LC-ESI-MS/MS Identification of Biologically Active Phenolics in Different Extracts of Alchemilla acutiloba Opiz
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Analysis
2.2. Qualitative and Quantitative Analysis
2.3. Antioxidant Activities
2.4. Anti-Inflammatory Activity
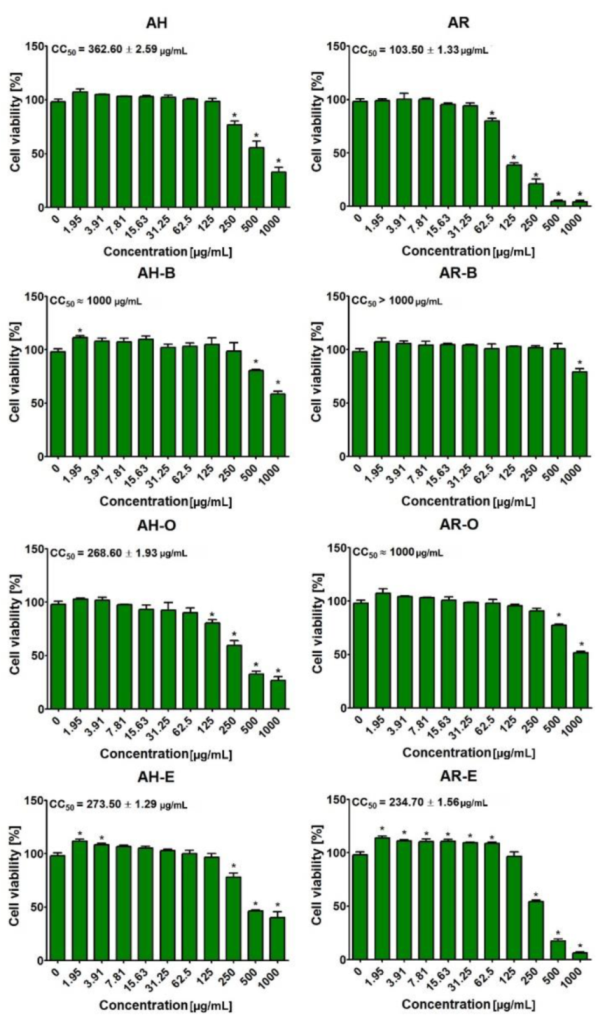
2.5. Evaluation of Cytotoxicity
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Extraction Procedure
3.4. Total Flavonoid, Phenolic and Phenolic Acids Content
3.5. LC-ESI-MS/MS Analysis
3.6. Antioxidant Activity
3.7. Cyclooxygenase-1 (COX-1) and Cyclooxygenase-2 (COX-2) Inhibitory Activity
3.8. Evaluation of Cytotoxicity
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jarić, S.; Mačukanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An ethnobotanical survey of traditionally used plants on Suva planina mountain (south-eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef]
- Ilić-Stojanović, S.; Nikolić, V.; Kundaković, T.; Savić, I.; Savić-Gajić, I.; Jocić, E.; Nikolić, L. Thermosensitive hydrogels for modified release of ellagic acid obtained from Alchemilla vulgaris L. extract. Int. J. Polym. Mater. 2018, 67, 553–563. [Google Scholar] [CrossRef]
- Ożarowski, A. Ziołolecznictwo. Poradnik dla Lekarzy, 3rd ed.; PZWL: Warsaw, Poland, 1982; pp. 53–54. [Google Scholar]
- Ożarowski, A.; Jaroniewski, W. Rośliny Lecznicze i ich Praktyczne Zastosowanie, 1st ed.; IWZZ: Warsaw, Poland, 1987; pp. 318–319. [Google Scholar]
- Shrivastava, R.; Cucuat, N.; John, G.W. Effects of Alchemilla vulgaris and glycerine on epithelial and myofibroblast cell growth and cutaneous lesion healing in rats. Phytother. Res. 2007, 21, 369–373. [Google Scholar] [CrossRef]
- Vitkova, A.; Nikolova, M.; Delcheva, M.; Tashev, A.; Gavrilova, A.; Aneva, I.; Dimitrov, D. Influence of species composition on total phenolic content and antioxidant properties of Herba Alchemillae. Bulg. J. Agric. Sci. 2015, 21, 990–997. [Google Scholar]
- Ertürk, S.; Karatoprak, G.; Koşar, M. Antioxidant properties and phenolic composition of Alchemilla mollis from Turkey. Planta Med. 2011, 77, 1383–1384. [Google Scholar]
- Kaya, B.; Menemen, Y.; Saltan, Z.F. Flavonoids in the endemic species of Alchemilla L. (Section Alchemilla L. Subsection Calycanthum Rothm. Ser. Elatae Rothm.) from Northeast Black sea region in Turkey. Pal. J. Bot. 2012, 44, 595–597. [Google Scholar]
- Duckstein, S.; Lotter, E.; Mayer, U.; Lindequist, U.; Stintzing, F. Phenolic constituents from Alchemilla vulgaris L. and Alchemilla mollis (Buser) Rothm. at different dates of harvest. Z. Naturforsch. C. 2013, 68, 529–540. [Google Scholar]
