Chemical Profile of Lipophilic Fractions of Different Parts of Zizyphus lotus L. by GC-MS and Evaluation of Their Antiproliferative and Antibacterial Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Yield
2.2. Lipophilic Composition
2.2.1. Fatty Acids
2.2.2. Monoglycerides
2.2.3. Long-Chain Aliphatic Alcohols
2.2.4. Pentacyclic Triterpenic Compounds
2.2.5. Sterols
2.2.6. Aromatic and Other Compounds
2.3. Biological Activities of Lipophilic Z. lotus Extracts
2.3.1. Antiproliferative Activity
2.3.2. Antibacterial Activity
3. Materials and Methods
3.1. Reagents
3.2. Samples
3.3. Extraction
3.4. GC–MS Analysis
3.4.1. Derivatization
3.4.2. GC–MS Conditions
3.4.3. Quantitative Analysis
3.5. Antiproliferative Activity
3.5.1. Cell Culture
3.5.2. Cell Viability Assay
3.5.3. Statistical Analysis
3.6. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Maraghni, M.; Gorai, M.; Neffati, M. Seed germination at different temperatures and water stress levels, and seedling emergence from different depths of Ziziphus lotus. S. Afr. J. Bot. 2010, 76, 453–459. [Google Scholar] [CrossRef]
- Sheng, J.P.; Shen, L. 13-Chinese jujube (Ziziphus jujuba Mill.) and Indian jujube (Ziziphus mauritiana Lam.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Woodhead Publishing Limited: Oxford, UK, 2011; pp. 299–326. [Google Scholar]
- Regehr, D.L.; El Brahli, A. Wild Jujube (Ziziphus lotus) Control in Morocco. Weed Technol. 1995, 9, 326–330. [Google Scholar] [CrossRef]
- Benammar, C.; Hichami, A.; Yessoufou, A.; Simonin, A.M.; Belarbi, M.; Allali, H.; Khan, N.A. Zizyphus lotus L. (Desf.) modulates antioxidant activity and human T-cell proliferation. BMC Complement. Altern. Med. 2010, 10, 1–9. [Google Scholar] [CrossRef]
- Borgi, W.; Ghedira, K.; Chouchane, N. Antiinflammatory and analgesic activities of Zizyphus lotus root barks. Fitoterapia 2007, 78, 16–19. [Google Scholar] [CrossRef]
- Abdeddaim, M.; Lombarkia, O.; Bacha, A.; Fahloul, D.; Abdeddaim, D.; Farhat, R.; Saadoudi, M.; Noui, Y.; Lekbir, A. Biochemical characterization and nutritional properties of Zizyphus lotus L. fruits in Aures region, northeastern of Algeria. Food Sci. Technol. 2014, 15, 75–81. [Google Scholar]
- Maciuk, A.; Ghedira, K.; Thepenier, P.; Lavaud, C.; Zeches-Hanrot, M. A new flavonol glycoside from leaves of Zizyphus Lotus. Die Pharm.-Int. J. Pharm. Sci. 2003, 58, 158–159. [Google Scholar] [CrossRef]
- Rached, W.; Barros, L.; Ziani, B.E.C.; Bennaceur, M.; Calhelha, R.C.; Heleno, S.A.; Alves, M.J.; Marouf, A.; Ferreira, I.C.F.R. HPLC-DAD-ESI-MS/MS screening of phytochemical compounds and the bioactive properties of different plant parts of Zizyphus lotus (L.) Desf. Food Funct. 2019, 10, 5898–5909. [Google Scholar] [CrossRef]
- Benammar, C.; Baghdad, C. Antidiabetic and Antioxidant Activities of Zizyphus lotus L. Aqueous Extracts in Wistar Rats. J. Nutr. Food Sci. 2014, s8, 8–13. [Google Scholar] [CrossRef]
