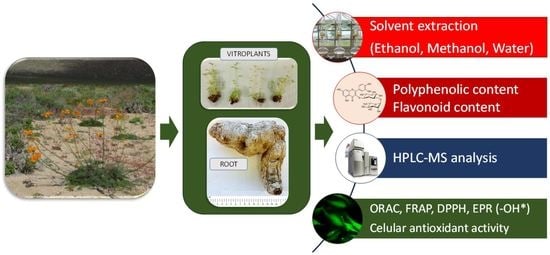
Polyphenolic Composition and Antioxidant Activity (ORAC, EPR and Cellular) of Different Extracts of Argylia radiata Vitroplants and Natural Roots
Abstract
:1. Introduction
2. Results and Discussion
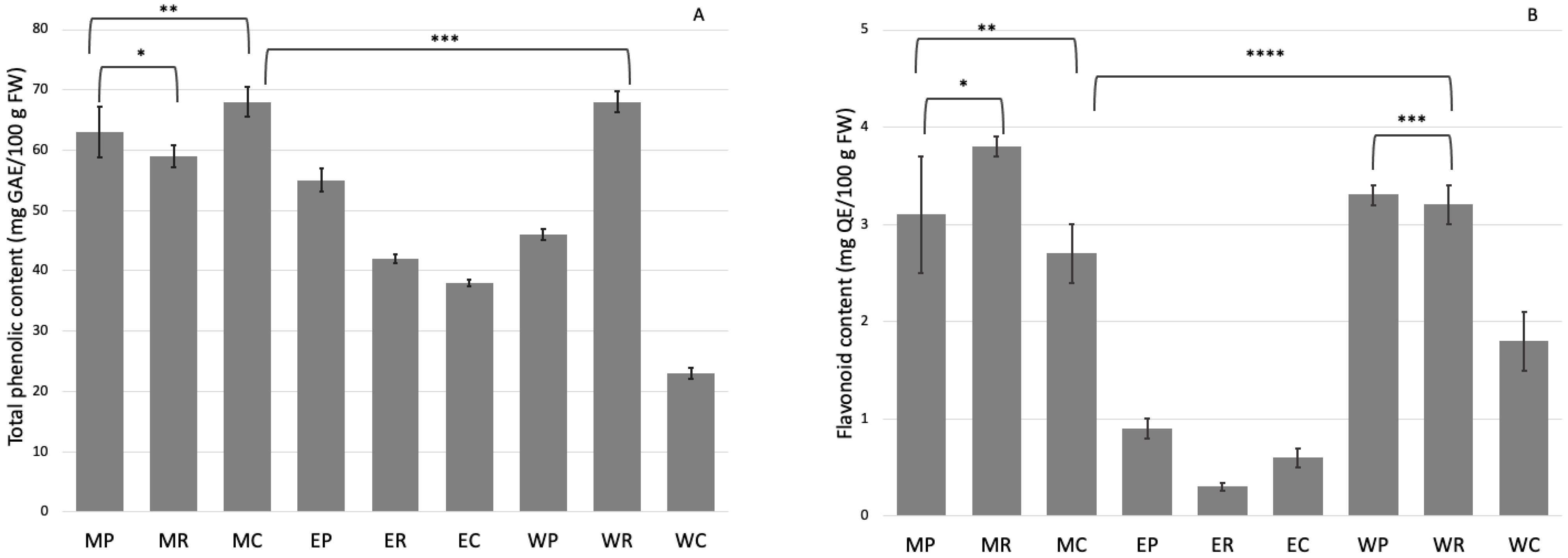
2.1. Extraction from Tissue Culture Plant
2.2. Phenolic Compounds Analysis
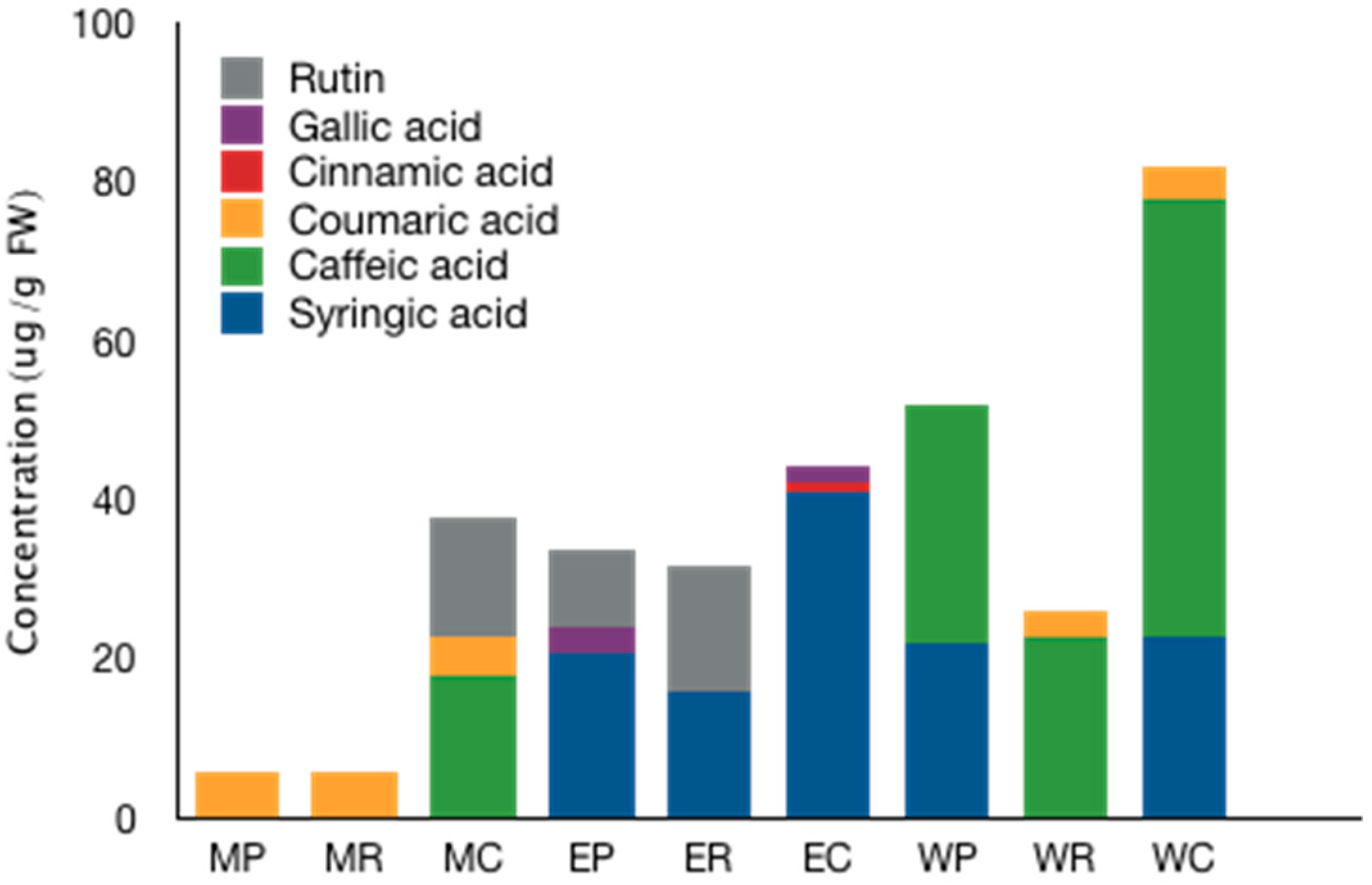
2.3. UPLC-MS/MS Analysis
2.4. Antioxidant Capacity Studies
2.4.1. Evaluation of ORAC-FL
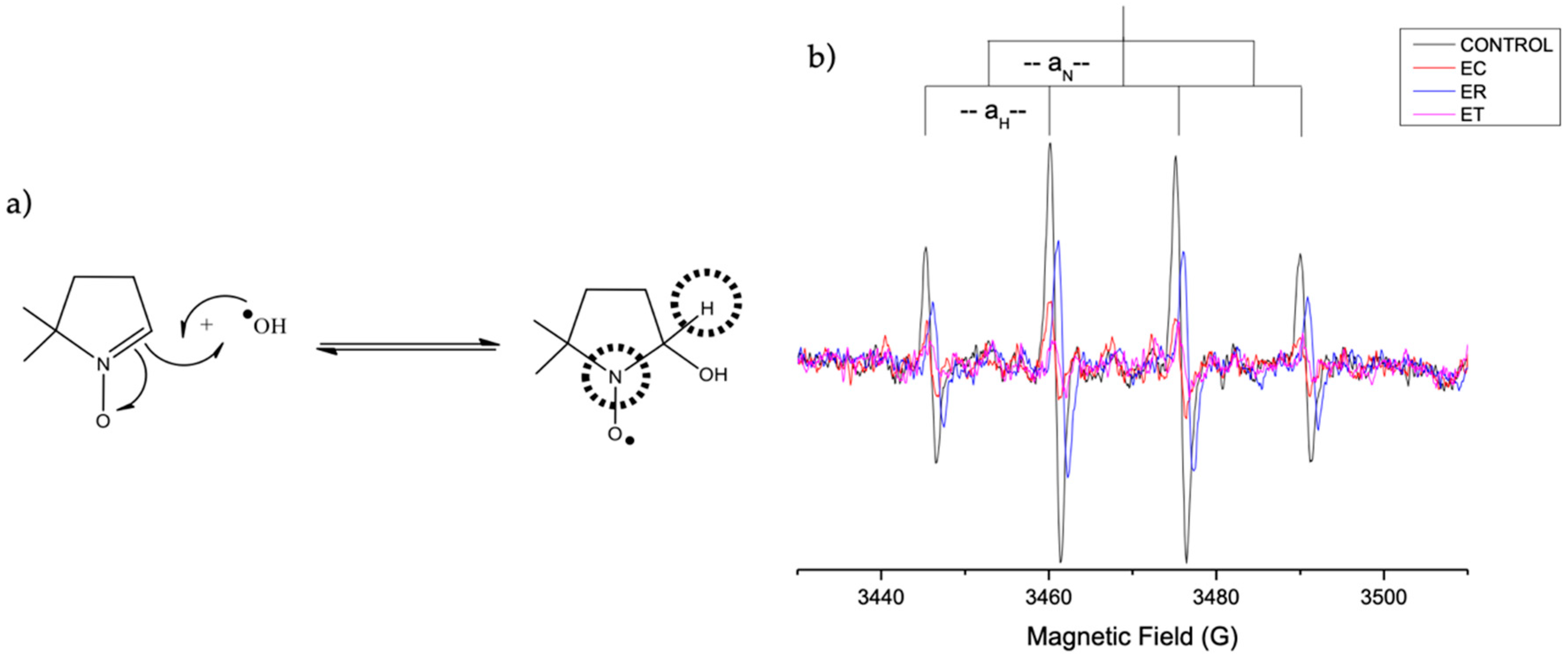
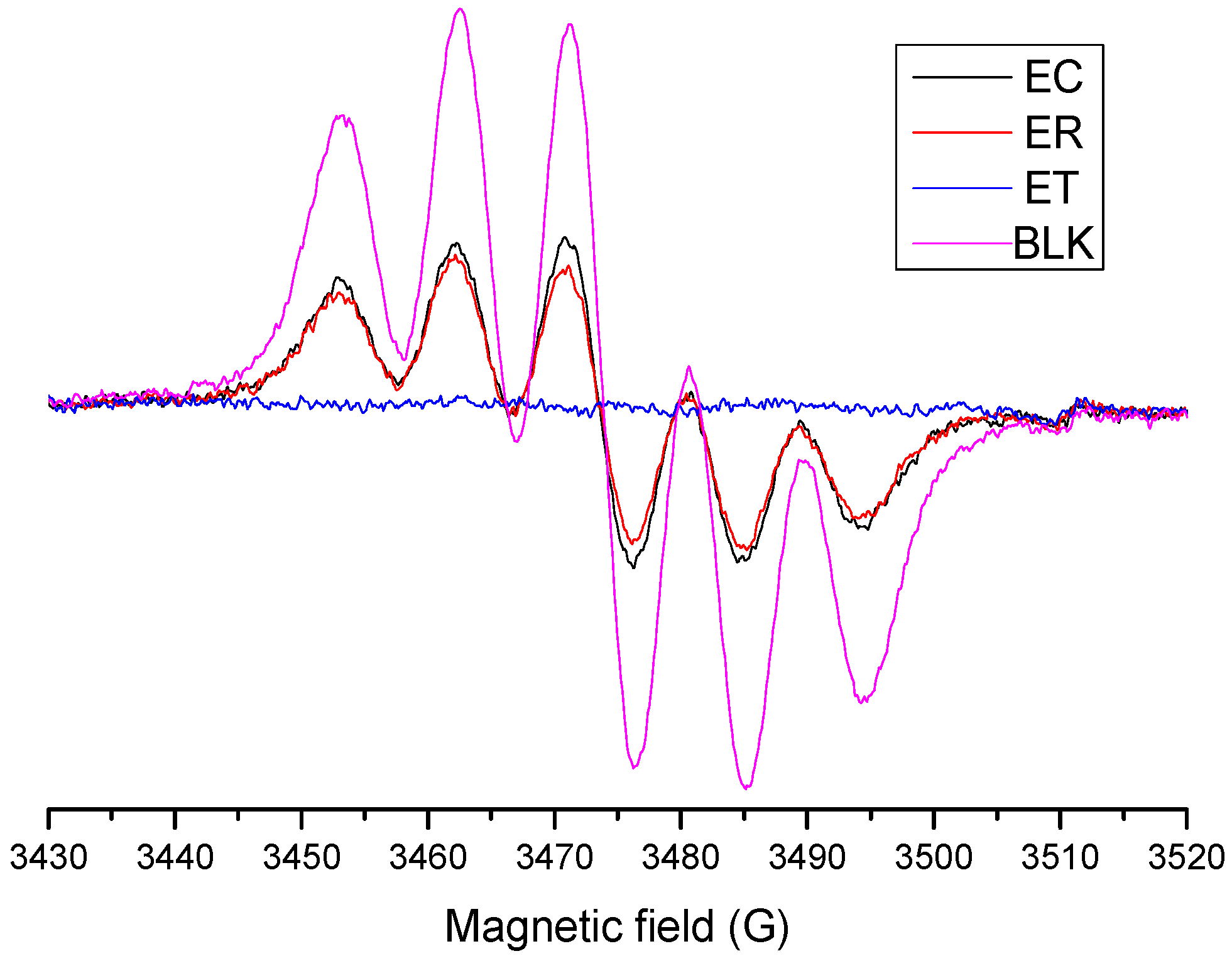
2.4.2. Evaluation of OH· Scavenging by ESR
2.4.3. Evaluation of DPPH∙ Radical-Scavenging Activity by the EPR Method
2.5. Evaluation of Cellular Antioxidant Activity (CAA)
3. Materials and Methods
3.1. Obtaining of Plant Material
3.2. Plant Extract Preparation
3.3. Total Phenolic Determination
3.4. Flavonoids Determination
3.5. UPLC-MS/MS Determination
3.6. Antioxidant Assays
3.6.1. Oxygen Radical Antioxidant Capacity-Fluorescein (ORAC-FL)
3.6.2. Evaluation of OH· Scavenging by ESR
3.6.3. DPPH Radical Scavenging Activity Evaluated by the ESR Method
3.6.4. Evaluation of the Antioxidant Activity in RAW 264.7 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Riedemann, P.; Aldunate, G.; Teillier, S. Flora Nativa de Valor Ornamental; Identificación y Propagación, Chile Zona Norte; Segunda, Ed.; Corporación Jardín Botánico Chagual: Santiago, Chile, 2016; ISBN 9789569479038. [Google Scholar]
- Morales-Tapia, P.; Gambardella, M.; Gómez, M.; Montenegro, G. Morpho-anatomical adaptations of Argylia radiata (L.) D. Don to an arid environment. Flora Morphol. Distrib. Funct. Ecol. Plants 2019, 258, 151440. [Google Scholar] [CrossRef]
- Lino Von Poser, G.; Schripsema, J.; Henriques, A.T.; Rosendal Jensen, S. The distribution of iridoids in Bignoniaceae. Biochem. Syst. Ecol. 2000, 28, 351–366. [Google Scholar] [CrossRef]
