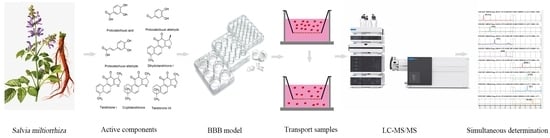
Simultaneous Determination of Seven Lipophilic and Hydrophilic Components in Salvia miltiorrhiza Bunge by LC-MS/MS Method and Its Application to a Transport Study in a Blood-Brain-Barrier Cell Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Development
2.2. Method Validation
2.2.1. Specificity
2.2.2. Linearity and LLOQ
2.2.3. Precision and Accuracy
2.2.4. Matrix Effect
2.2.5. Stability
2.3. BBB Cell Model Transport Study Application
3. Materials and Methods
3.1. Chemicals
3.2. Cell Culture
3.3. Instrumentation and Conditions
3.4. Stock Solutions, Calibration Solutions, and Quality Control Samples
3.5. Sample Preparation
3.6. Validation Procedure
3.7. Transport Study in a BBB Cell Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paraskevaidi, M.; Allsop, D.; Karim, S.; Martin, F.L.; Crean, S. Diagnostic Biomarkers for Alzheimer’s Disease Using Non-Invasive Specimens. J. Clin. Med. 2020, 9, 1673. [Google Scholar] [CrossRef]
- Polis, B.; Samson, A.O. Role of the metabolism of branched-chain amino acids in the development of Alzheimer’s disease and other metabolic disorders. Neural Regen. Res. 2020, 15, 1460–1470. [Google Scholar] [CrossRef]
- Tonnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.L.; Samant, N.P. Current druggable targets for therapeutic control of Alzheimer’s disease. Contemp. Clin. Trials 2021, 109, 106549–106560. [Google Scholar] [CrossRef]
- Soheili, M.; Karimian, M.; Hamidi, G.; Salami, M. Alzheimer’s disease treatment: The share of herbal medicines. Iran. J. Basic Med. Sci. 2021, 24, 123–135. [Google Scholar]
- Howes, M.J.R.; Fang, R.; Houghton, P.J. Effect of Chinese Herbal Medicine on Alzheimer’s Disease. Int. Rev. Neurobiol. 2017, 135, 29–56. [Google Scholar]
- Su, C.Y.; Ming, Q.L.; Rahman, K.; Han, T.; Qin, L.P. Salvia miltiorrhiza: Traditional medicinal uses, chemistry, and pharmacology. Chin. J. Nat. Med. 2015, 13, 163–182. [Google Scholar] [CrossRef]
- Guo, Y.; Dong, X.; Zhang, R.; Zhong, Y.; Yang, P.; Zhang, S.Y. Salvia miltiorrhiza improves Alzheimer’s disease: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e21924–e21928. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Liu, M.; Liu, B.; Li, N.; Dong, X.; Hong, Z.; Chai, Y. UHPLC-QTOF/MS-based metabolomics investigation for the protective mechanism of Danshen in Alzheimer’s disease cell model induced by Aβ1–42. Metabolomics 2019, 15, 13. [Google Scholar] [CrossRef]
