arendt, E.; Becker, T. Contributions of hop hard resins to beer quality. Brew. Sci. 2012, 65, 118–129. [Google Scholar]
- Avramia, I.; Amariei, S. Spent Brewer’s Yeast as a Source of Insoluble β-Glucans. Int. J. Mol. Sci. 2021, 22, 825. [Google Scholar] [CrossRef] [PubMed]
- Kemp, O.; Hofmann, S.; Braumann, I.; Jensen, S.; Fenton, A.; Oladokun, O. Changes in key hop-derived compounds and their impact on perceived dry-hop flavour in beers after storage at cold and ambient temperature. J. Inst. Brew. 2021, 127, 367–384. [Google Scholar] [CrossRef]
- Kobus, Z.; Wilczyński, K.; Nadulski, R.; Rydzak, L.; Guz, T. Effect of solvent polarity on the efficiency of ultrasound-assisted extraction of polyphenols from apple pomace. In Proceedings of the IX International Scientific Symposium “Farm Machinery and Processes Management in Sustainable Agriculture”, Lublin, Poland, 158–163 November 2017. [Google Scholar] [CrossRef]
- Bayliak, M.M.; Burdylyuk, N.I.; Lushchak, V.I. Quercetin increases stress resistance in the yeast Saccharomyces cerevisiae not only as an antioxidant. Ann. Microbiol. 2016, 66, 569–576. [Google Scholar] [CrossRef]
- Chong, S.Y.; Chiang, H.Y.; Chen, T.H.; Liang, Y.-J.; Lo, Y.-C. Green tea extract promotes DNA repair in a yeast model. Sci. Rep. 2019, 9, 3842. [Google Scholar] [CrossRef] [PubMed]
- Gernet, M.V.; Gribkova, I.N.; Borisenko, O.A.; Zakharov, M.A.; Zakharova, V.A. Migration of Hop Polyphenols in Beer Technology: Model Solution for Various Hopping Methods. Food Proc. Tech. Technol. 2021, 51, 628–638. [Google Scholar] [CrossRef]
- Gomes, F.O.; Guimarães, B.P.; Ceola, D.; Ghesti, G.F. Advances in dry hopping for industrial brewing: A review. Food Sci. Technol. 2021, 1–8. [Google Scholar] [CrossRef]
- Oladokun, O.; James, S.; Cowley, T.; Smart, K.; Hort, J.; Cook, D. Dry-Hopping: The effects of temperature and hop variety on the bittering profiles and properties of resultant beers. Brew. Sci. 2017, 70, 187–196. [Google Scholar] [CrossRef]
- Forster, A.; Gahr, A. On the Fate of Certain Hop Substances during Dry Hopping. Brew. Sci. 2013, 66, 93–103. [Google Scholar]
- Kim, J.S.; Lee, Y.-S. The Influence of pH on the Color Development of Melanoidins Formed from Fructose/Amino Acid Enantiomer Model Systems. Prev. Nutr. Food Sci. 2008, 13, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Doulias, P.T.; Gould, N.S. Analysis of Cysteine Post Translational Modifications Using Organic Mercury Resin. Curr. Protoc. Protein Sci. 2018, 94, e69. [Google Scholar] [CrossRef]
- Ardejani, M.S.; Powers, E.T.; Kelly, J.W. Using Cooperatively Folded Peptides to Measure Interaction Energies and Conformational Propensities. Acc. Chem. Res. 2018, 50, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Plazinski, W.; Plazinska, A. Molecular dynamics study of the interactions between phenolic compounds and alginate/alginic acid chains. New J. Chem. 2011, 35, 1607–1614. [Google Scholar] [CrossRef]
- Humia, B.V.; Santos, K.S.; Barbosa, A.M.; Sawata, M.; Mendonça, M.; Padilha, F.F. Beer Molecules and Its Sensory and Biological Properties: A Review. Molecules 2019, 24, 1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhães, P.; Dostálek, P.; Cruz, J.; Guido, L.; Barros, A. The Impact of a Xanthohumol-Enriched Hop Product on the Behavior of Xanthohumol and Isoxanthohumol in Pale and Dark Beers: A Pilot Scale Approach. J. Inst. Brew. 2008, 114, 246–256. [Google Scholar] [CrossRef]