- Falchero, L.; Coppa, M.; Esposti, S.; Tava, A. Essential oil composition of Alchemilla alpina L. em. Buser from Western Alpine Pastures. J. Essent. Oil Res. 2008, 20, 542–545. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Ayirlioglu-Ayaz, S.; Beyazoglu, O. Fatty acid composition of leaf lipids of some Alchemilla L (Rosaceae) species from Northeast Anatolia (Turkey). Grasas Aceites. 1999, 50, 341–344. [Google Scholar] [CrossRef]
- Matthaus, B.; Özcan, M.M. Fatty acid, tocopherol and squalene contents of Rosaceae seed oils. Bot. Stud. 2014, 55, 48. [Google Scholar] [CrossRef] [PubMed]
- Ngawhirunpat, T.; Opanasopi, P.; Sukma, M.; Sittisombut, C.; Kat, A.; Adachi, I. Antioxidant, free radical-scavenging activity and cytotoxicity of different solvent extracts and their phenolic constituents from the fruit hull of mangosteen (Garcinia mangostana). Pharm. Biol. 2010, 48, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Neagu, E.; Paun, G.; Albu, C.; Radu, G.-L. Assessment of acetylcholinesterase and tyrosinase inhibitory and antioxidant activity of Alchemilla vulgaris and Filipendula ulmaria extracts. J. Taiwan Inst. Chem. Eng. 2015, 52, 1–6. [Google Scholar] [CrossRef]
- Boroja, T.; Mihailović, V.; Katanić, J.; Pan, S.-P.; Nikles, S.; Imbimbo, P.; Monti, D.M.; Stanković, N.; Stanković, M.S.; Bauer, R. The biological activities of roots and aerial parts of Alchemilla vulgaris L. S. Afr. J. Bot. 2018, 116, 175–184. [Google Scholar] [CrossRef]
- Karatoprak, G.Ş.; Ilgün, S.; Koşar, M. Phenolic composition, anti-inflammatory, antioxidant, and antimicrobial activities of Alchemilla mollis (BUSER) ROTHM. Chem. Biodivers. 2017, 14, e1700150. [Google Scholar] [CrossRef]
- Szewczyk, K.; Bogucka-Kocka, A.; Vorobets, N.; Grzywa-Celińska, A.; Granica, S. Phenolic composition of the leaves of Pyrola rotundifolia L. and their antioxidant and cytotoxic activity. Molecules 2020, 25, 1749. [Google Scholar] [CrossRef]
- Nikolova, M.; Dincheva, I.; Vitkova, A.; Badjakov, I. Phenolic acids and free radical scavenging activity of Alchemilla jumrukczalica Pawl. Int. J. Pharm. Sci. Res. 2012, 3, 802–804. [Google Scholar]
- Eldeen, I.M.S.; Van Staden, J. Cyclooxygenase inhibition and antimycobacterial effects of extracts from Sudanese medicinal plants. S. Afr. J. Bot. 2008, 74, 225–229. [Google Scholar] [CrossRef][Green Version]
- Trouillas, P.; Calliste, C.; Allais, D.; Simon, A.; Marfak, A.; Delage, C.; Duroux, J. Antioxidant, anti-inflammatory and antiproliferative properties of sixteen water plant extracts used in the Limousin countryside as herbal teas. Food Chem. 2003, 80, 399–407. [Google Scholar] [CrossRef]
- Polish Pharmacopoeia IX, PTFarm; Polish Pharmaceutical Society: Warsaw, Poland, 2011; p. 150.