- Bagchi, S.; Chhibber, T.; Lahooti, B.; Verma, A.; Borse, V.; Jayant, R.D. In-vitro blood-brain barrier models for drug screening and permeation studies: An overview. Drug Des. Dev. Ther. 2019, 13, 3591–3605. [Google Scholar] [CrossRef] [Green Version]
- Harilal, S.; Jose, J.; Parambi, D.G.T.; Kumar, R.; Unnikrishnan, M.K.; Uddin, M.S.; Mathew, G.E.; Pratap, R.; Marathakam, A.; Mathew, B. Revisiting the blood-brain barrier: A hard nut to crack in the transportation of drug molecules. Brain Res. Bull. 2020, 160, 121–140. [Google Scholar] [CrossRef]
- Yu, P.F.; Wang, W.Y.; Eerdun, G.; Wang, T.; Zhang, L.M.; Li, C.; Fu, F.H. The Role of P-Glycoprotein in Transport of Danshensu across the Blood-Brain Barrier. Evid.-Based Complement. Altern. Med. 2011, 2011, 713523. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Hou, Z.N.; Su, F.; Chen, J.P.; Zhang, X.D.; Xu, L.; Yang, D.F.; Liang, Z.S. Quantitative Determination and Validation of Four Ketones in Salvia miltiorrhiza Bunge Using Quantitative Proton Nuclear Magnetic Resonance Spectroscopy. Molecules 2020, 25, 2043. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Qian, S.S.; Zhang, Y.J.; Wang, R.Q. Salvia miltiorrhiza: A source for anti-Alzheimer’s disease drugs. Pharm. Biol. 2016, 54, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yu, Y.; Cen, Y.; Yang, D.; Qi, Z.; Hou, Z.; Han, S.; Cai, Z.; Liu, K. Bivariate Correlation Analysis of the Chemometric Profiles of Chinese Wild Salvia miltiorrhiza Based on UPLC-Qqq-MS and Antioxidant Activities. Molecules 2018, 23, 538. [Google Scholar] [CrossRef] [Green Version]
- Xie, Q.; Yuan, H.; Liu, Y.; Qiu, Y.; Jian, Y.; Li, B.; Peng, C.; Zhou, S.; Yang, C.; Wang, W. Simultaneous Determination of 19 Bioactive Constituents in QishenYiqi Dropping Pills by Ultra-Performance Liquid Chromatography Coupled with Triple Quadrupole Mass Spectrometry. J. AOAC Int. 2019, 102, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Liu, Q.T.; Luo, H.L.; Chen, P.; Wu, X.R.; Yang, M.H.; Kong, W.J.; Guo, W.Y. UFLC-MS/MS analysis of four tanshinone components in Salvia miltiorrhizae after ultrasound-assisted extraction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1017, 204–210. [Google Scholar] [CrossRef] [Green Version]
- Eigenmann, D.E.; Xue, G.; Kim, K.S.; Moses, A.V.; Hamburger, M.; Oufir, M. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS 2013, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Smith, D.; Artursson, P.; Avdeef, A.; Di, L.; Ecker, G.F.; Faller, B.; Houston, J.B.; Kansy, M.; Kerns, E.H.; Kramer, S.D.; et al. Passive Lipoidal Diffusion and Carrier-Mediated Cell Uptake Are Both Important Mechanisms of Membrane Permeation in Drug Disposition. Mol. Pharm. 2014, 11, 1727–1738. [Google Scholar] [CrossRef]