- Wunderlich, S.; Zürcher, A.; Back, W. Enrichment of xanthohumol in the brewing process. Mol. Nutr. Food Res. 2005, 49, 874–881. [Google Scholar] [CrossRef]
- Zhang, B.; Chu, W.; Wei, P.; Liu, Y.; Wei, T. Xanthohumol induces generation of reactive oxygen species and triggers apoptosis through inhibition of mitochondrial electron transfer chain complex I. Free Radic. Biol. Med. 2015, 89, 486–497. [Google Scholar] [CrossRef]
- Martinez-Gomez, A.; Caballero, I.; Blanco, C.A. Phenols and Melanoidins as Natural Antioxidants in Beer. Structure, Reactivity and Antioxidant Activity. Biomolecules 2020, 10, 400. [Google Scholar] [CrossRef] [Green Version]
- Dvorakova, M.; Hulin, P.; Karabin, M.; Dostálek, P. Determination of polyphenols in beer by an effective method based on solid-phase extraction and high-performance liquid chromatography with diode-array detection. Czech J. Food Sci. 2007, 25, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Wannenmacher, J.; Gastl, M.; Becker, T. Phenolic Substances in Beer: Structural Diversity, Reactive Potential and Relevance for Brewing Process and Beer Quality. Compr. Rev. Food Sci. Food Saf. 2018, 17, 953–988. [Google Scholar] [CrossRef] [Green Version]
- Dai, Q.; He, Y.; Ho, C.T.; Wang, J.; Wang, S.; Yang, Y.; Gao, L.; Xia, T. Effect of interaction of epigallocatechin gallate and flavonols on color alteration of simulative green tea infusion after thermal treatment. J. Food Sci. Technol. 2017, 54, 2919–2928. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Iemma, F.; Puoci, F.; Cirillo, G.; Curcio, M.; Parisi, O.I.; Picci, N. Synthesis of antioxidant polymers by grafting of gallic acid and catechin on gelatin. Biomacromolecules 2009, 10, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Radonjić, S.; Maraš, V.; Raičević, J.; Košmerl, T. Wine or Beer? Comparison, Changes and Improvement of Polyphenolic Compounds during Technological Phases. Molecules 2020, 25, 4960. [Google Scholar] [CrossRef] [PubMed]
- Clippeleer, J.; Cooman, L.; Aerts, G. Beer’s bitter compounds—A detailed review on iso-a-acids: Current knowledge of the mechanisms for their formation and degradation. Brew. Sci. 2014, 67, 167–182. [Google Scholar]
- Hao, J.; Speers, R.A.; Fan, H.; Deng, Y.; Dai, Z. A Review of Cyclic and Oxidative Bitter Derivatives of Alpha, Iso-Alpha and Beta-Hop Acids. J. Am. Soc. Brew. Chem. 2020, 78, 89–102. [Google Scholar] [CrossRef]
- Wackerbauer, K.; Balzer, U. Hop Bitter Compounds in Beer. Part II: The Influence of Cohumulone on Beer Quality. Brew. Int. 1993, 11, 116–118. [Google Scholar]
- Rettberg, N.; Biendl, M.; Garbe, L. Hop Aroma and Hoppy Beer Flavor: Chemical Backgrounds and Analytical Tools—A Review. J. Am. Soc. Brew. Chem. 2018, 76, 1–20. [Google Scholar] [CrossRef]
- Intelmann, D.; Hofmann, T. On the Autoxidation of Bitter-Tasting Iso-α-acids in Beer. J. Agric. Food Chem. 2010, 58, 5059–5067. [Google Scholar] [CrossRef]
- Kishimoto, T.; Teramoto, S.; Fujita, A.; Yamada, O. Evaluation of Components Contributing to the International Bitterness Unit of Wort and Beer. J. Am. Soc. Brew. Chem. 2021, 80, 53–61. [Google Scholar] [CrossRef]
