Prion Strains Differ in Susceptibility to Photodynamic Oxidation
Abstract
1. Introduction
2. Results
2.1. Phthalocyanine Derivatives Characterization
2.1.1. Differences in the Phthalocyanine Structure
2.1.2. Spectroscopic Properties and Production of Singlet Oxygen
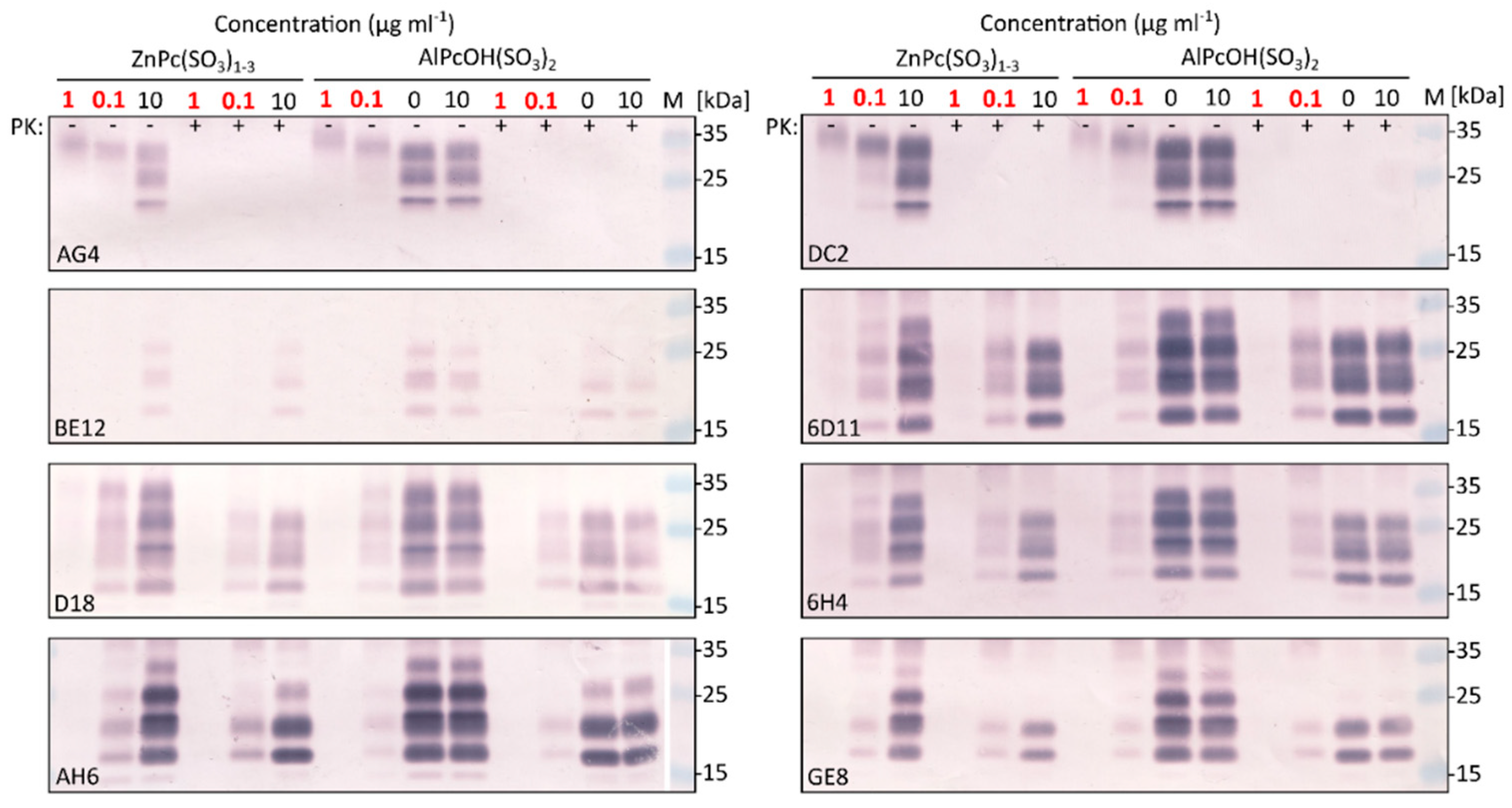
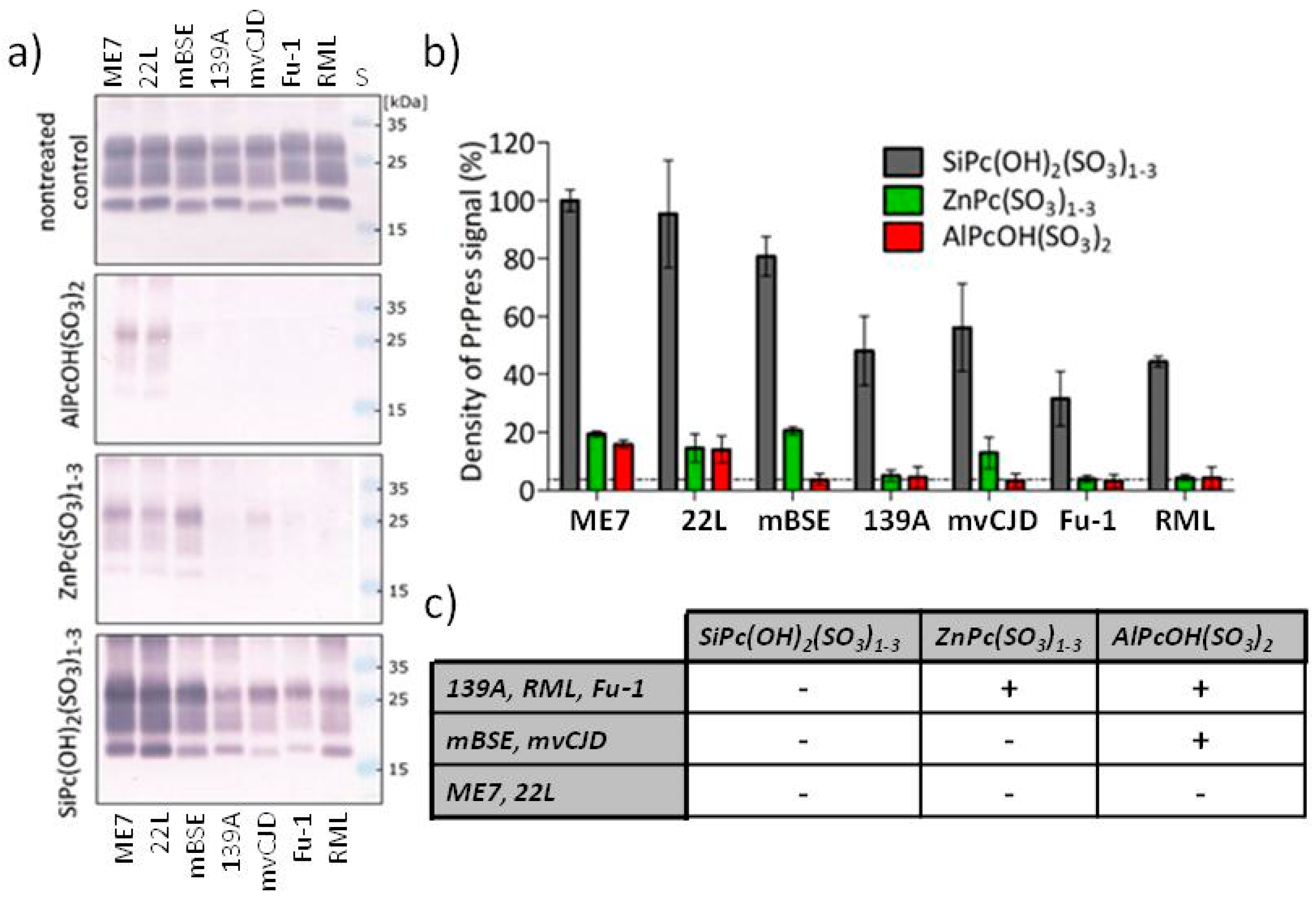
2.2. Photodynamic Elimination of Prion Epitopes
2.2.1. Demonstration of Epitopes Elimination by a Panel of Prion Antibodies
2.2.2. Differences in the Potency of Phthalocyanine Derivatives and in the Sensitivity of Prion Strains
3. Discussion
4. Materials and Methods
4.1. Phthalocyanine Derivatives Characterization
4.2. Tissue Samples
4.3. Photodynamic Oxidation of PrPTSE
4.4. Western Blotting
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Morales, R.; Abid, K.; Soto, C. The prion strain phenomenon: Molecular basis and unprecedented features. Biochim. Biophys. Acta 2007, 1772, 681–691. [Google Scholar] [CrossRef]
- Carta, M.; Aguzzi, A. Molecular foundations of prion strain diversity. Curr. Opin. Neurobiol. 2021, 72, 22–31. [Google Scholar] [CrossRef]
- Taylor, D.M. Resistance of transmissible spongiform encephalopathy agents to decontamination. Contrib. Microbiol. 2004, 11, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Hughson, A.G.; Race, B.; Kraus, A.; Sangare, L.R.; Robins, L.; Groveman, B.R.; Saijo, E.; Phillips, K.; Contreras, L.; Dhaliwal, V.; et al. Inactivation of Prions and Amyloid Seeds with Hypochlorous Acid. PLoS Pathog. 2016, 12, e1005914. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Pastore, M.; Rogez-Kreuz, C.; Richard, M.; Belondrade, M.; Rauwel, G.; Durand, F.; Yousfi, R.; Criquelion, J.; Clayette, P.; et al. New hospital disinfection processes for both conventional and prion infectious agents compatible with thermosensitive medical equipment. J. Hosp. Infect. 2009, 72, 342–350. [Google Scholar] [CrossRef]
- Peretz, D.; Supattapone, S.; Giles, K.; Vergara, J.; Freyman, Y.; Lessard, P.; Safar, J.G.; Glidden, D.V.; McCulloch, C.; Nguyen, H.O.; et al. Inactivation of prions by acidic sodium dodecyl sulfate. J. Virol. 2006, 80, 322–331. [Google Scholar] [CrossRef]
- Marin-Moreno, A.; Aguilar-Calvo, P.; Moudjou, M.; Espinosa, J.C.; Beringue, V.; Torres, J.M. Thermostability as a highly dependent prion strain feature. Sci. Rep.-UK 2019, 9, 11396. [Google Scholar] [CrossRef] [PubMed]
- Thackray, A.M.; Hopkins, L.; Klein, M.A.; Bujdoso, R. Mouse-adapted ovine scrapie prion strains are characterized by different conformers of PrPSc. J. Virol. 2007, 81, 12119–12127. [Google Scholar] [CrossRef]
- Janouskova, O.; Rakusan, J.; Karaskova, M.; Holada, K. Photodynamic inactivation of prions by disulfonated hydroxyaluminium phthalocyanine. J. Gen. Virol. 2012, 93, 2512–2517. [Google Scholar] [CrossRef][Green Version]
- Kostelanska, M.; Freisleben, J.; Backovska Hanusova, Z.; Mosko, T.; Vik, R.; Moravcova, D.; Hamacek, A.; Mosinger, J.; Holada, K. Optimization of the photodynamic inactivation of prions by a phthalocyanine photosensitizer: The crucial involvement of singlet oxygen. J. Biophotonics 2019, 12, e201800340. [Google Scholar] [CrossRef] [PubMed]