- Wilhelm, I.; Krizbai, I.A. In vitro models of the blood-brain barrier for the study of drug delivery to the brain. Mol. Pharm. 2014, 11, 1949–1963. [Google Scholar] [CrossRef]
- Liew, K.F.; Hanapi, N.A.; Chan, K.L.; Yusof, S.R.; Lee, C.Y. Assessment of the Blood-Brain Barrier Permeability of Potential Neuroprotective Aurones in Parallel Artificial Membrane Permeability Assay and Porcine Brain Endothelial Cell Models. J. Pharm. Sci. 2017, 106, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yan, J.; Feng, J. Treatment with Tanshinone Iia Suppresses Disruption of the Blood-Brain Barrier and Reduces Expression of Adhesion Molecules and Chemokines in Experimental Autoimmune Encephalomyelitis. Eur. J. Pharm. 2016, 771, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Lin, S.G.; Chen, X.; Zhou, Z.W.; Liang, J.; Duan, W.; Chowbay, B.; Wen, J.Y.; Chan, E.; Cao, J.; et al. Transport of Cryptotanshinone, a Major Active Triterpenoid in Salvia Miltiorrhiza Bunge Widely Used in the Treatment of Stroke and Alzheimer’s Disease, across the Blood-Brain Barrier. Curr. Drug Metab. 2007, 4, 365–377. [Google Scholar] [CrossRef] [PubMed]


| Analytes | Calibration Curve | Weighting | Linear Range (ng/mL) | r2 | LLOQ (ng/mL) |
|---|---|---|---|---|---|
| TS I | Y = 0.1068X + 0.0102 | 1/X2 | 0.1–8 | 0.9934 | 0.1 |
| DTS I | Y = 0.2497X + 0.0043 | 1/X | 0.1–8 | 0.9988 | 0.1 |
| TS IIA | Y = 0.8962X + 0.0426 | 1/X2 | 0.2–8 | 0.9922 | 0.2 |
| CTS | Y = 0.2353X + 0.0537 | 1/X2 | 1–80 | 0.9837 | 1 |
| PAL | Y = 0.0024X + 0.0092 | 1/X | 20–800 | 0.9965 | 20 |
| PCTA | Y = 0.0124X + 0.0451 | 1/X2 | 10–4000 | 0.9965 | 10 |
| CFA | Y = 0.0195X + 0.0250 | 1/X | 20–800 | 0.9989 | 20 |
| Analytes | Con. (ng/mL) | Validation Run 1 | Validation Run 2 | Validation Run 3 | Between-Run | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (ng/mL) | Accuracy (%) | RSD (%) | Mean ± SD (ng/mL) | Accuracy (%) | RSD (%) | Mean ± SD (ng/mL) | Accuracy (%) | RSD (%) | Mean ± SD (ng/mL) | Accuracy (%) | RSD (%) | ||
| TS I | 0.2 | 0.17 ± 0.02 | 86.46 ± 9.86 | 9.83 | 0.22 ± 0.01 | 107.58 ± 6.93 | 5.77 | 0.21 ± 0.01 | 104.97 ± 8.08 | 6.87 | 0.20 ± 0.03 | 99.67 ± 12.44 | 12.88 |
| 1 | 1.00 ± 0.03 | 100.21 ± 2.91 | 2.59 | 1.13 ± 0.03 | 113.05 ± 2.91 | 2.67 | 1.09 ± 0.06 | 108.61 ± 6.61 | 5.44 | 1.07 ± 0.07 | 107.29 ± 6.97 | 6.49 | |