- Bettenhausen, H.M.; Benson, A.; Fisk, S.; Herb, D.; Hernandez, J.; Lim, J.; Queisser, S.H.; Shellhammer, T.H.; Vega, V.; Yao, L.; et al. Variation in Sensory Attributes and Volatile Compounds in Beers Brewed from Genetically Distinct Malts: An Integrated Sensory and Non-Targeted Metabolomics Approach. J. Am. Soc. Brew. Chem. 2020, 78, 136–152. [Google Scholar] [CrossRef] [Green Version]
- Loredana, L.; Giovanni, D.F.; Donatella, A.; Antonio, M.; Giuseppe, P.; Marisa, D.M.; Paola, R. Impact of Osmotic Distillation on the Sensory Properties and Quality of Low Alcohol Beer. J. Food Qual. 2018, 2018, 8780725. [Google Scholar] [CrossRef] [Green Version]
- Bamforth, C. Eyes, nose, and throat, the quality of beer. In Beer: Tap into the Art and Science of Brewing, 2nd ed.; Oxford University Press: New York, NY, USA, 2003; pp. 78–79. [Google Scholar]
- Biendl, M.; Engelhard, B.; Forster, A.; Gahr, A.; Lutz, A.; Mitter, W.; Schmidt, R.; Schönberger, C. Hops: Their Cultivation, Composition and Usage; Fachverlag Hans Carl: Nürnberg, Germany, 2015; ISBN 978-3-418-00823-3. [Google Scholar]
- Aron, P.M.; Shellhammer, T.H. Profiling of Hop-Derived Flavan-3-ols from Lager Beer in Relation to Hopping Technology. J. Am. Soc. Brew. Chem. 2017, 75, 276–282. [Google Scholar] [CrossRef]
- Tedesco, I.; Spagnuolo, C.; Bilotto, S.; Izzo, A.A.; Borrelli, F.; Rigano, D.; Russo, M.; Tarricone, F.; Russo, G.L. Antioxidant and Chemopreventive Effect of Aliophen® Formulation Based on Malts and Hops. Antioxidants 2021, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Kallithraka, S.; Bakker, J.; Clifford, M.N. Evaluation of bitterness and astringency of (+)-catechin and (−)-epicatechin in red wine and in model solution. J. Sens. Stud. 1997, 12, 25–37. [Google Scholar] [CrossRef]
- Thorngate, J.H.; Noble, A.C. Sensory evaluation of bitterness and astringency of 3R(−)-epicatechin and 3S(+)-catechin. J. Sci. Food Agric. 1995, 67, 531–535. [Google Scholar] [CrossRef]
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, S330–S335. [Google Scholar] [CrossRef] [Green Version]
- Vidal, S.; Cheynier, V.; Waters, E.; Noble, A.C. Effect of Tannin Composition and Wine Carbohydrates on Astringency and Bitterness. In 5th Pangborn Sensory Science Symposium; Meiselman, H., Ed.; Elsevier: Boston, MA, USA, 2003. [Google Scholar]
- Birch, G.G.; Ogunmoyela, G. Taste properties of cocoa drinks with an added bitter/sweet sugar: Intensity/time effects. Int. J. Food Sci. Technol. 1980, 15, 549–555. [Google Scholar] [CrossRef]
- Calvino, A.M.; García-Medina, M.R.; Cometto-Munoz, J.E. Interactions in caffeine-sucrose and coffee-sucrose mixtures: Evidence of taste and flavor suppression. Chem. Senses 1990, 15, 505–519. [Google Scholar] [CrossRef]
- Burns, D.J.W.; Noble, A.C. Evaluation of the separate contribution of viscosity and sweetness of sucrose to perceived viscosity, sweetness and bitterness of vermouth. J. Texture Stud. 1985, 16, 365–380. [Google Scholar] [CrossRef]
- Speegle, K. The Effect of Flavor and Sweetness on the Temporal Perception of Astringency and Fruitiness in Red Wine. Master’s Thesis, University of California, Berkeley, CA, USA, 2002. [Google Scholar]
- Ishikawa, T.; Noble, A.C. Temporal perception of astringency and sweetness in red wine. Food Qual. Prefer. 1995, 6, 27–33. [Google Scholar] [CrossRef]