- Bianco, A.; Passacantilli, P.; Righi, G.; Nicoletti, M.; Serafini, M.; Garbarino, J.A.; Gambaro, V. Argylioside, a dimeric iridoid glucoside from Argylia radiata. Phytochemistry 1986, 25, 946–948. [Google Scholar] [CrossRef]
- Bianco, A.; Passacantilli, P.; Rispoli, C. Radiatoside, a new bisiridoid from Argylia radiata. J. Nat. Prod. 1986, 49, 519–521. [Google Scholar] [CrossRef]
- Bianco, A.; Passacantilli, P.; Righi, G.; Nicoletti, M.; Serafini, M.; Garbarino, J.A.; Gambaro, V.; Chamy, M.C. Radiatoside B and C, Two New Bisiridoid Glucosides from Argylia radiata. Planta Med. 1987, 53, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Passacantilli, P.; Garbarino, J.A.; Gambaro, V.; Serafini, M.; Nicoletti, M.; Rispoli, C.; Righi, G. A New Non-Glycosidic Iridoid and a New Bisiridoid from Argylia radiata. Planta Med. 1991, 57, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Marini, E.; Nicoletti, M.; Foddai, S.; Garbarino, J.A.; Piovano, M.; Chamy, M.T. Bis-iridoid glucosides from the roots of Argylia radiata. Phytochemistry 1992, 31, 4203–4206. [Google Scholar] [CrossRef]
- Bianco, A.; Bonadies, F.; Cianciolo, V.; Melchioni, C.; Ramunno, A.; Dezzi, S.; Nicoletti, M.; Serafini, M.; Ballero, M. Monoterpene alkaloids from Argylia radiata. Nat. Prod. Lett. 2002, 16, 77–80. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, C.; Li, Y.; Guan, S.; Han, F.; Zhang, S. Catalpol improves cholinergic function and reduces inflammatory cytokines in the senescent mice induced by d-galactose. Food Chem. Toxicol. 2013, 58, 50–55. [Google Scholar] [CrossRef]
- Zou, G.L.; Zhong, W.L.; Wu, F.; Wang, X.X.; Liu, L. Catalpol attenuates cardiomyocyte apoptosis in diabetic cardiomyopathy via Neat1/miR-140–5p/HDAC4 axis. Biochimie 2019, 165, 90–99. [Google Scholar] [CrossRef]