- Bakhtaoui, F.Z.; Lakmichi, H.; Megraud, F.; Chait, A.; Gadhi, C.E.A. Gastro-protective, anti-Helicobacter pylori and, antioxidant properties of Moroccan Zizyphus lotus L. J. Appl. Pharm. Sci. 2014, 4, 81–87. [Google Scholar] [CrossRef]
- Borgi, W.; Chouchane, N. Anti-spasmodic effects of Zizyphus lotus (L.) Desf. extracts on isolated rat duodenum. J. Ethnopharmacol. 2009, 126, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Chouaibi, M.; Mahfoudhi, N.; Rezig, L.; Donsì, F.; Ferrari, G.; Hamdi, S. Nutritional composition of Zizyphus lotus L. seeds. J. Sci. Food Agric. 2012, 92, 1171–1177. [Google Scholar] [CrossRef]
- Ghazghazi, H.; Aouadhi, C.; Riahi, L.; Maaroufi, A.; Hasnaoui, B. Fatty acids composition of Tunisian Ziziphus lotus L. (Desf.) fruits and variation in biological activities between leaf and fruit extracts. Nat. Prod. Res. 2014, 28, 1106–1110. [Google Scholar] [CrossRef]
- Cadi, H.E.; Bouzidi, H.E.; Selama, G.; Cadi, A.E.; Ramdan, B.; Oulad El Majdoub, Y.; Alibrando, F.; Dugo, P.; Mondello, L.; Lanjri, A.F.; et al. Physico-Chemical and Phytochemical Characterization of Moroccan Wild Jujube “Zizyphus lotus (L.)” Fruit Crude Extract and Fractions. Molecules 2020, 25, 5237. [Google Scholar] [CrossRef]
- Ghedira, K.; Chemli, R.; Richard, B.; Nuzillard, J.-M.; Zeches, M.; Men-Olivier, L. Le Two cyclopeptide alkaloids from Zizyphus lotus. Phytochemistry 1993, 32, 1591–1594. [Google Scholar] [CrossRef]
- Ghedira, K.; Chemli, R.; Caron, C.; Nuzilard, J.M.; Zeches, M.; Le Men-Olivier, L. Four cyclopeptide alkaloids from Zizyphus lotus. Phytochemistry 1995, 38, 767–772. [Google Scholar] [CrossRef]
- Le Crouéour, G.; Thépenier, P.; Richard, B.; Petermann, C.; Ghédira, K.; Zèches-Hanrot, M. Lotusine G: A new cyclopeptide alkaloid from Zizyphus lotus. Fitoterapia 2002, 73, 63–68. [Google Scholar] [CrossRef]
- Maciuk, A.; Lavaud, C.; Thépenier, P.; Jacquier, M.J.; Ghédira, K.; Zèches-Hanrot, M. Four new dammarane saponins from Zizyphus lotus. J. Nat. Prod. 2004, 67, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Jmiai, A.; El Ibrahimi, B.; Tara, A.; Chadili, M.; El Issami, S.; Jbara, O.; Khallaayoun, A.; Bazzi, L. Application of Zizyphus Lotuse—Pulp of Jujube extract as green and promising corrosion inhibitor for copper in acidic medium. J. Mol. Liq. 2018, 268, 102–113. [Google Scholar] [CrossRef]
- El Aloui, M.; Mguis, K.; Laamouri, A.; Albouchi, A.; Cerny, M.; Mathieu, C.; Vilarem, G.; Hasnaoui, B. Fatty acid and sterol oil composition of four Tunisian ecotypes of Ziziphus zizyphus (L.) H.Karst. Acta Bot. Gall. 2012, 159, 25–31. [Google Scholar] [CrossRef]
- Borgi, W.; Recio, M.C.; Ríos, J.L.; Chouchane, N. Anti-inflammatory and analgesic activities of flavonoid and saponin fractions from Zizyphus lotus (L.) Lam. S. Afr. J. Bot. 2008, 74, 320–324. [Google Scholar] [CrossRef]
- Wahida, B.; Abderrahman, B.; Nabil, C. Antiulcerogenic activity of Zizyphus lotus (L.) extracts. J. Ethnopharmacol. 2007, 112, 228–231. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.A.; Tang, Y.P.; Yang, N.Y.; Qian, D.W.; Su, S.L.; Shang, E.X. Characterization of triterpenic acids in fruits of ziziphus species by HPLC-ELSD-MS. J. Agric. Food Chem. 2010, 58, 6285–6289. [Google Scholar] [CrossRef]