- Smith, A.K.; June, H.; Noble, A.C. Effects of viscosity on the bitterness and astringency of grape seed tannin. Food Qual. Prefer. 1996, 7, 161–166. [Google Scholar] [CrossRef]
- Smith, A.K.; Noble, A.C. Effects of increased viscosity on the sourness and astringency of aluminum sulfate and citric acid. Food Qual. Pref. 1998, 9, 139–144. [Google Scholar] [CrossRef]
- Breslin, P.A.; Gilmore, M.; Beauchamp, G.K.; Green, B.G. Psychophysical evidence that oral astringency is a tactile sensation. Chem. Senses 1993, 18, 405–417. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; De Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef] [PubMed]
- Middle European Brewing Analysis Commission. Brautechnische Analysenmethoden, 3rd ed.; Band I Selbstverlag der MEBAK; Freising-Weihenstephan: Nüremberg, Germany, 1997. [Google Scholar]
- Institute of Brewing (IOB). Institute of Brewing (IOB) Recommended methods of analysis. EBC Method 4.9.3. J. Inst. Brew. 2007, 7, 54–76. [Google Scholar]
- Gutova, S.; Novoseltseva, M.; Kagan, E. Mathematical Modelling of Isohumulone Extraction Process in Beer Wort Hopping. In Proceedings of the 2019 International Russian Automation Conference, Sochi, Russia, 8 September 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Gottumukkala, R.V.; Nadimpalli, N.; Sukala, K.; Subbaraju, G.V. Determination of Catechin and Epicatechin Content in Chocolates by High-Performance Liquid Chromatography. Int. Sch. Res. Not. 2014, 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.C.; dos Anjos, J.P.; Guarieiro, L.L.N.; Machado, B.A.S. A Simple Method for Evaluating the Bioactive Phenolic Compounds’ Presence in Brazilian Craft Beers. Molecules 2021, 26, 4716. [Google Scholar] [CrossRef]
- Institute of Brewing (IOB). Institute of Brewing (IOB) Recommended methods of analysis. EBC Method 8.13.1. J. Inst. Brew. 2007, 7, 156–185. [Google Scholar]
- Koren, D.; Vecseri, B.H.; Kun-Farkas, G.; Urbin, Á.; Nyitrai, Á.; Sipos, L. How to objectively determine the color of beer? J. Food Sci. Technol. 2020, 57, 1183–1189. [Google Scholar] [CrossRef] [Green Version]
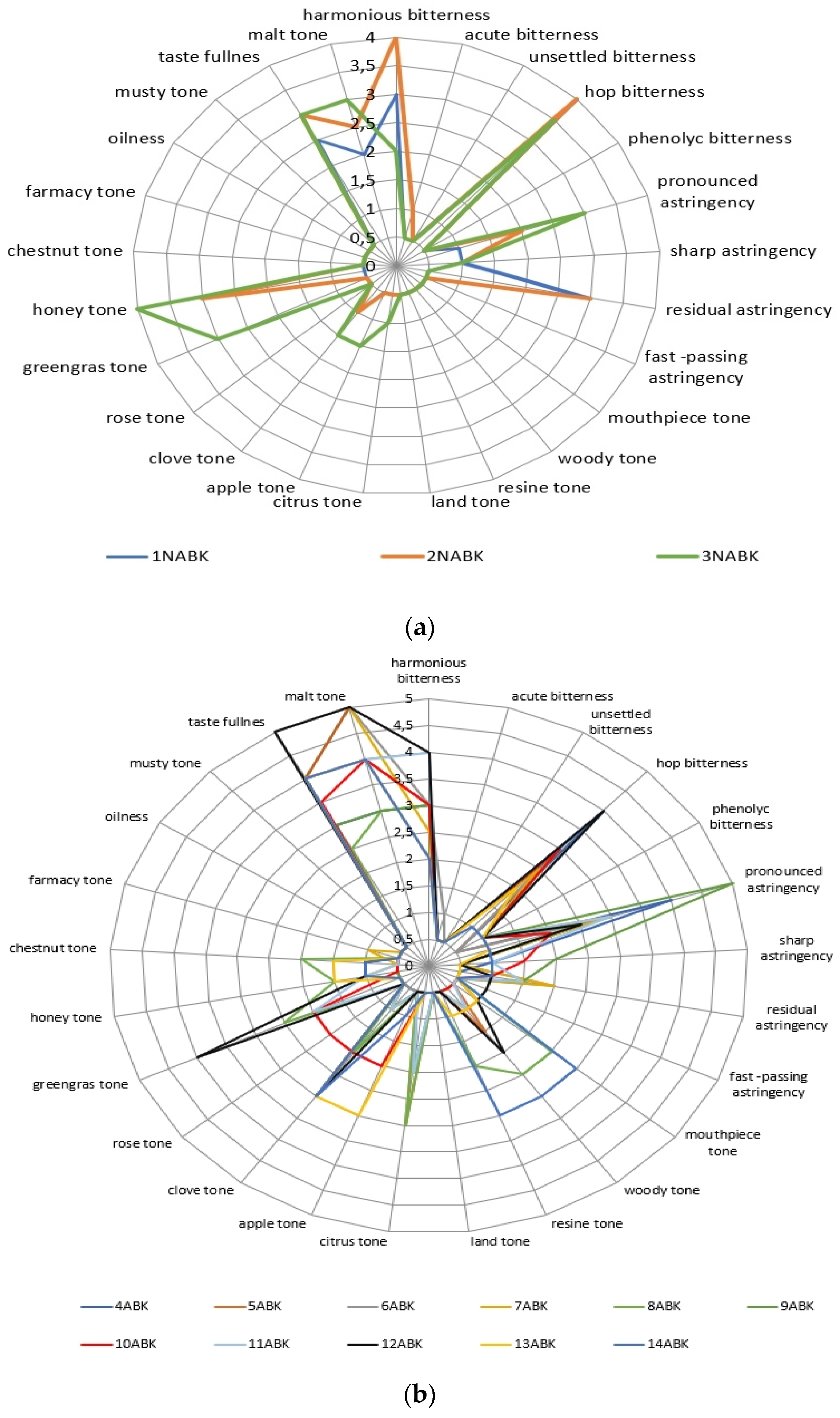
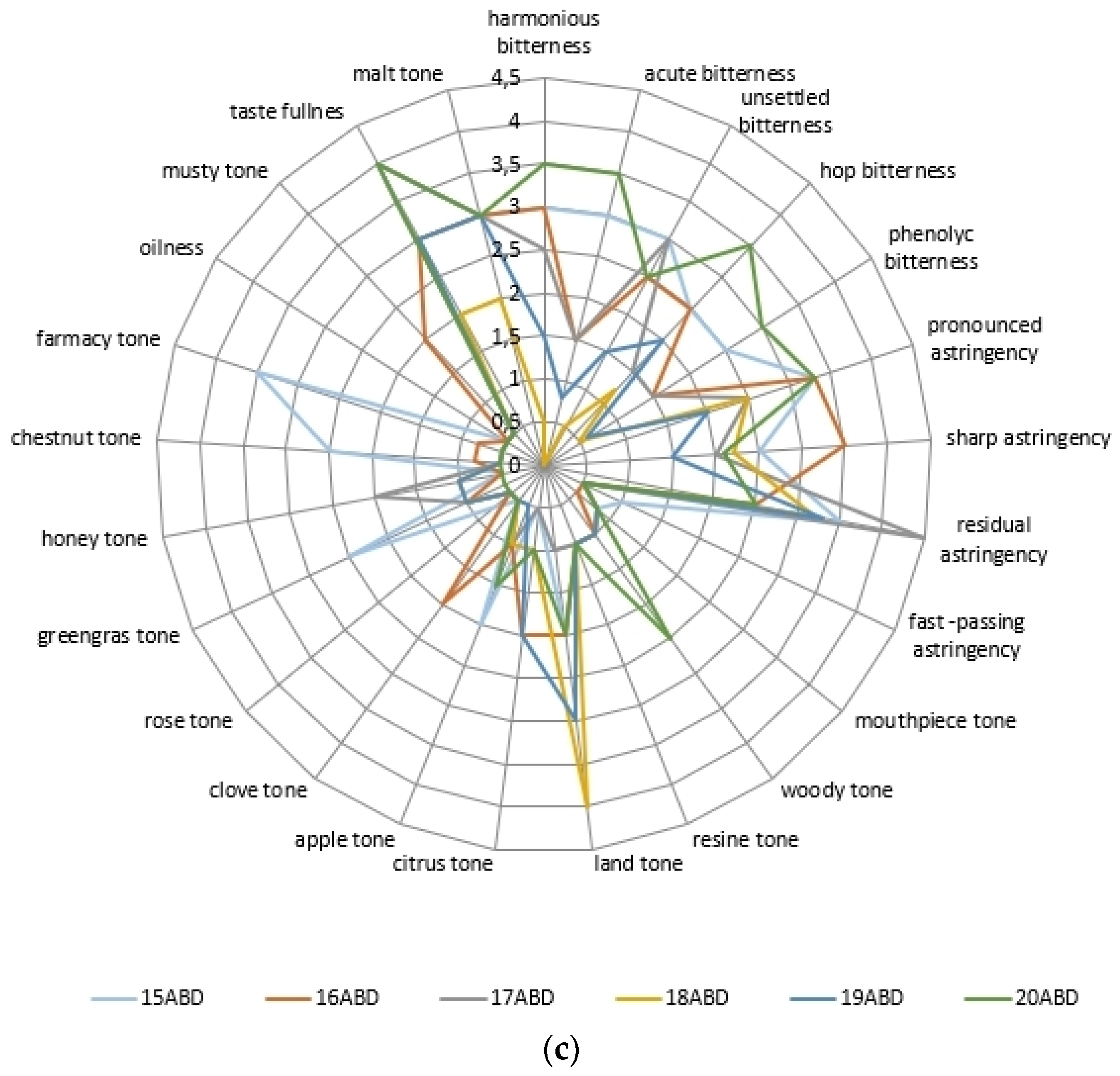
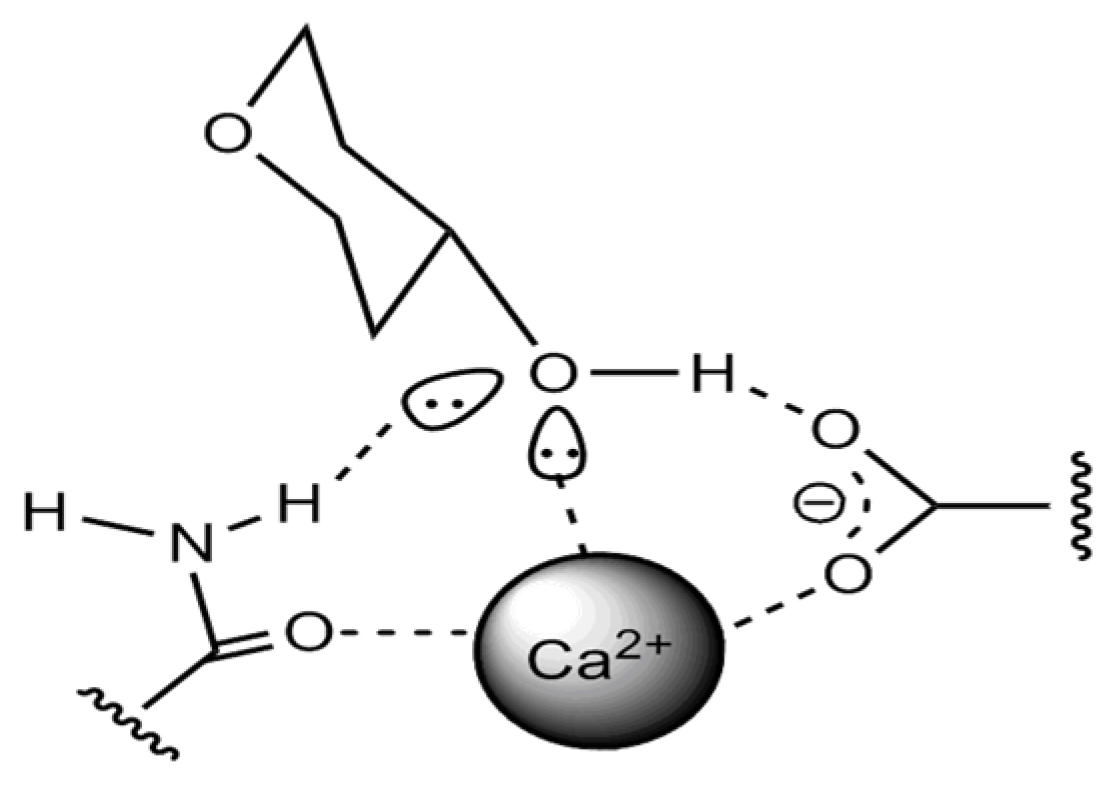
| Phenol Class/Compound | Associate Compounds | Plant Issue | Teste Contribution | References |
|---|---|---|---|---|
| Catechins (flavan-3-ols) | bitterness harsh, medicinal, and metallic | [9,10,11,12,13] | ||
| (+)-catechin | not associated | cereal/hop | ||
| (−)-epicatechin | not associated | hop | ||
| (+)-catechin | gallic acid | cereal | ||
| (−)-epicatechin | gallic acid, 4′-O-Methyl, glucuronic acid | hop | ||
| Proanthocyanidins (condensation products of flavan-3-ol monomers) Procianidins (di-, tri-, and tetra-catechin and epicatechin associated monomers) | gallic acid, 4′-O-Methyl, glucuronic acid | hop | bitterness | [9,10,11,12,13] |
| Prodelfinidins (gallocatec hin, epigallocatechin, and di-, tri-, and tetramers) | cereal | |||
| Flavanones Isoxanthohumol 6- and 8-prenylnaringenin 6-geranylnarin-genin | residual glucose | hop | bitterness | [14] |
| Flavones apigenin chrysoeriol tricin | residual glucose | cereals | astringency | [15] |
| Flavonols kaempferol quercetin rutin | residual glucose | cereals hop | bitterness | [16] |