- Klimek, K.; Strubińska, J.; Czernel, G.; Ginalska, G.; Gagoś, M. In vitro evaluation of antifungal and cytotoxic activities as also the therapeutic safety of the oxidized form of amphotericin B. Chem. Biol. Interact. 2016, 256, 47–54. [Google Scholar] [CrossRef]
- Gallol, L.C.; Bucalá, V.; Rigo, M.V.R.; Piña, J. Herbal Medicine: Dry extracts production and applications. In Plant Extracts: Role in Agriculture, Health Effects and Medical Applications; Giordano, A., Costs, A., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; pp. 171–198. [Google Scholar]
- Kicel, A.; Kolodziejczyk-Czepas, J.; Owczarek, A.; Marchelak, A.; Sopinska, M.; Ciszewski, P.; Nowak, P.; Olszewska, M.A. Polyphenol-rich extracts from Cotoneaster leaves inhibit pro-inflammatory enzymes and protect human plasma components against oxidative stress in vitro. Molecules 2018, 23, 2472. [Google Scholar] [CrossRef]
- Pietrzak, W.; Nowak, R.; Olech, M. Effect of extraction method on phenolic content and antioxidant activity of mistletoe extracts from Viscum album subsp. abietis. Chem. Pap. 2014, 68, 976–982. [Google Scholar] [CrossRef]
- Olech, M.; Nowak, R. Influence of different extraction procedures on the antiradical activity and phenolic profile of Rosa rugosa petals. Acta Pol. Pharm. 2012, 69, 501–507. [Google Scholar] [PubMed]

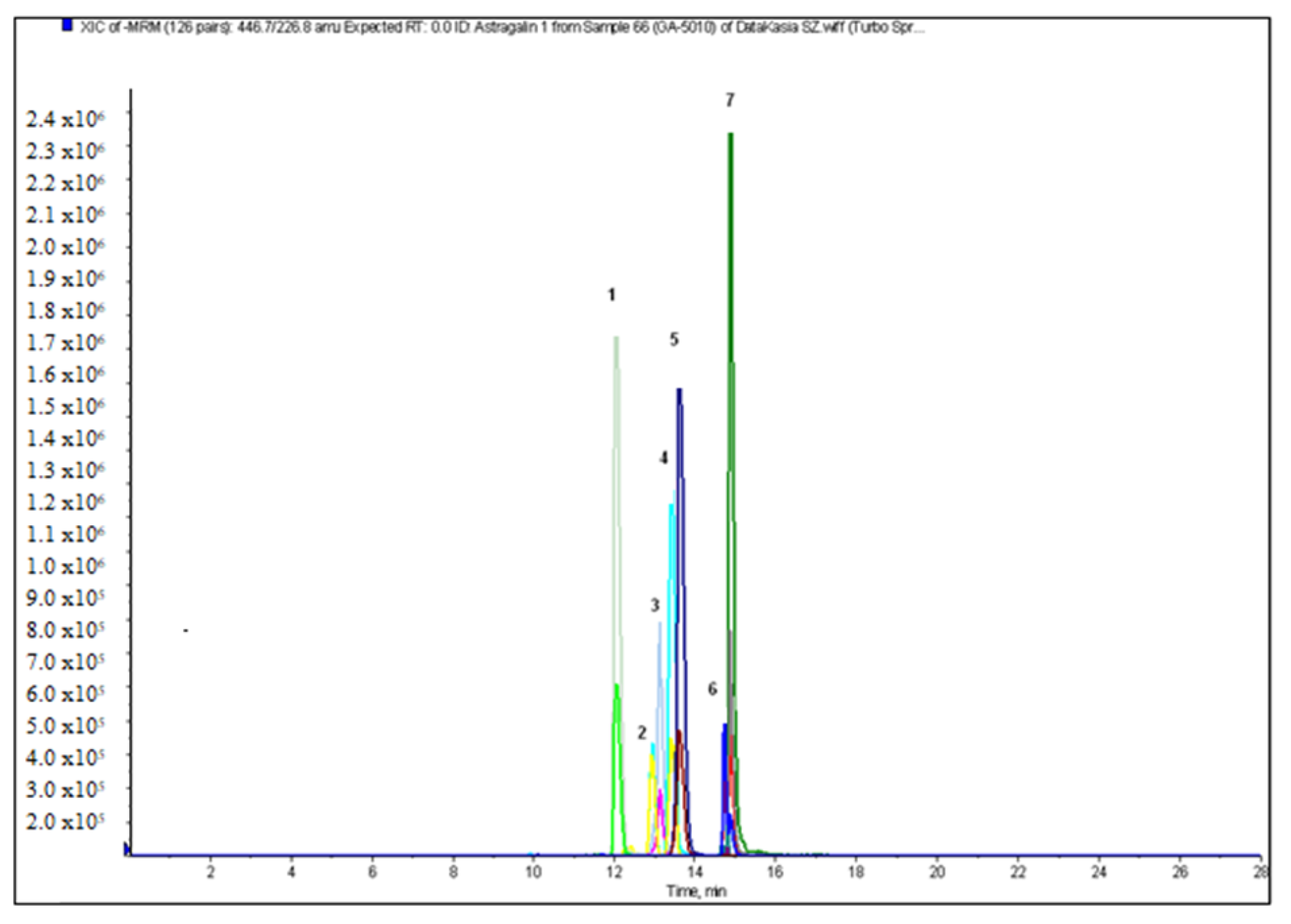
| Sample | Total Flavonoid Content [mg QE/g DE] | Total Phenolic Content [mg GAE/g DE] | Total Phenolic Acids [mg CAE/g DE] |
|---|---|---|---|
| AH | 113.79 ± 1.09 | 279.82 ± 1.52 | 39.15 ± 0.18 |
| AH-B | 57.09 ± 0.31 | 215.61 ± 2.10 | 72.19 ± 0.39 |
| AH-O | 189.25 ± 0.95 | 154.29 ± 1.09 | 11.09 ± 0.01 |
| AH-E | 1.57 ± 0.02 | 95.72 ± 0.89 | nd |
| AR | 19.85 ± 0.19 | 192.54 ± 0.49 | 6.52 ± 0.02 |
| AR-B | 4.63 ± 0.10 | 175.19 ± 1.15 | 9.32 ± 0.03 |
| AR-O | 23.18 ± 0.50 | 93.15 ± 0.95 | 2.15 ± 0.10 |
| AR-E | 1.92 ± 0.01 | 128.37 ± 1.10 | 5.89 ± 0.05 |
| Compound | AH | AR | AH-B | AR-B | AH-O | AR-O | AH-E | AR-E |