| 8 | 7.52 ± 0.24 | 94.06 ± 3.34 | 3.17 | 7.61 ± 0.25 | 95.10 ± 3.54 | 3.32 | 8.16 ± 0.56 | 102.06 ± 8.10 | 6.87 | 7.74 ± 0.47 | 96.72 ± 5.91 | 6.10 | |
| TS IIA | 0.4 | 0.34 ± 0.01 | 85.18 ± 3.95 | 4.25 | 0.42 ± 0.01 | 104.73 ± 4.04 | 3.46 | 0.35 ± 0.01 | 87.88 ± 3.55 | 3.51 | 0.37 ± 0.04 | 92.93 ± 9.86 | 10.44 |
| 2 | 1.90 ± 0.07 | 95.14 ± 4.08 | 3.82 | 2.13 ± 0.09 | 106.48 ± 5.15 | 4.34 | 2.07 ± 0.04 | 103.54 ± 2.12 | 1.93 | 2.03 ± 0.12 | 101.72 ± 6.19 | 6.09 | |
| 8 | 8.57 ± 0.17 | 107.08 ± 2.33 | 1.95 | 8.98 ± 0.20 | 112.23 ± 2.79 | 2.22 | 8.36 ± 0.70 | 104.47 ± 9.85 | 8.43 | 8.63 ± 0.52 | 107.93 ± 6.53 | 6.05 | |
| DTS I | 0.2 | 0.18 ± 0.01 | 87.28 ± 4.63 | 4.45 | 0.20 ± 0.01 | 102.02 ± 5.19 | 4.54 | 0.18 ± 0.02 | 91.9013.54 | 13.16 | 0.19 ± 0.02 | 93.73 ± 10.34 | 10.86 |
| 1 | 0.97 ± 0.03 | 97.16 ± 3.27 | 3.01 | 1.07 ± 0.04 | 107.054.69 | 3.93 | 0.93 ± 0.04 | 93.10 ± 4.91 | 4.72 | 0.99 ± 0.07 | 99.10 ± 7.28 | 7.35 | |
| 8 | 7.14 ± 0.21 | 89.29 ± 2.88 | 2.88 | 7.91 ± 0.93 | 98.89 ± 13.01 | 11.75 | 6.81 ± 0.51 | 85.17 ± 7.11 | 7.47 | 7.29 ± 0.80 | 91.12 ± 10.03 | 11.00 | |
| CTS | 2 | 1.83 ± 0.05 | 91.60 ± 2.26 | 2.46 | 2.03 ± 0.06 | 101.61 ± 3.48 | 3.06 | 2.23 ± 0.05 | 111.61 ± 3.18 | 2.45 | 2.03 ± 0.17 | 100.89 ± 8.74 | 8.54 |
| 20 | 17.73 ± 0.32 | 88.67 ± 1.77 | 1.80 | 19.00 ± 0.67 | 94.99 ± 3.75 | 3.53 | 22.26 ± 0.54 | 111.30 ± 3.04 | 2.44 | 19.66 ± 2.05 | 98.32 ± 10.24 | 10.43 | |
| 80 | 69.54 ± 1.78 | 86.92 ± 2.49 | 2.55 | 71.83 ± 1.22 | 89.78 ± 1.77 | 1.70 | 70.83 ± 1.39 | 88.53 ± 1.97 | 1.96 | 70.65 ± 1.83 | 99.31 ± 2.29 | 2.58 | |
| PAL | 40 | 39.61 ± 6.39 | 99.01 ± 3.53 | 3.09 | 40.43 ± 1.56 | 101.09 ± 4.53 | 3.87 | 45.40 ± 1.28 | 113.50 ± 3.58 | 2.82 | 42.09 ± 3.09 | 105.22 ± 7.72 | 7.33 |
| 200 | 215.23 ± 6.35 | 107.61 ± 3.57 | 2.95 | 217.97 ± 4.90 | 108.99 ± 2.75 | 2.25 | 224.32 ± 3.71 | 113.15 ± 2.75 | 1.65 | 219.18 ± 6.59 | 109.58 ± 3.30 | 3.01 | |
| 800 | 811.61 ± 12.22 | 101.46 ± 1.71 | 1.51 | 808.07 ± 4.92 | 101.02 ± 0.70 | 0.61 | 778.96 ± 53.75 | 98.63 ± 7.73 | 6.81 | 803.87 ± 32.47 | 100.49 ± 1.05 | 4.04 | |
| PCTA | 20 | 22.87 ± 0.76 | 113.70 ± 3.79 | 3.31 | 18.30 ± 1.35 | 91.43 ± 4.78 | 7.37 | 18.34 ± 0.57 | 91.70 ± 3.18 | 3.12 | 19.62 ± 2.36 | 97.89 ± 11.01 | 12.01 |
| 200 | 228.23 ± 9.87 | 109.51 ± 4.96 | 1.26 | 204.31 ± 2.58 | 102.16 ± 1.44 | 1.26 | 206.55 ± 7.03 | 103.30 ± 3.93 | 3.40 | 213.03 ± 13.40 | 104.99 ± 4.82 | 6.29 | |
| 4000 | 3710.42 ± 288.72 | 92.76 ± 8.07 | 7.78 | 3556.74 ± 44.97 | 88.91 ± 1.25 | 1.26 | 3688.29 ± 34.90 | 92.20 ± 0.99 | 0.95 | 3651.82 ± 189.36 | 91.29 ± 4.74 | 5.19 | |