- Cervenakova, L.; Akimov, S.; Vasilyeva, I.; Yakovleva, O.; McKenzie, C.; Cervenak, J.; Piccardo, P.; Asher, D.M. Fukuoka-1 strain of transmissible spongiform encephalopathy agent infects murine bone marrow-derived cells with features of mesenchymal stem cells. Transfusion 2011, 51, 1755–1768. [Google Scholar] [CrossRef]
- Giles, K.; Berry, D.B.; Condello, C.; Hawley, R.C.; Gallardo-Godoy, A.; Bryant, C.; Oehler, A.; Elepano, M.; Bhardwaj, S.; Patel, S.; et al. Different 2-Aminothiazole Therapeutics Produce Distinct Patterns of Scrapie Prion Neuropathology in Mouse Brains. J. Pharmacol. Exp. Ther. 2015, 355, 2–12. [Google Scholar] [CrossRef]
- Oelschlegel, A.M.; Fallahi, M.; Ortiz-Umpierre, S.; Weissmann, C. The extended cell panel assay characterizes the relationship of prion strains RML, 79A, and 139A and reveals conversion of 139A to 79A-like prions in cell culture. J. Virol. 2012, 86, 5297–5303. [Google Scholar] [CrossRef]
- Caughey, W.S.; Raymond, L.D.; Horiuchi, M.; Caughey, B. Inhibition of protease-resistant prion protein formation by porphyrins and phthalocyanines. Proc. Natl. Acad. Sci. USA 1998, 95, 12117–12122. [Google Scholar] [CrossRef]
- Priola, S.A.; Raines, A.; Caughey, W. Prophylactic and therapeutic effects of phthalocyanine tetrasulfonate in scrapie-infected mice. J. Infect. Dis. 2003, 188, 699–705. [Google Scholar] [CrossRef][Green Version]
- Caughey, W.S.; Priola, S.A.; Kocisko, D.A.; Raymond, L.D.; Ward, A.; Caughey, B. Cyclic tetrapyrrole sulfonation, metals, and oligomerization in antiprion activity. Antimicrob. Agents Chemother. 2007, 51, 3887–3894. [Google Scholar] [CrossRef][Green Version]
- Valiente-Gabioud, A.A.; Miotto, M.C.; Chesta, M.E.; Lombardo, V.; Binolfi, A.; Fernandez, C.O. Phthalocyanines as Molecular Scaffolds to Block Disease-Associated Protein Aggregation. Acc. Chem. Res. 2016, 49, 801–808. [Google Scholar] [CrossRef]