|---|---|---|---|---|---|---|---|---|
| gallic acid | 5.89 ± 0.05 | 2.45 ± 0.10 | <LOQ | <LOQ | <LOQ | nd | 0.00 | 0.00 |
| protocatechuic acid | 22.13 ± 0.18 | 0.66 ± 0.02 | 32.88 ± 0.18 | 5.71 ± 0.01 | 19.42 ± 0.12 | 0.80 ± 0.00 | 0.87 ± 0.01 | 2.41 ± 0.03 |
| caffeic acid | 23.50 ± 0.18 | 0.00 | 78.88 ± 0.18 | 9.71 ± 0.10 | 9.82 ± 0.02 | <LOQ | 0.28 ± 0.00 | 1.26 ± 0.01 |
| syringic acid | 4.98 ± 0.11 | 0.00 | 13.01 ± 0.02 | 1.81 ± 0.13 | 4.40 ± 1.25 | 0.00 | 0.00 | <LOQ |
| 4-hydroxybenzoic acid | 12.81 ± 0.09 | <LOQ | 70.36 ± 0.18 | 13.04 ± 0.05 | 5.58 ± 0.28 | < LOQ | 1.43 ± 0.02 | 2.16 ± 0.04 |
| vanilic acid | 12.56 ± 0.70 | 0.00 | 76.75 ± 0.35 | 9.32 ± 0.05 | <LOQ | 0.00 | 0.00 | 0.00 |
| gentisic acid | 0.00 | <LOQ | <LOQ | <LOQ | 3.10 ± 0.05 | 0.24 ± 0.01 | 0.00 | 0.00 |
| sinapic acid | 0.00 | 0.00 | <LOQ | <LOQ | 0.00 | 0.00 | 0.00 | 0.00 |
| p-coumaric acid | 20.88 ± 0.18 | <LOQ | 187.63 ± 0.18 | 24.79 ± 0.20 | 4.26 ± 0.01 | <LOQ | 1.84 ± 0.01 | 4.47 ± 0.05 |
| ferulic acid | 0.00 | 0.00 | 0.00 | 15.93 ± 0.10 | 0.00 | 0.00 | <LOQ | <LOQ |
| rosmarinic acid | 7.56 ± 0.52 | 0.14 ± 0.00 | 0.84 ± 0.01 | 0.02 ± 0.00 | 5.87 ± 0.02 | 0.31 ± 0.01 | 0.04 ± 0.00 | 0.05 ± 0.00 |
| salicylic acid | 11.59 ± 0.05 | <LOQ | 55.50 ± 0.35 | 6.01 ± 0.02 | 7.52 ± 0.12 | <LOQ | 0.17 ± 0.01 | 0.63 ± 0.00 |
| Total of Phenolic Acids | 121.90 | 3.25 | 515.85 | 86.34 | 59.97 | 1.35 | 4.63 | 10.98 |
| astragalin | 23.75 ± 0.18 | <LOQ | 7.48 ± 0.11 | 0.33 ± 0.04 | 22.42 ± 0.35 | <LOQ | 0.03 ± 0.00 | 2.25 ± 0.02 |
| quercitrin | 294.38 ± 4.12 | 1.19 ± 0.02 | 395.00 ± 3.54 | 57.36 ± 0.10 | 335.83 ± 1.18 | 4.02 ± 0.19 | 10.24 ± 0.12 | 18.58 ± 0.02 |
| isoquercitrin | 139.38 ± 0.88 | 1.21 ± 0.03 | 22.59 ± 0.16 | 0.66 ± 0.02 | 147.75 ± 1.06 | 5.86 ± 0.03 | 1.67 ± 0.02 | 4.85 ± 0.02 |
| isorhamnetin-3-glucoside | 4.36 ± 0.10 | <LOQ | <LOQ | <LOQ | 2.31±0.01 | <LOQ | <LOQ | <LOQ |