| CFA | 40 | 37.74 ± 1.59 | 94.66 ± 4.04 | 4.22 | 39.00 ± 0.78 | 97.48 ± 2.19 | 2.01 | 39.63 ± 2.31 | 99.07 ± 6.45 | 5.83 | 38.87 ± 1.92 | 97.07 ± 4.64 | 4.94 |
| 200 | 194.22 ± 6.35 | 97.12 ± 3.53 | 3.27 | 201.53 ± 13.94 | 100.78 ± 7.81 | 6.92 | 193.81 ± 10.40 | 96.90 ± 5.83 | 5.37 | 196.52 ± 11.66 | 98.27 ± 5.84 | 5.93 | |
| 800 | 652.11 ± 4.53 | 81.51 ± 0.64 | 0.69 | 728.80 ± 30.19 | 91.10 ± 4.37 | 4.14 | 695.55 ± 5.20 | 86.92 ± 0.78 | 0.75 | 688.53 ± 39.42 | 86.06 ± 4.93 | 5.73 | |
| Analytes | LQC | MQC | HQC | |||
|---|---|---|---|---|---|---|
| Mean ± SD (%) | RSD (%) | Mean ± SD (%) | RSD (%) | Mean ± SD (%) | RSD (%) | |
| TS I | 94.33 ± 4.46 | 4.73 | 99.28 ± 2.78 | 2.8 | 95.33 ± 1.86 | 1.95 |
| DTS I | 87.76 ± 3.89 | 4.43 | 88.34 ± 5.36 | 6.07 | 86.91 ± 3.96 | 4.55 |
| TS IIA | 86.30 ± 5.53 | 6.41 | 96.86 ± 6.81 | 7.03 | 89.81 ± 6.29 | 7.01 |
| CTS | 88.11 ± 7.13 | 8.09 | 91.26 ± 8.03 | 8.8 | 86.45 ± 5.86 | 6.78 |
| PAL | 106.06 ± 6.03 | 5.69 | 116.50 ± 14.41 | 12.37 | 118.96 ± 10.00 | 8.41 |
| PCTA | 101.00 ± 1.00 | 0.99 | 108.48 ± 14.50 | 13.36 | 106.06 ± 5.76 | 5.43 |
| CFA | 99.97 ± 9.51 | 9.51 | 103.06 ± 6.82 | 6.61 | 104.63 ± 6.77 | 6.47 |
| Analytes | Concentration (ng/mL) | Autosampler (24 h) | Long Term (−80 °C, 15 Days) | ||
|---|---|---|---|---|---|
| Mean ± SD (%) | RSD (%) | Mean ± SD (%) | RSD (%) | ||
| TS I | 0.2 | 104.83 ± 12.38 | 11.81 | 112.64 ± 2.51 | 2.23 |
| 1 | 94.45 ± 5.86 | 6.2 | 111.60 ± 4.40 | 3.94 | |
| 8 | 93.83 ± 3.75 | 4 | 110.22 ± 9.21 | 8.36 | |
| DTS I | 0.2 | 88.70 ± 3.67 | 4.14 | 113.43 ± 10.52 | 9.27 |
| 1 | 91.52 ± 3.06 | 3.34 | 114.08 ± 2.21 | 1.94 | |
| 8 | 89.13 ± 2.15 | 2.41 | 99.99 ± 6.54 | 6.54 | |
| TS IIA | 0.4 | 100.64 ± 14.49 | 14.39 | 108.42 ± 15.37 | 14.17 |
| 2 | 99.36 ± 13.77 | 13.86 | 113.91 ± 1.68 | 1.47 | |
| 8 | 93.08 ± 11.14 | 11.97 | 109.50 ± 5.61 | 5.12 | |
| CTS | 2 | 107.58 ± 4.68 | 4.35 | 110.59 ± 4.75 | 4.3 |
| 20 | 94.78 ± 3.77 | 3.97 | 110.62 ± 4.01 | 3.63 | |
| 80 | 94.90 ± 3.73 | 3.93 | 91.81 ± 5.50 | 5.99 | |
| PAL | 40 | 88.42 ± 5.31 | 6.01 | 106.24 ± 5.99 | 5.64 |
| 200 | 102.92 ± 11.45 | 11.12 | 101.76 ± 8.94 | 8.78 | |
| 800 | 101.06 ± 1.83 | 1.81 | 92.28 ± 4.85 | 5.25 | |
| PCTA | 20 | 96.14 ± 4.10 | 4.27 | 114.06 ± 2.31 | 2.03 |
| 200 | 102.10 ± 6.02 | 5.9 | 110.63 ± 1.60 | 1.45 | |
| 4000 | 91.74 ± 2.51 | 2.74 | 91.53 ± 5.04 | 5.5 | |
| CFA | 40 | 85.7 ± 0.42 | 0.5 | 113.78 ± 1.90 | 1.67 |
| 200 | 91.08 ± 8.57 | 9.41 | 111.44 ± 1.95 | 1.75 | |
| 800 | 91.58 ± 6.17 | 6.74 | 106.34 ± 6.10 | 5.74 | |