| Monophenols Gallic acid, protocatechic acid, caffeic acid, vanillic acid, ferulic acid, p-coumaric acid, syringic acid, and their aldehydes | bound form as esters, glycosides, and bound complexes | cereal hop | harsh, bitter–sweet, sour, astringent, peppery, medicinal woody, roasted | [17,18,19,20] |
| Sample Number | The Content in Samples, mg/L, Reliability Limit p ˂ 0.05 | |||||
|---|---|---|---|---|---|---|
| Alcohol, vol% | Original Extract, °P | β-Glucan (Gl) | Iso-α-Humulon (IBU) (IH) | Soluble Nitrogen (SN) | Color, °EBC | |
| 1NABK | (0.49 ± 0.05) * | 7.0 ± 0.70 | 65.0 ± 4.6 | 11.8 ± 0.06 | 300.4 ± 12 | 6.75 ± 0.20 |
| 2NABK | 0.48 ± 0.05 | 7.5 ± 0.70 | 69.8 ± 4.9 | 21.6 ± 0.11 | 439.8 ± 18 | 5.00 ± 0.15 |
| 3NABK | 0.48 ± 0.05 | 7.8 ± 0.80 | 108.6 ± 7.6 | 13.5 ± 0.07 | 630.6 ± 25 | 7.50 ± 0.22 |
| 4ABK | 4.6 ± 0.40 | 10.7 ± 1.00 | 62.1 ± 4.3 | 9.7 ± 0.05 | 459.4 ± 20 | 5.25 ± 0.16 |
| 5ABK | 4.5 ± 0.40 | 10.8 ± 1.00 | 124.1 ± 8.7 | 6.3 ± 0.03 | 445.4 ± 25 | 5.75 ± 0.17 |
| 6ABK | 5.1 ± 0.50 | 11.0 ± 1.00 | 62.0 ± 4.3 | 12.9 ± 0.06 | 984.0 ± 40 | 7.50 ± 0.22 |
| 7ABK | 4.7 ± 0.40 | 11.3 ± 1.00 | 77.6 ± 5.4 | 24.4 ± 0.12 | 1185.0 ± 47 | 106.3 ± 3.19 |
| 8ABK | 4.8 ± 0.50 | 11.6 ± 1.00 | 62.1 ± 4.3 | 26.3 ± 0.13 | 823.6 ± 33 | 6.75 ± 0.20 |
| 9ABK | 4.5 ± 0.40 | 11.8 ± 1.00 | 75.6 ± 5.3 | 12.3 ± 0.06 | 980.0 ± 40 | 25.0 ± 0.75 |
| 10ABK | 5.0 ± 0.50 | 11.9 ± 1.00 | 128.0 ± 9.0 | 14.1 ± 0.07 | 306.8 ± 12 | 5.25 ± 0.16 |
| 11ABK | 5.2 ± 0.50 | 12.0 ± 1.00 | 120.3 ± 8.4 | 12.2 ± 0.06 | 743.0 ± 30 | 7.25 ± 0.21 |
| 12ABK | 5.3 ± 0.50 | 12.8 ± 1.00 | 240.5 ± 16.8 | 4.9 ± 0.02 | 972.1 ± 39 | 9.50 ± 0.29 |
| 13ABK | 8.1 ± 0.80 | 16.5 ± 1.50 | 93.1 ± 6.5 | 26.5 ± 0.13 | 888.0 ± 36 | 5.25 ± 0.16 |
| 14ABK | 9.2 ± 0.90 | 18.6 ± 1.50 | 96.2 ± 6.7 | 12.7 ± 0.06 | 854.4 ± 34 | 17.5 ± 0.53 |
| 15ABD | 4.6 ± 0.40 | 10.0 ± 1.00 | 31.0 ± 2.2 | 29.1 ± 0.15 | 560.3 ± 22 | 9.50 ± 0.29 |
| 16ABD | 4.9 ± 0.50 | 12.0 ± 1.00 | 93.1 ± 6.5 | 28.7 ± 0.14 | 935.7 ± 37 | 5.25 ± 0.16 |
| 17ABD | 6.6 ± 0.70 | 14.5 ± 1.00 | 186.2 ± 13.0 | 32.4 ± 0.16 | 823.6 ± 33 | 12.5 ± 0.38 |
| 18ABD | 5.9 ± 0.60 | 15.0 ± 1.50 | 155.2 ± 10.9 | 58.3 ± 0.30 | 767.6 ± 30 | 16.5 ± 0.50 |
| 19ABD | 5.9 ± 0.60 | 16.0 ± 1.50 | 74.5 ± 5.2 | 42.6 ± 0.21 | 798.4 ± 32 | 5.25 ± 0.16 |
| 20ABD | 7.7 ± 0.80 | 17.5 ± 1.50 | 108.6 ± 7.6 | 76.2 ± 0.36 | 1075.7 ± 43 | 17.0 ± 0.51 |
| Sample Number | The Polyphenol Content in Samples, mg/L, Reliability Limit p ˂ 0.05 | ||||
|---|---|---|---|---|---|
| Total | Isoxanthohumol (IXG) | (+)Catechin (Ct) | Quercetin (Qv) | Rutin (Rt) | |
| 1NABK | (41.0 ± 3.7) * | 1.6 ± 0.02 | 1.24 ± 0.01 | 11.02 ± 0.10 | 3.53 ± 0.03 |
| 2NABK | 65.6 ± 6.0 | 1.1 ± 0.01 | 1.73 ± 0.01 | 2.78 ± 0.03 | 5.24 ± 0.05 |
| 3NABK | 82.0 ± 7.4 | 2.4 ± 0.02 | 3.96 ± 0.04 | 0.75 ± 0.01 | 6.56 ± 0.07 |
| 4ABK | 106.6 ± 9.6 | 2.2 ± 0.02 | 3.71 ± 0.04 | 9.90 ± 0.10 | 3.50 ± 0.04 |
| 5ABK | 98.4 ± 8.9 | 3.7 ± 0.04 | 2.97 ± 0.03 | 12.84 ± 0.10 | 7.39 ± 0.07 |
| 6ABK | 114.8 ± 10.3 | 4.5 ± 0.04 | 3.96 ± 0.04 | 12.09 ± 0.10 | 7.72 ± 0.08 |
| 7ABK | 139.4 ± 12.5 | 7.4 ± 0.07 | 21.78 ± 0.22 | 12.58 ± 0.10 | 6.05 ± 0.06 |
| 8ABK | 139.4 ± 12.5 | 3.8 ± 0.04 | 2.72 ± 0.03 | 11.94 ± 0.10 | 8.20 ± 0.08 |
| 9ABK | 172.2 ± 15.5 | 5.2 ± 0.05 | 21.78 ± 0.22 | 11.98 ± 0.10 | 7.83 ± 0.08 |
| 10ABK | 106.6 ± 9.6 | 3.0 ± 0.03 | 3.96 ± 0.04 | 12.83 ± 0.10 | 6.41 ± 0.06 |