- Morales-Tapia, P.; Cabrera-Barjas, G.; Giordano, A. Polyphenolic distribution in organs of Argylia radiata, an extremophile plant from Chilean Atacama desert. Nat. Prod. Res. 2020, 35, 4143–4147. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Serwah Boateng, N.A.; Ma, H. Latest developments in polyphenol recovery and purification from plant by-products: A review. Trends Food Sci. Technol. 2020, 99, 375–388. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Ma, G.; Chen, Y. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis. J. Funct. Foods 2020, 66, 103829. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Curr. Knowl. Dir. Future Res. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef]
- Morales, P. Argylia radiata micropropagation, a biotechnological tool to domesticate a new ornamental crop. Acta Hortic. 2019, 1240, 69–72. [Google Scholar] [CrossRef]
- Driscoll, K.; Deshpande, A.; Chapp, A.; Li, K.; Datta, R.; Ramakrishna, W. Anti-inflammatory and immune-modulating effects of rice callus suspension culture (RCSC) and bioactive fractions in an in vitro inflammatory bowel disease model. Phytomedicine 2019, 57, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Li, J.; Li, J.; Liu, S.; Huang, L.; Gao, W. Production of Active Compounds in Medicinal Plants: From Plant Tissue Culture to Biosynthesis. Chin. Herb. Med. 2017, 9, 115–125. [Google Scholar] [CrossRef]
- Paek, K.Y.; Murthy, H.N.; Zhong, J.J. (Eds.) Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Springer: Dordrecht, The Netherlands, 2014; ISBN 9789401792226. [Google Scholar]
- Rodrigo, R.; Libuy, M. Modulation of Plant Endogenous Antioxidant Systems by Polyphenols. In Polyphenols in Plants: Isolation, Purification and Extract Preparation; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 65–85. ISBN 9780123979346. [Google Scholar]
- Larrazabal-Fuentes, M.; Palma, J.; Paredes, A.; Mercado, A.; Neira, I.; Lizama, C.; Sepulveda, B.; Bravo, J. Chemical composition, antioxidant capacity, toxicity and antibacterial activity of the essential oils from Acantholippia deserticola (Phil.) Moldenke (Rica rica) and Artemisia copa Phil. (Copa copa) extracted by microwave-assisted hydrodistillation. Ind. Crops Prod. 2019, 142, 111830. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Carrillo-Inungaray, M.L.; López, L.I.; Nevárez-Moorillón, G.V.; Aguilar, C.N. Total phenolic content, in vitro antioxidant activity and chemical composition of plant extracts from semiarid Mexican region. Asian Pac. J. Trop. Med. 2015, 8, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Encinar, J.A.; Rodríguez-Díaz, J.C.; Micol, V. Antimicrobial Capacity of Plant Polyphenols against Gram-positive Bacteria: A Comprehensive Review. Curr. Med. Chem. 2018, 27, 2576–2606. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef]
- Sharma, S.; Vig, A.P. Evaluation of in vitro antioxidant properties of methanol and aqueous extracts of Parkinsonia aculeata L. leaves. Sci. World J. 2013, 2013, 604865. [Google Scholar] [CrossRef] [Green Version]
- Mellado, M.; Soto, M.; Madrid, A.; Montenegro, I.; Jara-Gutiérrez, C.; Villena, J.; Werner, E.; Godoy, P.; Aguilar, L.F. In vitro antioxidant and antiproliferative effect of the extracts of Ephedra chilensis K Presl aerial parts. BMC Complement. Altern. Med. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Muthukumaran, J.; Srinivasan, S.; Venkatesan, R.S.; Ramachandran, V.; Muruganathan, U. Syringic acid, a novel natural phenolic acid, normalizes hyperglycemia with special reference to glycoprotein components in experimental diabetic rats. J. Acute Dis. 2013, 2, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Campos, F.M.; Couto, J.A.; Hogg, T.A. Influence of phenolic acids on growth and inactivation of Oenococcus oeni and Lactobacillus hilgardii. J. Appl. Microbiol. 2003, 94, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef]
- Dorta, E.; Fuentes-Lemus, E.; Aspée, A.; Atala, E.; Speisky, H.; Bridi, R.; Lissi, E.; López-Alarcón, C. The ORAC (oxygen radical absorbance capacity) index does not reflect the capacity of antioxidants to trap peroxyl radicals. RSC Adv. 2015, 5, 39899–39902. [Google Scholar] [CrossRef]