- Dee, D.R.; Gupta, A.N.; Anikovskiy, M.; Sosova, I.; Grandi, E.; Rivera, L.; Vincent, A.; Brigley, A.M.; Petersen, N.O.; Woodside, M.T. Phthalocyanine tetrasulfonates bind to multiple sites on natively-folded prion protein. Biochim. Biophys. Acta 2012, 1824, 826–832. [Google Scholar] [CrossRef]
- Grebenova, D.; Cajthamlova, H.; Holada, K.; Marinov, J.; Jirsa, M.; Hrkal, Z. Photodynamic effects of meso-tetra (4-sulfonatophenyl)porphine on human leukemia cells HEL and HL60, human lymphocytes and bone marrow progenitor cells. J. Photochem. Photobiol. B 1997, 39, 269–278. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Calvete, M.J.F. Phthalocyanines: An Old Dog Can Still Have New (Photo)Tricks! Molecules 2021, 26, 2823. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, N.A.; Gretsova, N.S.; Derkacheva, V.M.; Kaliya, O.L.; Lukyanets, E.A. Sulfonated phthalocyanines: Aggregation and singlet oxygen quantum yield in aqueous solutions. J. Porphyr. Phthalocya. 2003, 7, 147–154. [Google Scholar] [CrossRef]
- Mosinger, J.; Mosinger, B. Photodynamic sensitizers assay: Rapid and sensitive iodometric measurement. Experientia 1995, 51, 106–109. [Google Scholar] [CrossRef]
- Jensen, R.L.; Arnbjerg, J.; Ogilby, P.R. Temperature Effects on the Solvent-Dependent Deactivation of Singlet Oxygen. J. Am. Chem. Soc. 2010, 132, 8098–8105. [Google Scholar] [CrossRef]
- Klaper, M.; Fudickar, W.; Linker, T. Role of Distance in Singlet Oxygen Applications: A Model System. J. Am. Chem. Soc. 2016, 138, 7024–7029. [Google Scholar] [CrossRef]