- Masullo, M.; Montoro, P.; Autore, G.; Marzocco, S.; Pizza, C.; Piacente, S. Quali-quantitative determination of triterpenic acids of Ziziphus jujuba fruits and evaluation of their capability to interfere in macrophages activation inhibiting NO release and iNOS expression. Food Res. Int. 2015, 77, 109–117. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Guerra, A.R.; Duarte, M.; Freire, C.S.R.; Neto, C.P.; Silva, C.M.S.; Silvestre, A.J.D. Bioactive triterpenic acids: From agroforestry biomass residues to promising therapeutic tools. Mini. Rev. Org. Chem. 2014, 11, 382–399. [Google Scholar] [CrossRef]
- Rsaissi, N.; Kamili, E.L.; Bencharki, B.; Hillali, L.; Bouhache, M. Antimicrobial activity of fruits extracts of the wild jujube “Ziziphus lotus” (L.) Desf. Int. J. Sci. Eng. Res. 2013, 4, 1521–1528. [Google Scholar]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharm. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Annarao, S.; Sidhu, O.P.; Roy, R.; Tuli, R.; Khetrapal, C.L. Lipid profiling of developing Jatropha curcas L. seeds using (1)H NMR spectroscopy. Bioresour. Technol. 2008, 99, 9032–9035. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Valencia, V.A.; Us-Vázquez, R.A.; Larqué-Saavedra, F.A.; Barahona-Pérez, L.F. Naturally occurring fatty acid methyl esters and ethyl esters in the green microalga Chlamydomonas reinhardtii. Ann. Microbiol. 2012, 62, 865–870. [Google Scholar] [CrossRef]
- Widad, O.; Hamza, F.; Youcef, M.; Jean-claude, C.; Pierre, C.; Fadila, B.; Samir, B.; Université, B.; El, A. Chemical Composition and Antioxidant Activity of the Fruit Essential Oil of Zizyphus lotus (L.) Desf (Rhamnaceae). Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 228–232. [Google Scholar] [CrossRef][Green Version]
- Pinto, M.E.A.; Araújo, S.G.; Morais, M.I.; Sá, N.P.; Lima, C.M.; Rosa, C.A.; Siqueira, E.P.; Johann, S.; Lima, L.A.R.S. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. An. Acad. Bras. Cienc. 2017, 89, 1671–1681. [Google Scholar] [CrossRef]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.-R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Patinha, D.J.S.; Sousa, G.D.A.; Villaverde, J.J.; Silva, C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Eucalytpus biomass residues from agro-forest and pulping industries as sources of high-value triterpenic compounds. Cellullose Chem. Technol. 2011, 45, 475–481. [Google Scholar]
- Galgon, T.; Höke, D.; Dräger, B. Identification and quantification of betulinic acid. Phytochem. Anal. 1999, 10, 187–190. [Google Scholar] [CrossRef]