- Dorta, E.; Aspée, A.; Pino, E.; González, L.; Lissi, E.; López-Alarcón, C. Controversial alkoxyl and peroxyl radical scavenging activity of the tryptophan metabolite 3-hydroxy-anthranilic acid. Biomed. Pharmacother. 2017, 90, 332–338. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, E.; Campos, A.M.; Edwards, A.M.; Lissi, E.; López-Alarcón, C. Antioxidant capacity of herbal infusions and tea extracts: A comparison of ORAC-fluorescein and ORAC-pyrogallol red methodologies. Food Chem. 2008, 107, 1114–1119. [Google Scholar] [CrossRef]
- Pérez-Cruz, K.; Moncada-Basualto, M.; Morales-Valenzuela, J.; Barriga-González, G.; Navarrete-Encina, P.; Núñez-Vergara, L.; Squella, J.A.; Olea-Azar, C. Synthesis and antioxidant study of new polyphenolic hybrid-coumarins. Arab. J. Chem. 2018, 11, 525–537. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Aspée, A.; Lissi, E. Influence of the target molecule on the ORAC index. ACS Symp. Ser. 2012, 1093, 417–429. [Google Scholar] [CrossRef]
- Koroleva, O.; Torkova, A.; Nikolaev, I.; Khrameeva, E.; Fedorova, T.; Tsentalovich, M.; Amarowicz, R. Evaluation of the antiradical properties of phenolic acids. Int. J. Mol. Sci. 2014, 15, 16351–16380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villamena, F.A. EPR Spin Trapping. In Reactive Species Detection in Biology; Elsevier: Boston, UK, 2017; pp. 163–202. ISBN 9780124200807. [Google Scholar]
- Mura, F.; Silva, T.; Castro, C.; Borges, F.; Zuñiga, M.C.; Morales, J.; Olea-Azar, C. New insights into the antioxidant activity of hydroxycinnamic and hydroxybenzoic systems: Spectroscopic, electrochemistry, and cellular studies. Free. Radic. Res. 2014, 48, 1473–1484. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, B.; Shen, S.; Hou, J.; Hu, J.; Xin, W. ESR study on the structure-antioxidant activity relationship of tea catechins and their epimers. Biochim. Biophys. Acta-Gen. Subj. 1999, 1427, 13–23. [Google Scholar] [CrossRef]
