Identification of Antibacterial Metabolites from Endophytic Fungus Aspergillus fumigatus, Isolated from Albizia lucidior Leaves (Fabaceae), Utilizing Metabolomic and Molecular Docking Techniques
Abstract
:1. Introduction
2. Results
2.1. Endophytic Fungi Isolation and Cultivation
2.2. Identification of the Fungus Based on Phenotypic and Genotypic Characteristics
2.3. UHPLC–QTOF Analysis of A. fumigatus Ethyl Acetate Extract
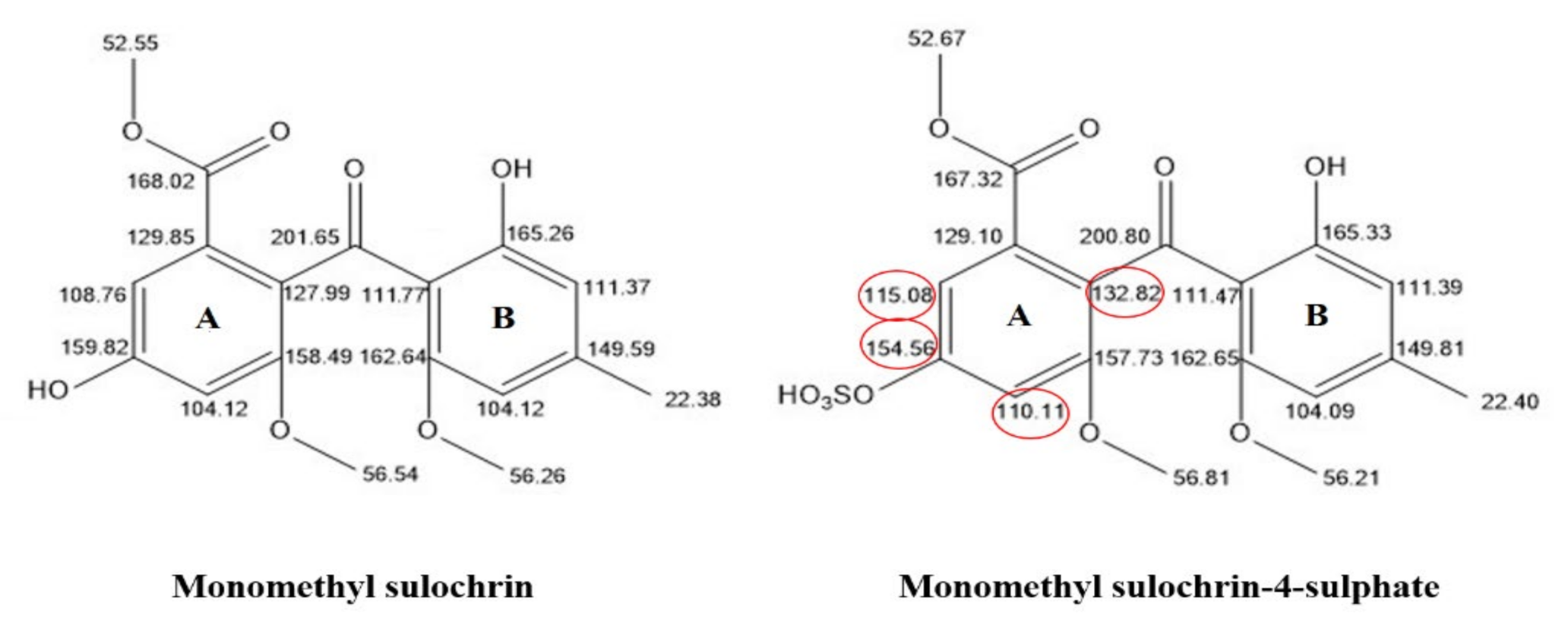