| Analytes | Papp (AP-BL) cm/s | Papp (BL-AP) cm/s | ER |
|---|---|---|---|
| TS IIA | (3.757 ± 1.723) × 10−8 | (2.528 ± 0.773) × 10−8 | 0.673 |
| DTS I | (4.643 ± 2.012) × 10−6 | (3.617 ± 1.082) × 10−6 | 0.779 |
| CFA | (2.147 ± 1.010) × 10−5 | (2.252 ± 0.954) × 10−5 | 1.049 |
| CTS | (4.977 ± 1.587) × 10−6 | (5.125 ± 1.584) × 10−6 | 1.030 |
| PAL | (1.516 ± 0.179) × 10−5 | (1.220 ± 0.021) × 10−5 | 0.805 |
| PCTA | (4.369 ± 1.410) × 10−5 | (4.132 ± 0.288) × 10−5 | 0.946 |
| Analytes | Precursor (m/z) | Product (m/z) | Frag. (V) | CE (eV) | Dwell | Cell Accelerator Voltage | Polarity |
|---|---|---|---|---|---|---|---|
| TS I | 277.2 | 249.2 | 130 | 25 | 100 | 8 | Positive |
| DTS I | 279.3 | 204.9 | 110 | 30 | 100 | 7 | Positive |
| TS IIA | 295.2 | 277.2 | 95 | 20 | 100 | 7 | Positive |
| CTS | 297.3 | 251.4 | 125 | 33 | 100 | 7 | Positive |
| PAL | 137.2 | 108.2 | 110 | 30 | 100 | 7 | Negative |
| PCTA | 153.2 | 109.1 | 90 | 15 | 100 | 5 | Negative |
| CFA | 179.1 | 135.1 | 100 | 15 | 100 | 7 | Negative |
| SMZ (IS) | 252.0 | 156.1 | 100 | 10 | 100 | 5 | Negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, M.; Fang, J.; He, Y.; Liu, M.; Hong, Z.; Chai, Y. Simultaneous Determination of Seven Lipophilic and Hydrophilic Components in Salvia miltiorrhiza Bunge by LC-MS/MS Method and Its Application to a Transport Study in a Blood-Brain-Barrier Cell Model. Molecules 2022, 27, 657. https://doi.org/10.3390/molecules27030657
Wang H, Zhang M, Fang J, He Y, Liu M, Hong Z, Chai Y. Simultaneous Determination of Seven Lipophilic and Hydrophilic Components in Salvia miltiorrhiza Bunge by LC-MS/MS Method and Its Application to a Transport Study in a Blood-Brain-Barrier Cell Model. Molecules. 2022; 27(3):657. https://doi.org/10.3390/molecules27030657
Chicago/Turabian StyleWang, Hui, Mingyong Zhang, Jiahao Fang, Yuzhen He, Min Liu, Zhanying Hong, and Yifeng Chai. 2022. "Simultaneous Determination of Seven Lipophilic and Hydrophilic Components in Salvia miltiorrhiza Bunge by LC-MS/MS Method and Its Application to a Transport Study in a Blood-Brain-Barrier Cell Model" Molecules 27, no. 3: 657. https://doi.org/10.3390/molecules27030657
APA StyleWang, H., Zhang, M., Fang, J., He, Y., Liu, M., Hong, Z., & Chai, Y. (2022). Simultaneous Determination of Seven Lipophilic and Hydrophilic Components in Salvia miltiorrhiza Bunge by LC-MS/MS Method and Its Application to a Transport Study in a Blood-Brain-Barrier Cell Model. Molecules, 27(3), 657. https://doi.org/10.3390/molecules27030657