- Foti, M.C.; Daquino, C.; Geraci, C. Electron-Transfer Reaction of Cinnamic Acids and Their Methyl Esters with the DPPH∙ Radical in Alcoholic Solutions. J. Org. Chem. 2004, 69, 2309–2314. [Google Scholar] [CrossRef]
- Espinoza, M.; Olea-Azar, C.; Speisky, H.; Rodríguez, J. Determination of reactions between free radicals and selected Chilean wines and transition metals by ESR and UV-vis technique. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2009, 71, 1638–1643. [Google Scholar] [CrossRef]
- Mothanka, D.; Habtemariam, S.; Houghton, P. Free radical scavenging activity of crude extracts and 4′-o-methylepigallocatechin isolated from roots of cassine transvaalensis burtt-davy from Botswana. Afr. J. Biomed. Res. 2008, 11, 55–63. [Google Scholar] [CrossRef]
- Yen, G.-C.; Duh, P.-D.; Tsai, H.-L. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002, 79, 307–313. [Google Scholar] [CrossRef]
- Zhou, K.; Yin, J.-J.; Yu, L. ESR determination of the reactions between selected phenolic acids and free radicals or transition metals. Food Chem. 2006, 95, 446–457. [Google Scholar] [CrossRef]
- Zúñiga-López, M.C.; Maturana, G.; Campmajó, G.; Saurina, J.; Núñez, O. Determination of Bioactive Compounds in Sequential Extracts of Chia Leaf (Salvia hispanica L.) Using UHPLC-HRMS (Q-Orbitrap) and a Global Evaluation of Antioxidant In Vitro Capacity. Antioxidants 2021, 10, 1151. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Rui, H.L. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Kang, X.; He, X.; Dong, M.; Zhang, Q.; Liu, R.H. Cellular Antioxidant Activity of Common Fruits. J. Agric. Food Chem. 2008, 56, 8418–8426. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Moncada-Basualto, M.; Lapier, M.; Maya, J.D.; Matsuhiro, B.; Olea-Azar, C.; Delogu, G.L.; Uriarte, E.; Santana, L.; Matose, M.J. Evaluation of Trypanocidal and Antioxidant Activities of a Selected Series of 3-amidocoumarins. Med. Chem. 2018, 14, 573–584. [Google Scholar] [CrossRef]