- Yang, B.; Yang, H.; Chen, F.; Hua, Y.; Jiang, Y. Phytochemical analyses of Ziziphus jujuba Mill. var. spinosa seed by ultrahigh performance liquid chromatography-tandem mass spectrometry and gas chromatography-mass spectrometry. Analyst 2013, 138, 6881–6888. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.J.; Sikder, M.A.A.; Kaisar, M.A.; Haque, M.R.; Chowdhury, A.A.; Rashid, M.A. Phytochemical and Biological Investigations of Methanol Extract of Leaves of Ziziphus mauritiana Lam. Bol. Latinoam. Y Del Caribe Plantas Med. Y Aromat. 2015, 14, 179–189. [Google Scholar]
- Jiang, L.; Zhao, X.; Xu, J.; Li, C.; Yu, Y.; Wang, W.; Zhu, L. The protective effect of dietary phytosterols on cancer risk: A systematic meta-analysis. J. Oncol. 2019, 2019, 7479518. [Google Scholar] [CrossRef]
- Souid, S.; Elsayed, H.E.; Ebrahim, H.Y.; Mohyeldin, M.M.; Siddique, A.B.; Karoui, H.; El Sayed, K.A.; Essafi-Benkhadir, K. 131-Oxophorbine protopheophorbide A from Ziziphus lotus as a novel mesenchymal-epithelial transition factor receptor inhibitory lead for the control of breast tumor growth in vitro and in vivo. Mol. Carcinog. 2018, 57, 1507–1524. [Google Scholar] [CrossRef]
- Sousa, J.L.C.; Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S. Recent Developments in the Functionalization of Betulinic Acid and Its Natural Analogues: A Route to New Bioactive Compounds. Molecules 2019, 24, 355. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Soares, B.; Guerreiro, O.; Ramos, P.; Oliveira, H.; Silvestre, A.; Freire, C.; Duarte, M.F. Anti-tumoral activity of lipophilic Eucalyptus bark extracts, enriched on triterpenic acids, against breast cancer cells. Planta Med. 2014, 80, SL41. [Google Scholar] [CrossRef]
- Weber, D.; Zhang, M.; Zhuang, P.; Zhang, Y.; Wheat, J.; Currie, G.; Al-eisawi, Z. The efficacy of betulinic acid in triple-negative breast cancer. SAGE Open Med. 2014, 2, 1–12. [Google Scholar] [CrossRef]
- Tagne, R.S.; Telefo, B.P.; Talla, E.; Nyemb, J.N.; Njina, S.N.; Asrar, M.F.M.; Kamdje, A.H.N.; Moundipa, P.F.; Ahsana Dar Farooq, M.I.C. Bio-guided fractionation of methanol extract of Ziziphus mauritiana Lam. (bark) and effect of the most active fraction on cancer cell lines. Asian Pac. J. Trop. Dis. 2015, 5, 307–312. [Google Scholar] [CrossRef]
- Barber, M.D.; Fearon, K.C.H.; Tisdale, M.J.; Mcmillan, D.C.; Ross, A.; Ross, J.A. Effect of a Fish Oil-Enriched Nutritional Supplement on Metabolic Mediators in Patients With Pancreatic Cancer Cachexia Effect of a Fish Oil-Enriched Nutritional Supplement on Metabolic Mediators in Patients With Pancreatic Cancer Cachexia. Nutr. Cancer 2009, 40, 157–164. [Google Scholar] [CrossRef]
- Awad, A.B.; Fink, C.S. Phytosterols as Anticancer Dietary Components: Evidence and Mechanism of Action. J. Nutr. 2000, 130, 2127–2130. [Google Scholar] [CrossRef] [PubMed]
- Naili, M.B.; Alghazeer, R.O.; Saleh, N.A.; Al-Najjar, A.Y. Evaluation of antibacterial and antioxidant activities of Artemisia campestris (Astraceae) and Ziziphus lotus (Rhamnacea). Arab. J. Chem. 2010, 3, 79–84. [Google Scholar] [CrossRef]