- Lee, B.I.; Lee, S.; Suh, Y.S.; Lee, J.S.; Kim, A.K.; Kwon, O.Y.; Yu, K.; Park, C.B. Photoexcited Porphyrins as a Strong Suppressor of beta-Amyloid Aggregation and Synaptic Toxicity. Angew. Chem. Int. Ed. 2015, 54, 11472–11476. [Google Scholar] [CrossRef]
- Mitra, K.; Hartman, M.C.T. Silicon phthalocyanines: Synthesis and resurgent applications. Org. Biomol. Chem. 2021, 19, 1168–1190. [Google Scholar] [CrossRef]
- Panigaj, M.; Glier, H.; Wildova, M.; Holada, K. Expression of prion protein in mouse erythroid progenitors and differentiating murine erythroleukemia cells. PLoS ONE 2011, 6, e24599. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]

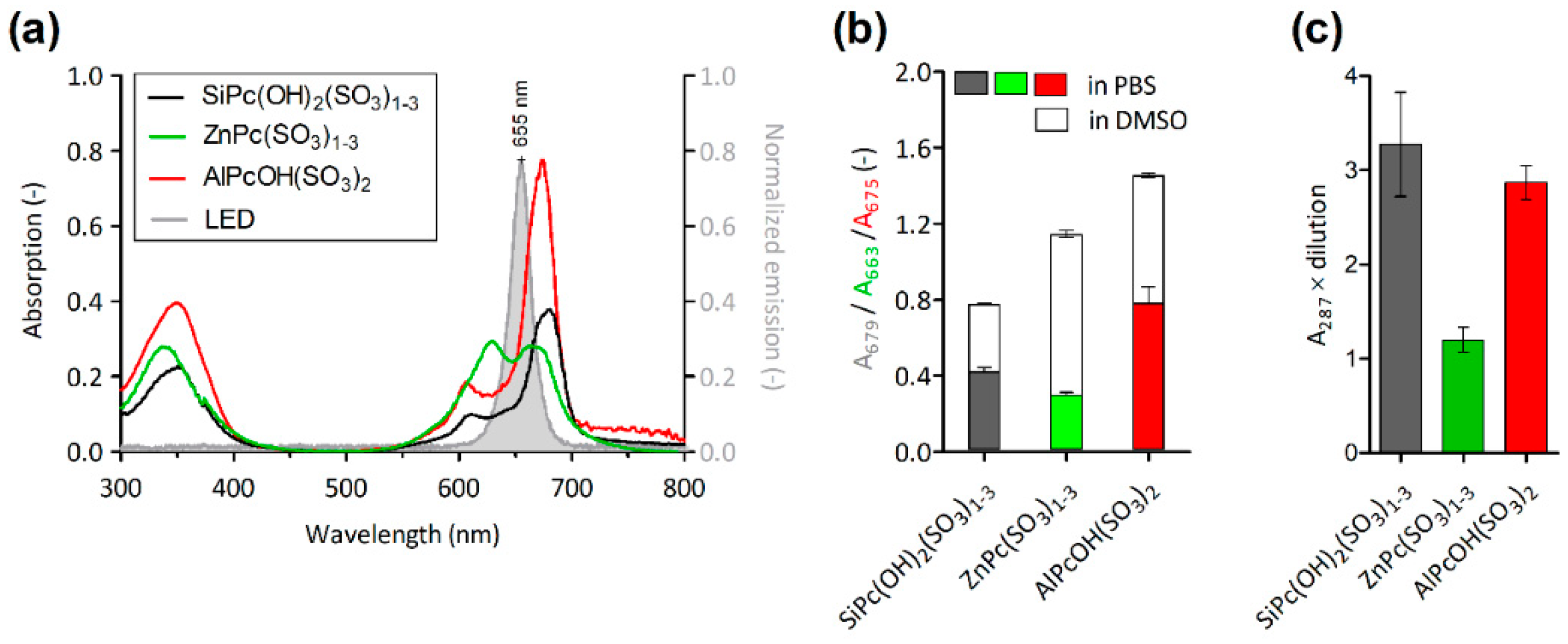

| Phthalocyanine Derivative | Coordinated Metal | Peripheral Sulfonation |
|---|---|---|
| SiPc(OH)2(SO3)1-3 | HO–Si–OH | R1 ᴧ R2 ᴧ R3 1 |
| ZnPc(SO3)1-3 | Zn | R1 ᴧ R2 ᴧ R3 1 |
| AlPcOH(SO3)2 | Al–OH | R1 ᴧ R2 or R1 ᴧ R3 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostelanska, M.; Holada, K. Prion Strains Differ in Susceptibility to Photodynamic Oxidation. Molecules 2022, 27, 611. https://doi.org/10.3390/molecules27030611
Kostelanska M, Holada K. Prion Strains Differ in Susceptibility to Photodynamic Oxidation. Molecules. 2022; 27(3):611. https://doi.org/10.3390/molecules27030611
Chicago/Turabian StyleKostelanska, Marie, and Karel Holada. 2022. "Prion Strains Differ in Susceptibility to Photodynamic Oxidation" Molecules 27, no. 3: 611. https://doi.org/10.3390/molecules27030611
APA StyleKostelanska, M., & Holada, K. (2022). Prion Strains Differ in Susceptibility to Photodynamic Oxidation. Molecules, 27(3), 611. https://doi.org/10.3390/molecules27030611