- Matos, M.J.; Mura, F.; Vazquez-Rodriguez, S.; Borges, F.; Santana, L.; Uriarte, E.; Olea-Azar, C. Study of coumarin-resveratrol hybrids as potent antioxidant compounds. Molecules 2015, 20, 3290–3308. [Google Scholar] [CrossRef] [Green Version]

| Samples | ORAC-FL (µM TE/g FW) | Scavenging OH∙ (%) * | Scavenging DPPH∙ (%) * |
|---|---|---|---|
| MP | 1031.3 ± 4.2 | 81.7 ± 1.1 | 87.6 ± 2.0 |
| MR | 982.3 ± 2.5 | 73.5 ± 2.1 | 76.7 ± 1.9 |
| MC | 917.3 ± 3.1 | 67.4 ± 1.3 | 68.9 ± 1.2 |
| EP | 1103.4 ± 3.6 | 97.1 ± 1.1 | 97.6 ± 2.0 |
| ER | 837.8 ± 2.6 | 59.3 ± 1.2 | 56.7 ± 1.9 |
| EC | 989.3 ± 3.4 | 81.9 ± 1.2 | 61.9 ± 1.2 |
| WP | 869.1 ± 1.8 | 57.6 ± 2.1 | 27.6 ± 2.1 |
| WR | 798.5 ± 1.9 | 56.7 ± 2.1 | 16.7 ± 1.1 a |
| WC | 805.4 ± 2.1 | 58.9 ± 1.2 | 18.9 ± 1.0 a |
| Samples | Cellular Antioxidant Activity (%) | |
|---|---|---|
| 1 (µg/mL) | 10 (µg/mL) | |
| MP | 44.6 ± 3.2 | 51.0 ± 1.1 |
| MR | 33.8 ± 1.3 | 45.5 ± 1.3 |
| MC | 21.7 ± 2.0 | 37.4 ± 2.1 |
| EP | 23.4 ± 1.1 | 39.7 ± 1.8 |
| ER | 20.3 ±1.5 | 32.5 ± 1.6 |
| EC | 20.1 ± 1.3 | 33.1 ± 1.8 |
| WP | 21.6 ± 1.8 | 41.1 ± 1.3 |
| WR | 17.6 ± 1.4 | 34.3 ± 1.5 |
| WC | 20.4 ± 1.9 | 27.8 ± 1.3 |
| Standard | Mass Q1 | Mass Q3 | DP | CE |
|---|---|---|---|---|
| Apigenin | 268,958 | 117,000 | −130,000 | −130,000 |
| Caffeic Acid | 178,949 | 135,000 | −70,000 | −70,000 |
| Catechin | 289,038 | 245,000 | −100,000 | −100,000 |
| Chlorogenic Acid | 353,111 | 191,000 | −75,000 | −75,000 |
| Chrysin | 253,048 | 142,900 | −120,000 | −120,000 |
| Cinnamic Acid | 146,974 | 103,100 | −55,000 | −55,000 |
| Cumaric Acid | 162,913 | 119,000 | −70,000 | −70,000 |
| Epicatechin | 288,985 | 244,900 | −110,000 | −110,000 |
| Ferulic Acid | 192,974 | 146,900 | −50,000 | −50,000 |
| Gallic Acid | 168,922 | 124,900 | −70,000 | −70,000 |
| 3-Hydroxybenzoic Acid | 136,958 | 92,900 | −50,000 | −50,000 |