- Tlili, H.; Marino, A.; Ginestra, G.; Cacciola, F.; Mondello, L.; Miceli, N.; Taviano, M.F.; Najjaa, H.; Nostro, A. Polyphenolic profile, antibacterial activity and brine shrimp toxicity of leaf extracts from six Tunisian spontaneous species. Nat. Prod. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.-J. Antibacterial free fatty acids and monoglycerides: Biological activities, experimental testing, and therapeutic applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Tribedi, P.; Mukhopadhyay, B.; Sil, A.K. Antibacterial activity of long-chain fatty alcohols against mycobacteria. FEMS Microbiol. Lett. 2013, 338, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Togashi, N.; Shiraishi, A.; Nishizaka, M.; Matsuoka, K.; Endo, K.; Hamashima, H.; Inoue, Y. Antibacterial activity of long-chain fatty alcohols against Staphylococcus aureus. Molecules 2007, 12, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.Y.; Chung, L.Y.; Navaratnam, P. Potential targets by pentacyclic triterpenoids from Callicarpa farinosa against methicillin-resistant and sensitive Staphylococcus aureus. Fitoterapia 2014, 94, 48–54. [Google Scholar] [CrossRef]
- Rabah, S.; Kouachi, K.; Ramos, P.A.B.; Gomes, A.P.; Almeida, A.; Haddadi-Guemghar, H.; Madani, K.; Silvestre, A.J.D.; Santos, S.A.O. Unveiling the bioactivity of Allium triquetrum L. lipophilic fractions: Chemical characterization and in vitro antibacterial activity against methicillin-resistant Staphylococcus aureus. Food Funct. 2020, 11, 5257–5265. [Google Scholar] [CrossRef]
- Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Identification of new hydroxy fatty acids and ferulic acid esters in the wood of Eucalyptus globulus. Holzforschung 2002, 56, 143–149. [Google Scholar] [CrossRef]
- Villaverde, J.J.; Domingues, R.M.A.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Ligero, P.; Vega, A. Miscanthus x giganteus extractives: A source of valuable phenolic compounds and sterols. J. Agric. Food Chem. 2009, 57, 3626–3631. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.M.A.; Sousa, G.D.A.; Silva, C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. High value triterpenic compounds from the outer barks of several Eucalyptus species cultivated in Brazil and in Portugal. Ind. Crops Prod. 2011, 33, 158–164. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Guerra, Â.R.; Guerreiro, O.; Freire, C.S.R.; Silva, A.M.S.; Duarte, M.F.; Silvestre, A.J.D. Lipophilic extracts of Cynara cardunculus L. var. altilis (DC): A source of valuable bioactive terpenic compounds. J. Agric. Food Chem. 2013, 61, 8420–8429. [Google Scholar] [CrossRef] [PubMed]
- Silvério, F.O.; Barbosa, L.C.A.; Silvestre, A.J.D.; Piló-Veloso, D.; Gomide, J.L. Comparative study on the chemical composition of lipophilic fractions from three wood tissues of Eucalyptus species by gas chromatography-mass spectrometry analysis. J. Wood Sci. 2007, 53, 533–540. [Google Scholar] [CrossRef]
- Coelho, D.; Marques, G.; Gutiérrez, A.; Silvestre, A.J.D.; del Río, J.C. Chemical characterization of the lipophilic fraction of giant reed (Arundo donax) fibres used for pulp and paper manufacturing. Ind. Crops Prod. 2007, 26, 229–236. [Google Scholar] [CrossRef]