| kaempferol-3-rutinoside | 191.88 ± 0.88 | 37.74 ± 0.07 | 7.41 ± 0.16 | 0.63 ± 0.10 | 244.17 ± 3.54 | 53.91 ± 0.04 | 8.35 ± 0.00 | 12.82 ± 0.16 |
| rutin | 235.00 ± 3.54 | 83.16 ± 0.00 | 5.05 ± 0.60 | < LOQ | 332.50 ± 1.18 | 97.94 ± 0.42 | 15.99 ± 0.08 | 24.97 ± 0.14 |
| narcissoside | 145.00 ± 3.54 | 47.71 ± 0.04 | 5.21 ± 0.12 | 0.58 ± 0.01 | 206.67 ± 4.71 | 61.76 ± 0.00 | 8.05 ± 0.02 | 13.45 ± 0.07 |
| hyperoside | 0.00 | 0.00 | 2.47 ± 0.08 | 0.66 ± 0.02 | 0.00 | 0.00 | 0.00 | 1.52 ± 0.01 |
| tiliroside | 0.00 | 0.00 | <LOQ | <LOQ | 0.00 | 0.00 | 0.00 | 0.00 |
| naringenin-7-glucoside | <LOQ | 0.00 | 0.00 | 0.00 | <LOQ | 0.00 | 0.00 | 0.00 |
| Total of Flavonoid Glycosides | 1033.75 | 171.01 | 445.21 | 60.22 | 1291.65 | 223.49 | 44.33 | 78.44 |
| luteolin | 0.05 ± 0.00 | 0.00 | 0.51 ± 0.00 | 0.02 ± 0.00 | <LOQ | 0.00 | <LOQ | <LOQ |
| kaempferol | 0.00 | 0.00 | 1.23 ± 0.00 | <LOQ | 0.00 | 0.00 | 0.00 | 0.04 ± 0.00 |
| 3-O-methylquercetin | 0.00 | 0.00 | <LOQ | 0.00 | 0.00 | <LOQ | 0.00 | <LOQ |
| naringenin | <LOQ | 0.00 | <LOQ | 0.00 | <LOQ | 0.00 | 0.00 | 0.00 |
| eriodictyol | <LOQ | 0.00 | 0.17 ± 0.00 | <LOQ | <LOQ | 0.00 | 0.00 | 0.00 |
| quercetin | 0.15 ± 0.00 | <LOQ | 22.38 ± 0.39 | 1.04 ± 0.01 | 0.14 ± 0.00 | <LOQ | 0.00 | 0.00 |
| isorhamnetin | <LOQ | 0.00 | 1.89 ± 0.00 | 0.04 ± 0.00 | <LOQ | 0.00 | 0.00 | 0.00 |
| rhamnetin | 0.00 | 0.00 | <LOQ | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total of Flavonoid Aglycones | 0.20 | 0.00 | 26.18 | 1.10 | 0.14 | 0.00 | 0.00 | 0.04 |
| AH | AR | AH-B | AR-B | AH-O | AR-O | AH-E | AR-E | AA | Trolox | Na2EDTA*2H2O | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | 18.69 ± 0.04 | 29.87 ± 0.15 | 8.96 ± 0.10 | 12.08 ± 0.18 | 8.83 ± 0.37 | 15.37 ± 0.19 | 41.46 ± 0.32 | 51.42 ± 0.18 | 4.90 ± 0.09 | - | - |
| ABTS | 6.17 ± 0.24 | 14.29 ± 0.06 | 1.42 ± 0.18 | 8.78 ± 0.01 | 6.54 ± 0.03 | 10.39 ± 0.15 | 16.28 ± 0.15 | 24.82 ± 0.20 | - | 3.07 ± 0.01 | - |