| 11ABK | 123.0 ± 11.1 | 4.0 ± 0.04 | 4.21 ± 0.04 | 12.71 ± 0.10 | 8.74 ± 0.09 |
| 12ABK | 188.6 ± 11.3 | 2.2 ± 0.02 | 5.94 ± 0.06 | 2.46 ± 0.02 | 12.98 ± 0.13 |
| 13ABK | 287.0 ± 25.8 | 6.1 ± 0.06 | 8.42 ± 0.08 | 31.02 ± 0.30 | 1.96 ± 0.02 |
| 14ABK | 237.8 ± 21.4 | 3.5 ± 0.04 | 12.87 ± 0.13 | 22.10 ± 0.20 | 1.84 ± 0.02 |
| 15ABD | 147.6 ± 13.3 | 4.2 ± 0.04 | 7.43 ± 0.07 | 21.55 ± 0.20 | 2.80 ± 0.03 |
| 16ABD | 164.0 ± 14.8 | 3.4 ± 0.03 | 6.44 ± 0.05 | 13.04 ± 0.10 | 13.64 ± 0.14 |
| 17ABD | 213.2 ± 19.2 | 4.7 ± 0.05 | 8.91 ± 0.09 | 14.57 ± 0.10 | 4.43 ± 0.04 |
| 18ABD | 131.2 ± 11.8 | 4.6 ± 0.05 | 7.92 ± 0.08 | 24.20 ± 0.20 | 2.14 ± 0.02 |
| 19ABD | 192.7 ± 17.3 | 5.3 ± 0.05 | 10.89 ± 0.11 | 33.41 ± 0.30 | 2.38 ± 0.02 |
| 20ABD | 328.0 ± 29.5 | 9.4 ± 0.10 | 10.40 ± 0.10 | 20.20 ± 0.20 | 4.11 ± 0.04 |
| Indicators | The Beer’s Samples Group | |
|---|---|---|
| Kettle Hoped | Dry Hoped | |
| Bitterness Descriptor: harmonious bitterness (hb) | ||
| significant compounds according to the elasticity coefficient (EC) | IH (EC = 0.86); IXG (EC = −0.84); Rt (EC = 0.57) | SN (EC = 1.73); Qv (EC = −1.34); IH (EC = −1.04); Gl (EC = −0.79); IXG (EC = 0.76) |
| the descriptor influencing factors and their correlation coefficient (Rc) | IXG/SN (Rc = 0.73); IH/Ct (Rc = 0.72); Ct/SN (Rc = 0.69); hb/Rt (Rc = 0.62) IXG/Qv (Rc = 0.58); hb/Qv (Rc = −0.52) hb-Rt/SN (Rc = 0.71); hb-Rt/IXG (Rc = 0.66) hb-Rt/Ct (Rc = 0.64); hb-Rt/Gl (Rc = 0.56) hb-Qv/SN (Rc = 0.50) | IH/IXG (Rc = 0.84); IXG/Ct (Rc = 0.75) IH/SN (Rc = 0.64); IH-IXG/hb (Rc = 0.96) hb-IXG/IH (Rc = 0.89); hb-IH/IXG (Rc = −0.85) IXG-Ct/hb (Rc = 0.77); IH-SN/hb (Rc = 0.71) hb-IXG/Rt (Rc = 0.69); IXG-SN/hb (Rc = 0.67) hb-IXG/Qv (Rc = 0.63); Qv-Gl/hb (Rc = −0.63) Ct-Rt/hb (Rc = −0.63); hb-IXG/Gl (Rc = 0.55) |
| the general correlation coefficient (GCC) | 0.78 | 1.0 |
| the general determination coefficient (GDC) | 0.62 | 1.0 |
| the unreported compounds contribution, % | 38.4 | 0.0 |
| Descriptor: acute bitterness (ab) | ||
| significant compounds according to the elasticity coefficient (EC) | Gl (EC = −0.57); IXG (EC = −0.54) Rt (EC = 0.52); SN (EC = 0.51) | SN (EC = −4.23); Ct (EC = 2.12) Qv (EC = −1.94); IXG (EC = 1.72) IH (EC = 0.77); Gl (EC = −0.64) |
| the descriptor influencing factors and their correlation coefficient (Rc) | Ct/SN (Rc = 0.69); IXG/SN (Rc = 0.62) ab/IH (Rc = 0.61); ab-IH/IXG (Rc = 0.54) ab-IH/Qv (Rc = 0.59); ab-IH/Ct (Rc = 0.52) IXG-Ct/Gl (Rc = 0.73); IXG-Ct/IH (Rc = 0.73) IXG-Ct/Rt (Rc = 0.72); IXG-Ct/Qv (Rc = 0.72) | ab/IXG (Rc = 0.72); IH/IXG (Rc = 0.84) IXG/Ct (Rc = 0.74); IXG/SN (Rc = 0.74) IH/SN (Rc = 0.64); IH-IXG/ab (Rc = 0.91) IXG-Ct/ab (Rc = 0.79); IXG-SN/ab (Rc = 0.71) IH-SN/ab (Rc = 0.69) |
| the general correlation coefficient (GCC) | 0.89 | 1.0 |
| the general determination coefficient (GDC) | 0.80 | 1.0 |
| the unreported compounds contribution, % | 19.6 | 0.0 |
| Descriptor: hop bitterness (hb) | ||
| significant compounds according to the elasticity coefficient (EC) | IXG (EC = 0.59); GL (EC = −0,29) Qv (EC = −0,22); Rt (EC = 0.20) SN (EC = −0.20) | SN (EC = −3.59); IH (EC = −1.23) Gl (EC = −1.13); Ct (EC = −1.02) IXG (EC = −0.92) |