- Saitta, M.; Salvo, F.; Di Bella, G.; Dugo, G.; La Torre, G.L. Minor compounds in the phenolic fraction of virgin olive oils. Food Chem. 2009, 112, 525–532. [Google Scholar] [CrossRef]
- Canini, A.; Alesiani, D.; D’Arcangelo, G.; Tagliatesta, P. Gas chromatography—Mass spectrometry analysis of phenolic compounds from Carica papaya L. leaf. J. Food Compos. Anal. 2007, 20, 584–590. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Fan, M.-X.; Wu, X.; Wang, H.-J.; Yang, J.; Si, N.; Bian, B.-L. Chemical profiling of the Chinese herb formula Xiao-Cheng-Qi Decoction using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Chromatogr. Sci. 2013, 51, 273–285. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. M07: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
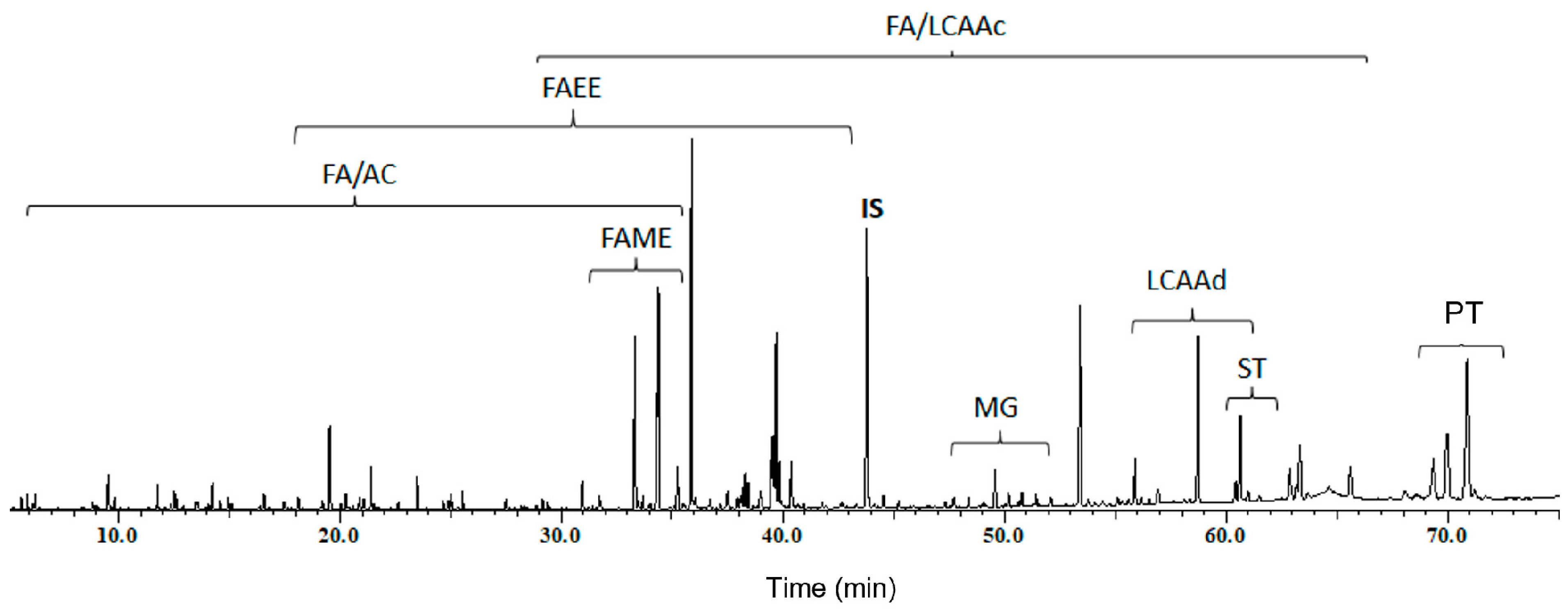
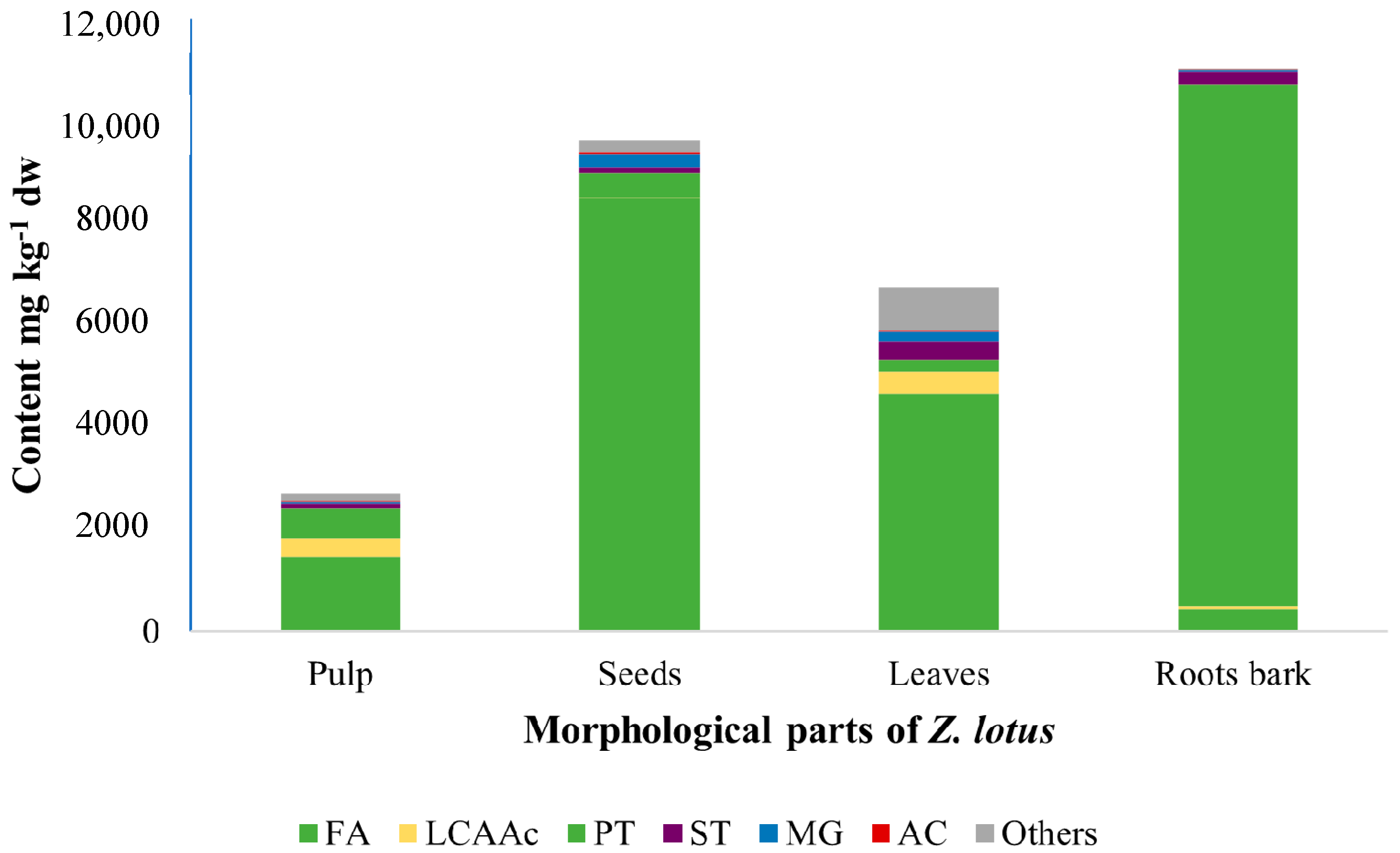
| RT (min) | Compound | Pulp | Seeds | Leaves | Root Bark |
|---|---|---|---|---|---|
| Fatty acids | 1469 | 8512 | 4643 | 434 | |
| Saturated fatty acids | 799 | 1260 | 1461 | 265 | |
| Decanoic acid | 19.50 | 67 | 5 | 5 | 1 |
| Undecanoic acid | 22.58 | 9 | n.d. | n.d. | n.d. |
| Dodecanoic acid | 25.49 | 17 | 2 | 4 | 2 |
| Tridecanoic acid | 28.25 | 4 | n.d. | n.d. | n.d. |
| Tetradecanoic acid | 30.89 | 28 | 10 | 52 | n.d. |
| Pentadecanoic acid | 33.40 | 9 | 4 | 5 | 2 |
| Hexadecanoic acid | 35.82 | 366 | 594 | 877 | 152 |
| Heptadecanoic acid | 38.10 | 22 | 7 | 8 | 7 |
| Octadecanoic acid | 40.32 | 42 | 570 | 276 | 49 |
| Nonadecanoic acid | 42.43 | 2 | n.d. | n.d. | 1 |
| Eicosanoic acid | 44.48 | 16 | 48 | 40 | 7 |
| Heneicosanoic acid | 46.48 | 5 | n.d. | 3 | 3 |
| Docosanoic acid | 48.34 | 10 | 19 | 25 | 13 |
| Tricosanoic acid | 50.16 | n.d. | n.d. | n.d. | 9 |
| Tetracosanoic acid | 52.04 | 10 | n.d. | 26 | 14 |
| Pentacosanoic acid | 54.02 | 3 | n.d. | 9 | 5 |
| Hexacosanoic acid | 56.10 | 10 | n.d. | 52 | n.d. |
| Heptacosanoic acid | 58.30 | 11 | n.d. | n.d. | n.d. |
| Octacosanoic acid | 60.56 | 114 | n.d. | 79 | n.d. |
| Triacontanoic acid | 65.51 | 54 | n.d. | tr | n.d. |
| Unsaturated fatty acids | 421 | 7222 | 3175 | 159 | |
| Tetradecenoic acid | 30.19 | 2 | n.d. | n.d. | n.d. |
| Hexadecenoic acid isomer a | 35.05 | 4 | 10 | 4 | 2 |
| Hexadecenoic acid isomer b | 35.19 | 40 | 11 | 23 | 2 |
| Hexadecenoic acid isomer c | 35.44 | 7 | n.d. | n.d. | 2 |
| Heptadecenoic acid isomer a | 37.44 | 24 | 7 | n.d. | n.d. |
| Heptadecenoic acid isomer b | 37.52 | n.d. | n.d. | n.d. | 1 |
| Heptadecenoic acid isomer c | 37.60 | n.d. | n.d. | n.d. | 1 |
| (9Z,12Z)-Octadeca-9,12-dienoic acid | 39.42 | 60 | 737 | 544 | 50 |
| (9Z,12Z,15Z)-Octadeca-9,12,15-trienoic acid | 39.50 | 45 | n.d. | 2431 | 9 |
| (9Z)-Octadec-9-enoic acid | 39.62 | 179 | 6255 | 120 | 59 |
| (9E)-Octadec-9-enoic acid | 39.78 | 50 | 135 | 54 | 18 |
| Nonadecenoic acid | 41.72 | 7 | n.d. | n.d. | n.d. |
| Eicos-11-enoic acid | 43.83 | 2 | 66 | <0.5 | 14 |
| Diacids | 7 | n.d. | n.d. | n.d. | |
| Hexadecanedioic acid | 45.19 | 7 | n.d. | n.d. | n.d. |