| CHEL | 21.60 ± 0.39 | 25.76 ± 0.03 | 11.43 ± 0.18 | 12.33 ± 0.33 | 18.89 ± 0.94 | 19.30 ± 0.22 | 25.51 ± 0.89 | 44.12 ± 0.24 | - | - | 9.45 ± 0.03 |
| COX-1 Inibition [%] ±SD | COX-2 Inhibition [%] ±SD | |||
|---|---|---|---|---|
| Extract | 50 µg/mL | 100 µg/mL | 50 µg/mL | 100 µg/mL |
| AH | 50.17 ± 0.85 | 71.32 ± 2.73 | 43.65 ± 1.36 | 78.52 ± 2.18 |
| AR | 31.27 ± 0.76 | 47.80 ± 1.12 | 48.59 ± 2.19 | 54.25 ± 1.23 |
| AH-B | 76.82 ± 0.69 | 83.14 ± 1.08 | 79.75 ± 1.29 | 95.10 ± 1.81 |
| AR-B | 63.17 ± 1.28 | 78.29 ± 2.25 | 72.64 ± 1.93 | 90.93 ± 2.65 |
| AH-O | 52.65 ± 2.19 | 74.50 ± 1.29 | 60.23 ± 0.67 | 80.12 ± 1.73 |
| AR-O | 45.73 ± 1.45 | 64.49 ± 2.74 | 58.08 ± 1.79 | 79.15 ± 3.10 |
| AH-E | nd | 10.43 ± 0.19 | nd | 28.09 ± 0.13 |
| AR-E | nd | 28.71 ± 0.35 | nd | 41.96 ± 1.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dos Santos Szewczyk, K.; Pietrzak, W.; Klimek, K.; Grzywa-Celińska, A.; Celiński, R.; Gogacz, M. LC-ESI-MS/MS Identification of Biologically Active Phenolics in Different Extracts of Alchemilla acutiloba Opiz. Molecules 2022, 27, 621. https://doi.org/10.3390/molecules27030621
Dos Santos Szewczyk K, Pietrzak W, Klimek K, Grzywa-Celińska A, Celiński R, Gogacz M. LC-ESI-MS/MS Identification of Biologically Active Phenolics in Different Extracts of Alchemilla acutiloba Opiz. Molecules. 2022; 27(3):621. https://doi.org/10.3390/molecules27030621
Chicago/Turabian StyleDos Santos Szewczyk, Katarzyna, Wioleta Pietrzak, Katarzyna Klimek, Anna Grzywa-Celińska, Rafał Celiński, and Marek Gogacz. 2022. "LC-ESI-MS/MS Identification of Biologically Active Phenolics in Different Extracts of Alchemilla acutiloba Opiz" Molecules 27, no. 3: 621. https://doi.org/10.3390/molecules27030621
APA StyleDos Santos Szewczyk, K., Pietrzak, W., Klimek, K., Grzywa-Celińska, A., Celiński, R., & Gogacz, M. (2022). LC-ESI-MS/MS Identification of Biologically Active Phenolics in Different Extracts of Alchemilla acutiloba Opiz. Molecules, 27(3), 621. https://doi.org/10.3390/molecules27030621