| the descriptor influencing factors and their correlation coefficient (Rc) | hb/Qv (Rc = 0.74); hb/IXG (Rc = 0.58) IXG/SN (Rc = 0.79); IXG/Ct (Rc = 0.72) IH/Gl (Rc = 0.50); hb-IXG/Rt (Rc = 0.76) hb-Qv/IH (Rc = −0.71); hb-IXG/Gl (Rc = −0.57); hb-IXG/IH (Rc = 0.52) | IH/IXG (Rc = 0.80); IXG/Ct (Rc = 0.77) IXG/SN (Rc = 0.76); IH/SN (Rc = 0.62) hb-IH/IXG (Rc = −0.87); hb-IXG/IH (Rc = 0.81) IXG-Ct/hb (Rc = 0.79); hb-Qv/Gl (Rc = −0.77) hb-IXG/Rt (Rc = 0.61); Qv-Gl/hb (Rc = 0.73) IH-SN/hb (Rc = 0.72); IXG-SN/hb (Rc = 0.71) |
| the general correlation coefficient (GCC) | 0.84 | 1.0 |
| the general determination coefficient (GDC) | 0.70 | 1.0 |
| the unreported compounds contribution, % | 29.9 | 0.0 |
| Descriptor: phenolic bitterness (pb) | ||
| significant compounds according to the elasticity coefficient (EC) | IXG (EC = −0.55): Qv (EC = 0.52) Rt (EC = 0.55) | SN (EC = 2.52); Qv (EC = −2.31); Gl (EC = −1.24); Rt (EC = −0.80) |
| the descriptor influencing factors and their correlation coefficient (Rc) | pb/Qv (Rc = 0.43); IXG/SN (Rc = 0.73) IXG/Ct (Rc = 0.72); IXG/Rt (Rc = 0.59) Ct-SN/pb (Rc = 0.64); Rt-Gl/pb (Rc = 0.63) IH-Gl/pb (Rc = −0.59); pb-Qv/Rt (Rc = 0.56); pb-Qv/Gl (Rc = 0.51) | IH/IXG (Rc = 0.78); IH/Ct (Rc = 0.52) IH-IXG/pb (Rc = 0.90); IXG-Ct/pb (Rc = 0.85); pb-IH/IXG (Rc = −0.71); IXG-SN/pb (Rc = 0.70) Qv-Rt/pb (Rc = 0.69); pb-IXG/Ct (Rc = 0.64) IH-SN/pb (Rc = 0.59) |
| the general correlation coefficient (GCC) | 0.81 | 1.0 |
| the general determination coefficient (GDC) | 0.65 | 1.0 |
| the unreported compounds contribution, % | 34.5 | 0.0 |
| Astringency Descriptor: pronounced astringency (pa) | ||
| significant compounds according to the elasticity coefficient (EC) | Gl (EC = −0.58); Rt (EC = 0.55); Ct (EC = 0.50); IXG (EC= −0.46) | SN (EC = −6.93); Ct (EC = 6.00); IH (EC = 3.14) Qv (EC = −2.36); Gl (EC= −1.26); Rt (EC = 1.17) |
| the descriptor influencing factors and their correlation coefficient (Rc) | pa/Rt (Rc = 0.55); pa-IXG/Rt (Rc = 0.56) pa-Qv/IXG (Rc = −0.64); pa-Rt/Gl (Rc = 0.64); Ct-SN/pa (Rc = 0.67) | pa-IXG/Ct (Rc = 0.89); pa-Qv/IH (Rc = 0.88); pa/Ct (Rc = −0.54); pa/Qv (Rc = −0.74); pa-Ct/SN (Rc = −0.70); IXG-SN/pa (Rc = 0.73) pa-IH/Ct (Rc = 0.69); pa/Rt (Rc = 0.50) |
| the general correlation coefficient (GCC) | 0.85 | 1.0 |
| the general determination coefficient (GDC) | 0.72 | 1.0 |
| the unreported compounds contribution, % | 27.9 | 0.0 |
| Descriptor: sharp astringency (sa) | ||
| significant compounds according to the elasticity coefficient (EC) | SN (EC = 1.08); IXG (EC = −0.97) Rt (EC = 0.85); Ct (EC = 0.72) | Ct (EC = 7.26); SN (EC = −6.36); IH (EC = 2.34); IXG (EC= −1.61); Qv (EC = −1.78); Rt (EC = 1.30) |
| the descriptor influencing factors and their correlation coefficient (Rc) | sa-Ct/SN (Rc = 0.68); sa-IXG/Ct (Rc = 0.60) Ct-SN/sa (Rc = 0.83); IH-Ct/sa (Rc = 0.82) Rt-Gl/sa (Rc = 0.62); IH-Gl/sa (Rc = 0.56) | sa/Gl (Rc = 0.77); sa-Gl/IH (Rc = 0.91); sa-Gl/Ct,Qv, Rt,SN (Rc = 0.86); sa-IH/Gl (Rc = −0.80); sa-Gl/IXG (Rc = 0.79); IXG-Ct/sa (Rc = 0.76); IXG-SN/sa (Rc = 0.75) |
| the general correlation coefficient (GCC) | 0.85 | 1.0 |
| the general determination coefficient (GDC) | 0.72 | 1.0 |
| the unreported compounds contribution, % | 28.4 | 0.0 |
| Descriptor: residual astringency (ra) | ||
| significant compounds according to the elasticity coefficient (EC) | IXG (EC= 0.69); IH (EC = −0.66) Gl (EC = −0.62); SN (EC = 0.53) Qv (EC = −0.52) | SN (EC = 65.56); Ct (EC = −77.84); IH (EC = −33.37); IXG (EC= 23.67); Qv (EC = 26.106); Rt (EC = −11.44) |