| ω-Hydroxy fatty acids | 10 | n.d. | 7 | 9 | |
| 22-Hydroxydocosanoic acid | 55.04 | 5 | n.d. | 7 | 9 |
| 2-Hydroxytetracosanoic acid | 55.24 | 5 | n.d. | n.d. | n.d. |
| Fatty acid ethyl esters | 228 | 16 | n.d. | n.d. | |
| Ethyl decanoate | 17.08 | 1 | n.d. | n.d. | n.d. |
| Ethyl tetradecanoate | 29.11 | 11 | n.d. | n.d. | n.d. |
| Ethyl pentadecanoate | 31.74 | 4 | n.d. | n.d. | n.d. |
| Ethyl hexadec-9-enoate isomer a | 33.62 | 12 | n.d. | n.d. | n.d. |
| Ethyl hexadec-9-enoate isomer b | 33.86 | 3 | n.d. | n.d. | n.d. |
| Ethyl hexadecanoate | 34.26 | 104 | 5 | n.d. | n.d. |
| Ethyl (9Z)-octadec-9-enoate | 38.24 | 33 | 11 | n.d. | n.d. |
| Ethyl (9E)-octadec-9-enoate | 38.39 | 25 | n.d. | n.d. | n.d. |
| Ethyl octadecanoate | 38.95 | 29 | n.d. | n.d. | n.d. |
| Ethyl eicosanoate | 43.27 | 6 | n.d. | n.d. | n.d. |
| Fatty acid methyl esters | 5 | 15 | n.d. | 1 | |
| Methyl hexadecanoate | 32.53 | 5 | n.d. | n.d. | n.d. |
| Methyl (9Z)-octadec-9-enoate | 36.67 | n.d. | 15 | n.d. | 1 |
| Monoglycerides | 27 | 255 | 189 | 24 | |
| 2-Palmitoylglycerol | 47.05 | n.d. | 3 | 5 | n.d. |
| 1-Palmitoylglycerol | 47.67 | 13 | 44 | 47 | 12 |
| 1-Linoleoylglycerol | 50.61 | n.d. | 35 | 30 | 3 |
| 1-Linolenoylglycerol | 50.72 | n.d. | n.d. | 84 | n.d. |
| 1-Oleoylglycerol | 50.73 | 14 | 155 | n.d. | 4 |
| 1-Stearoylglycerol | 51.28 | n.d. | 17 | 24 | 4 |
| Long chain aliphatic alcohols | 340 | 2 | 438 | 51 | |
| Tetradecan-1-ol | 28.89 | n.d. | n.d. | n.d. | 2 |
| Hexadecan-1-ol | 33.96 | 4 | 2 | 4 | 9 |
| (9Z)-Octadec-9-en-1-ol | 37.87 | 9 | n.d. | 11 | 14 |
| Octadecan-1-ol | 38.60 | 4 | n.d. | 2 | 6 |
| Docosan-1-ol | 46.83 | 3 | n.d. | n.d. | 3 |
| Tetracosan-1-ol | 50.51 | n.d. | n.d. | 5 | 3 |
| Hexacosan-1-ol | 54.36 | 7 | n.d. | 118 | 3 |
| Heptacosan-1-ol | 56.46 | 8 | n.d. | 23 | n.d. |
| Octacosan-1-ol | 58.65 | 207 | n.d. | 230 | 11 |
| Nonacosan-1-ol | 60.91 | 19 | n.d. | 13 | n.d. |
| Triacontan-1-ol | 63.24 | 79 | n.d. | 31 | n.d. |
| Pentacyclic triterpenic compounds | 608 | 483 | 248 | 10230 | |
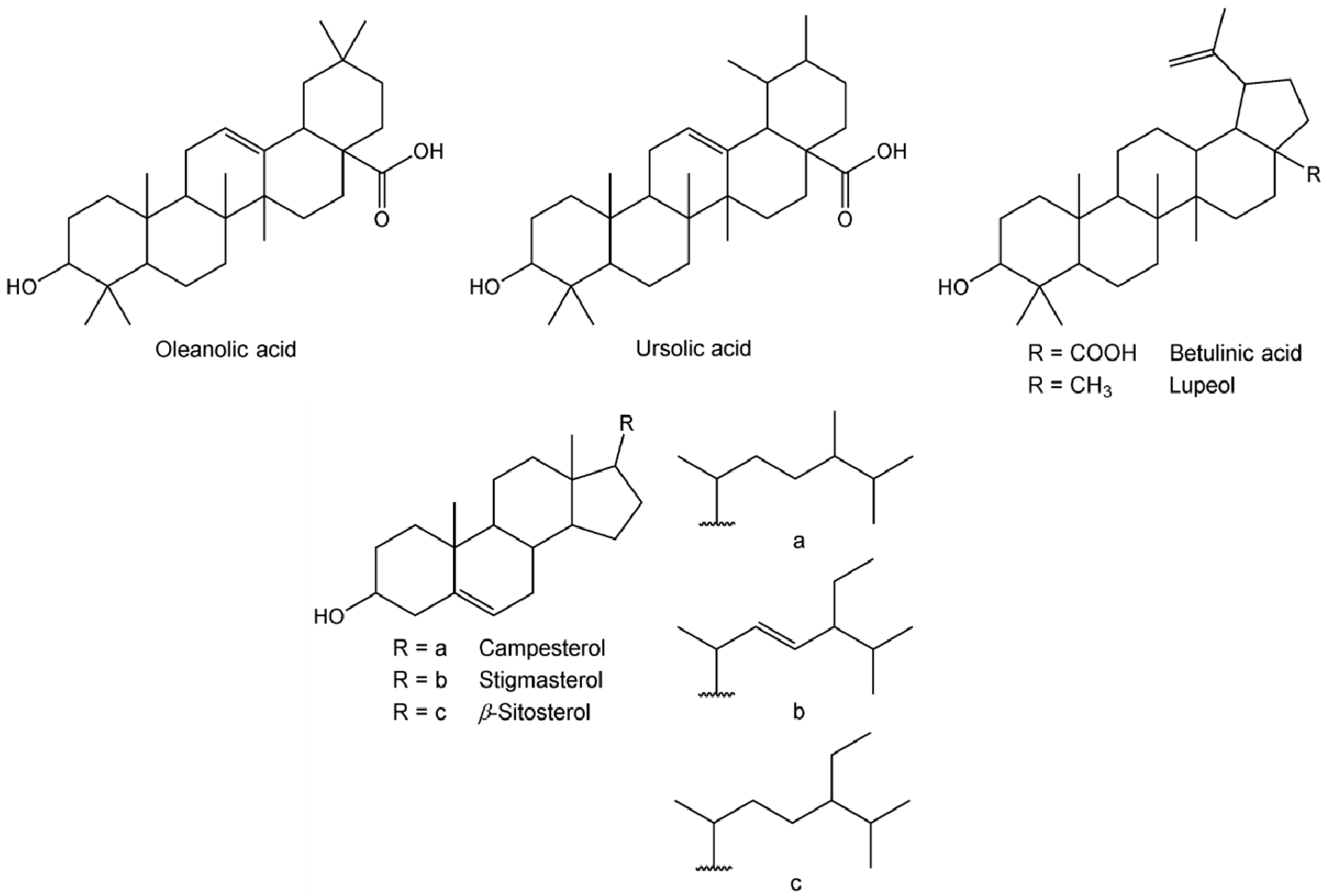
| Lupeol | 63.86 | n.d. | n.d. | 78 | 105 |
| Oleanolic acid | 69.21 | 103 | 164 | 51 | 287 |
| Betulinic acid | 69.82 | 160 | 238 | 119 | 9838 |
| Ursolic acid | 70.72 | 345 | 81 | n.d. | n.d. |
| Sterols | 81 | 96 | 355 | 257 | |
| Campesterol | 60.70 | n.d. | n.d. | 28 | 4 |
| Stigmasterol | 61.41 | 13 | n.d. | 119 | 126 |
| β-Sitosterol | 62.79 | 68 | 96 | 208 | 127 |
| Aromatic compounds | 29 | 31 | 21 | 11 | |
| Benzoic acid | 11.71 | 23 | 2 | 5 | n.d. |
| Vanillin | 21.00 | n.d. | 20 | n.d. | 3 |
| Salicylic acid | 21.02 | n.d. | n.d. | 4 | n.d. |
| Vanillyl alcohol | 24.99 | n.d. | 5 | n.d. | 1 |
| Syringaldehyde | 25.93 | n.d. | n.d. | n.d. | 1 |
| Homovanillyl alcohol | 27.00 | n.d. | n.d. | n.d. | 2 |
| Vanillic acid | 28.39 | 4 | 4 | n.d. | 2 |
| Hydroxytyrosol | 28.96 | n.d. | n.d. | n.d. | 2 |
| Protocatechuic acid | 30.42 | n.d. | n.d. | n.d. | <0.5 |