| Luteolin | 285,022 | 133,000 | −125,000 | −125,000 |
| Myricetin | 316,934 | 150,900 | −120,000 | −120,000 |
| Pinocembrin | 255,062 | 212,900 | −95,000 | −95,000 |
| Quercetin | 301,010 | 150,900 | −115,000 | −115,000 |
| Resveratrol | 227,004 | 185,000 | −100,000 | −100,000 |
| Rutin | 609,007 | 299,800 | −170,000 | −170,000 |
| Sinapic Acid | 223,021 | 207,900 | −75,000 | −75,000 |
| Syringic Acid | 196,981 | 181,900 | −65,000 | −65,000 |
| Vanillic Acid | 166,844 | 122.900 | −50,000 | −50,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, A.; Morales-Tapia, P.; Moncada-Basualto, M.; Pozo-Martínez, J.; Olea-Azar, C.; Nesic, A.; Cabrera-Barjas, G. Polyphenolic Composition and Antioxidant Activity (ORAC, EPR and Cellular) of Different Extracts of Argylia radiata Vitroplants and Natural Roots. Molecules 2022, 27, 610. https://doi.org/10.3390/molecules27030610
Giordano A, Morales-Tapia P, Moncada-Basualto M, Pozo-Martínez J, Olea-Azar C, Nesic A, Cabrera-Barjas G. Polyphenolic Composition and Antioxidant Activity (ORAC, EPR and Cellular) of Different Extracts of Argylia radiata Vitroplants and Natural Roots. Molecules. 2022; 27(3):610. https://doi.org/10.3390/molecules27030610
Chicago/Turabian StyleGiordano, Ady, Pablo Morales-Tapia, Mauricio Moncada-Basualto, Josué Pozo-Martínez, Claudio Olea-Azar, Aleksandra Nesic, and Gustavo Cabrera-Barjas. 2022. "Polyphenolic Composition and Antioxidant Activity (ORAC, EPR and Cellular) of Different Extracts of Argylia radiata Vitroplants and Natural Roots" Molecules 27, no. 3: 610. https://doi.org/10.3390/molecules27030610
APA StyleGiordano, A., Morales-Tapia, P., Moncada-Basualto, M., Pozo-Martínez, J., Olea-Azar, C., Nesic, A., & Cabrera-Barjas, G. (2022). Polyphenolic Composition and Antioxidant Activity (ORAC, EPR and Cellular) of Different Extracts of Argylia radiata Vitroplants and Natural Roots. Molecules, 27(3), 610. https://doi.org/10.3390/molecules27030610