| the descriptor influencing factors and their correlation coefficient (Rc) | IXG-SN/ra (Rc = 0.73); IXG-Ct/ra (Rc = 0.72) IH-IXG/ra (Rc = 0.71); IXG-Qv/ra (Rc = 0.65) | IH-IXG/ra (Rc = 0.83); ra-SN/Gl (Rc = −0.79); Qv-Rt/ra (Rc = −0.77); SN-Gl/ra (Rc = 0.75); ra-IH/Rt (Rc = −0.70); Ct-Qv/ra (Rc = 0.69); IXG-Ct/ra (Rc = 0.68); ra-Rt/Qv (Rc = −0.66); ra-Gl/IH (Rc = 0.65); IH-Rt/ra (Rc = −0.64); Ct-Rt/ra (Rc = −0.64); ra-IH/Ct (Rc = −0.55) |
| the general correlation coefficient (GCC) | 0.61 | 1.0 |
| the general determination coefficient (GDC) | 0.37 | 1.0 |
| the unreported compounds contribution, % | 62.8 | 0.0 |
| Sample Code | Raw Material List | Yeast Type | Color | Hopping Technology |
|---|---|---|---|---|
| 7 ÷ 8 °P | ||||
| 1NABK * | light barley malt, hop | lager | light | kettle hopping |
| 2NABK | light barley malt, hop products | lager | light | |
| 3NABK | wheat and barley malts, hop products | ale | light | |
| 11 ÷ 13 °P | ||||
| 4ABK ** | light barley malt, rice, hop, hop products | lager | light | kettle hopping |
| 5ABK | light barley malt, maize, hop, hop products | lager | light | |
| 6ABK | light barley malt, hop | lager | light | |
| 7ABK | light barley and caramel malts, hop and hop products | lager | dark | |
| 8ABK | light barley malt, hop | lager | light | |
| 9ABK | light barley and caramel malts, hop and hop products | lager | dark | |
| 10ABK | light barley malt, hop | lager | light | |
| 11ABK | light barley malt, hop and hop products | lager | light | |
| 12ABK | wheat and barley malts, hop and hop products | ale | light | |
| 14 ÷ 18 °P | ||||
| 13ABK | dark barley malt, wheat, sugar, hop, hop products | ale | light | kettle hopping |
| 14ABK | dark barley malt, wheat, hop, hop products | ale | dark | |
| 10 ÷ 18 °P (IPA) | ||||
| 15ABD *** | light barley malt, hop products | lager | light | dry hopping |
| 16ABD | light barley malt, hop products | lager | light | |
| 17ABD | light barley malt, hop products | ale | light | |
| 18ABD | light and caramel, wheat malts, hop products | lager | light | |
| 19ABD | light barley malt, hop | lager | light | |
| 20ABD | light and caramel barley malts, hop products | ale | light | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gribkova, I.N.; Kharlamova, L.N.; Lazareva, I.V.; Zakharov, M.A.; Zakharova, V.A.; Kozlov, V.I. The Influence of Hop Phenolic Compounds on Dry Hopping Beer Quality. Molecules 2022, 27, 740. https://doi.org/10.3390/molecules27030740
Gribkova IN, Kharlamova LN, Lazareva IV, Zakharov MA, Zakharova VA, Kozlov VI. The Influence of Hop Phenolic Compounds on Dry Hopping Beer Quality. Molecules. 2022; 27(3):740. https://doi.org/10.3390/molecules27030740
Chicago/Turabian StyleGribkova, Irina N., Larisa N. Kharlamova, Irina V. Lazareva, Maxim A. Zakharov, Varvara A. Zakharova, and Valery I. Kozlov. 2022. "The Influence of Hop Phenolic Compounds on Dry Hopping Beer Quality" Molecules 27, no. 3: 740. https://doi.org/10.3390/molecules27030740
APA StyleGribkova, I. N., Kharlamova, L. N., Lazareva, I. V., Zakharov, M. A., Zakharova, V. A., & Kozlov, V. I. (2022). The Influence of Hop Phenolic Compounds on Dry Hopping Beer Quality. Molecules, 27(3), 740. https://doi.org/10.3390/molecules27030740