| Syringic acid | 31.86 | n.d. | n.d. | n.d. | 1 |
| p-Coumaric acid | 32.88 | 2 | n.d. | 5 | n.d. |
| E-Ferulic acid | 36.54 | n.d. | n.d. | 7 | n.d. |
| Others | 151 | 228 | 832 | 9 | |
| Solerol | 13.45 | 6 | n.d. | n.d. | n.d. |
| Glycerol | 14.21 | 41 | 148 | 251 | 9 |
| Loliolide | 28.26 | n.d. | n.d. | 39 | n.d. |
| Neophytadiene isomer a | 30.76 | tr | n.d. | 141 | n.d. |
| Neophytadiene isomer b | 31.29 | n.d. | n.d. | 29 | n.d. |
| Neophytadiene isomer c | 31.75 | n.d. | n.d. | 49 | n.d. |
| Inositol | 36.94 | n.d. | n.d. | 13 | n.d. |
| Phytol | 39.04 | n.d. | n.d. | 117 | n.d. |
| Squalene | 51.53 | n.d. | 79 | 39 | n.d. |
| γ-Tocopherol | 55.21 | n.d. | n.d. | 54 | n.d. |
| Tetracosyl acetate | 55.33 | n.d. | n.d. | 26 | n.d. |
| Octacosanal | 55.82 | 52 | n.d. | n.d. | n.d. |
| Nonacosan-10-one | 56.86 | 24 | n.d. | n.d. | n.d. |
| α-Tocopherol | 58.19 | n.d. | n.d. | 74 | n.d. |
| Triacontanal | 60.35 | 27 | n.d. | n.d. | n.d. |
| Total | 2704 | 9607 | 6726 | 11016 | |
| Lipophilic Z. lotus Extract | MDA-MB-231 (IC50 µg mL−1) |
|---|---|
| Pulp | >50 |
| Seeds | >50 |
| Leaves | >50 |
| Root bark | 4.23 ± 0.18 a |
| Synthetic root bark mixture * | 15.27 ± 1.79 b |
| Z. lotus Extract | MIC (μg mL−1) | ||
|---|---|---|---|
| E. coli | Methicillin-Sensitive Staphylococcus aureus (MSSA) | S. epidermidis | |
| Pulp | >2048 | >2048 | >2048 |
| Seeds | >2048 | >2048 | 1024 |
| Leaves | 1024 | 2048 | 1024 |
| Root bark | >2048 | 2048 | 2048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zazouli, S.; Chigr, M.; Ramos, P.A.B.; Rosa, D.; Castro, M.M.; Jouaiti, A.; Duarte, M.F.; Santos, S.A.O.; Silvestre, A.J.D. Chemical Profile of Lipophilic Fractions of Different Parts of Zizyphus lotus L. by GC-MS and Evaluation of Their Antiproliferative and Antibacterial Activities. Molecules 2022, 27, 483. https://doi.org/10.3390/molecules27020483
Zazouli S, Chigr M, Ramos PAB, Rosa D, Castro MM, Jouaiti A, Duarte MF, Santos SAO, Silvestre AJD. Chemical Profile of Lipophilic Fractions of Different Parts of Zizyphus lotus L. by GC-MS and Evaluation of Their Antiproliferative and Antibacterial Activities. Molecules. 2022; 27(2):483. https://doi.org/10.3390/molecules27020483
Chicago/Turabian StyleZazouli, Sofia, Mohammed Chigr, Patrícia A. B. Ramos, Daniela Rosa, Maria M. Castro, Ahmed Jouaiti, Maria F. Duarte, Sónia A. O. Santos, and Armando J. D. Silvestre. 2022. "Chemical Profile of Lipophilic Fractions of Different Parts of Zizyphus lotus L. by GC-MS and Evaluation of Their Antiproliferative and Antibacterial Activities" Molecules 27, no. 2: 483. https://doi.org/10.3390/molecules27020483
APA StyleZazouli, S., Chigr, M., Ramos, P. A. B., Rosa, D., Castro, M. M., Jouaiti, A., Duarte, M. F., Santos, S. A. O., & Silvestre, A. J. D. (2022). Chemical Profile of Lipophilic Fractions of Different Parts of Zizyphus lotus L. by GC-MS and Evaluation of Their Antiproliferative and Antibacterial Activities. Molecules, 27(2), 483. https://doi.org/10.3390/molecules27020483