2.4. Isolation and Characterization of the Metabolites
2.5. Biological Activity
2.5.1. Antimicrobial Activity
2.5.2. In Vitro Enzyme Assessment
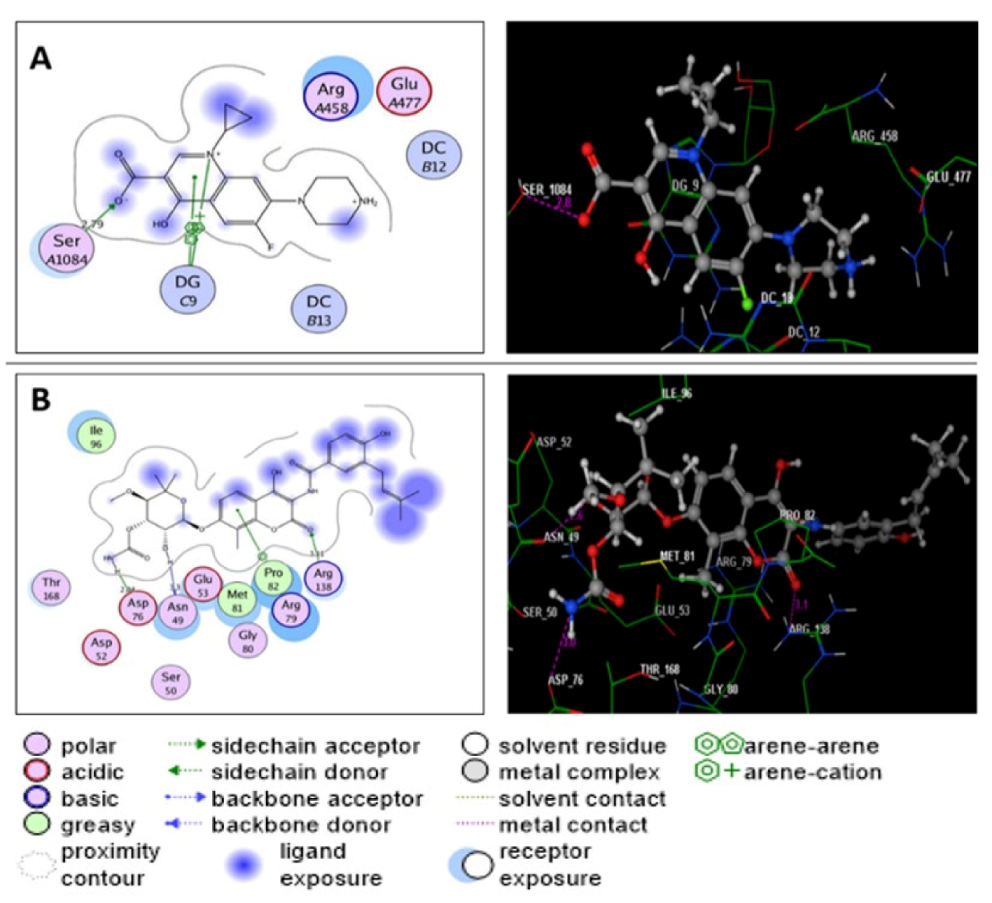
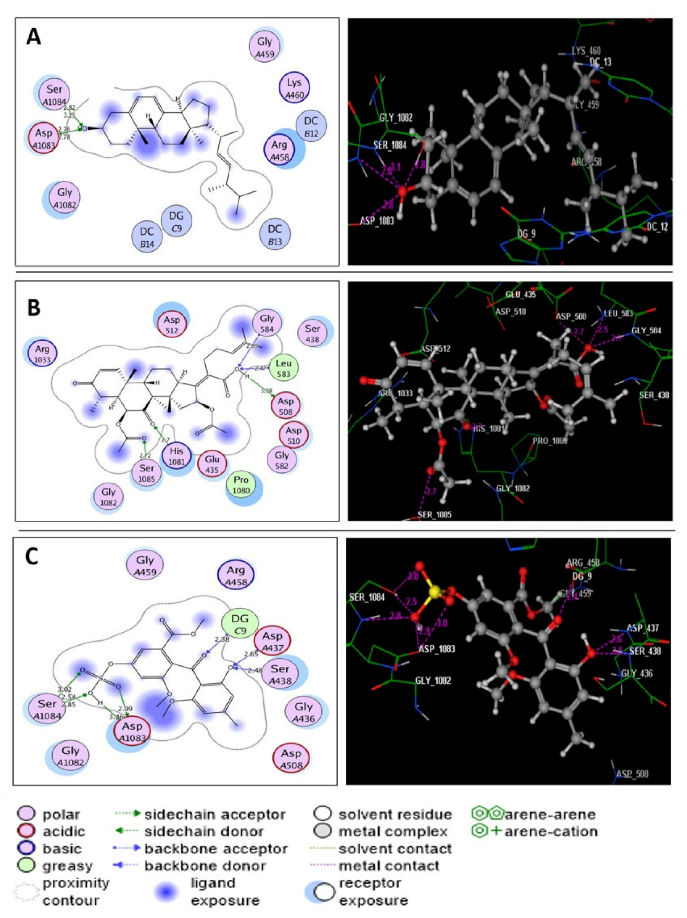
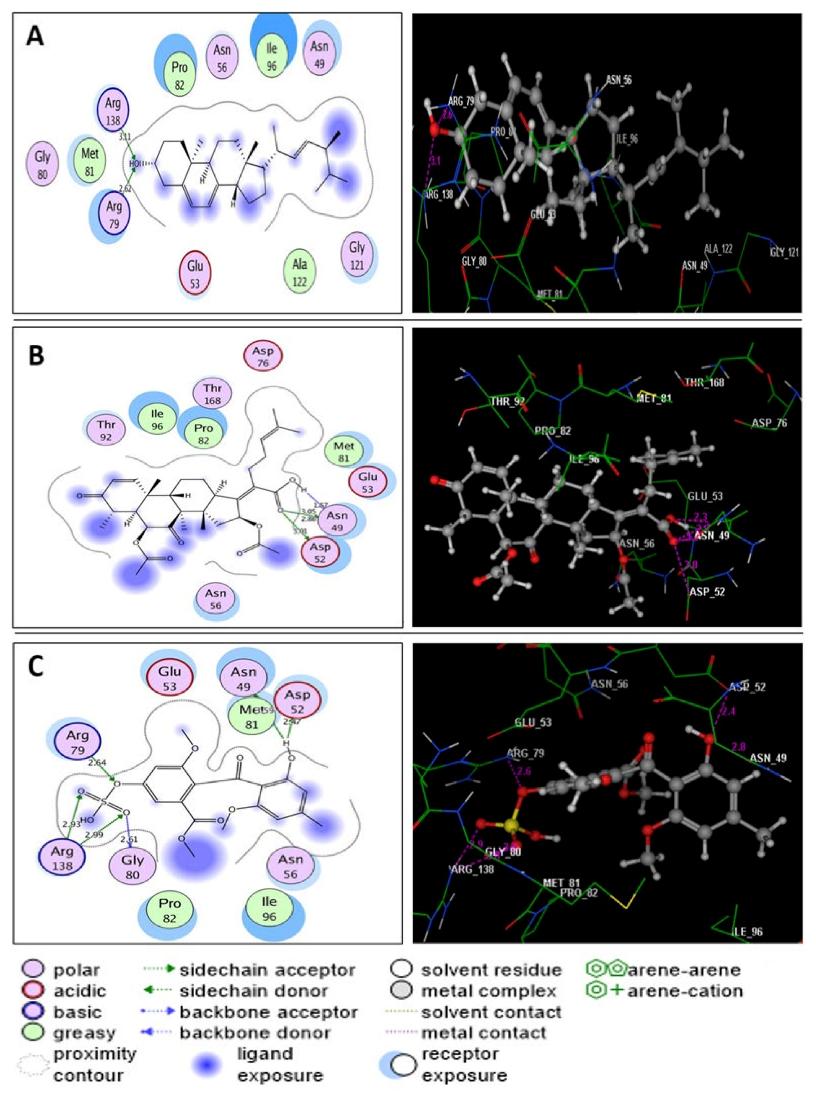
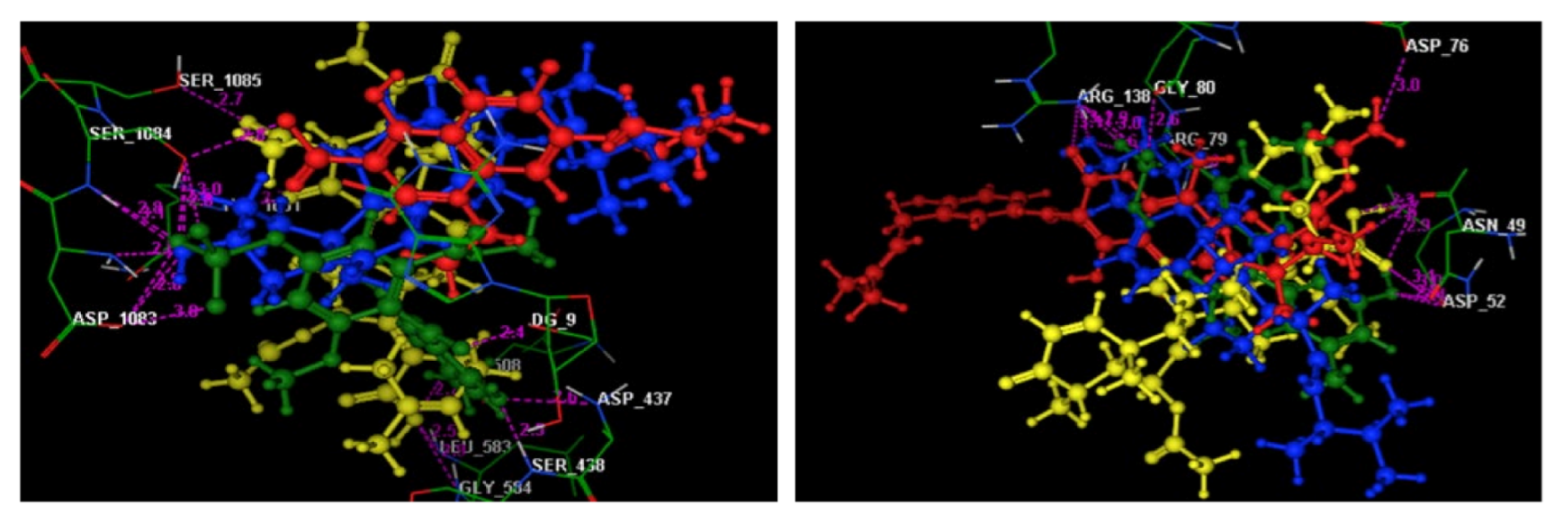
2.6. Molecular Modeling Study
3. Discussion
4. Materials and Methods
4.1. Plant Collection and Identification
4.2. Surface Sterilization and Isolation of Endophytic Fungi
4.3. Morphological and Taxonomic Identification of the Fungus
4.4. Scale-Up Fermentation and Extraction
4.5. UHPLC-QTOF-MS/MS Profiling of the Fungal Crude Extract
4.6. Fractionation and Purification of Metabolites
4.7. Screening of Antimicrobial Activity
4.7.1. Agar Disc Diffusion Method
4.7.2. Microplate Dilution Method
4.7.3. In Vitro Enzyme Assessment
4.8. Molecular Docking Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Mohamed, O.G.; Khalil, Z.G.; Salim, A.A.; Cui, H.; Blumenthal, A.; Capon, R.J. Lincolnenins A–D: Isomeric Bactericidal Bianthracenes from Streptomyces lincolnensis. J. Org. Chem. 2021, 86, 11011–11018. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, O.G.; Dorandish, S.; Lindow, R.; Steltz, M.; Shoukat, I.; Shoukat, M.; Chehade, H.; Baghdadi, S.; McAlister-Raeburn, M.; Kamal, A.; et al. Identification of a New Antimicrobial, Desertomycin H, Utilizing a Modified Crowded Plate Technique. Mar. Drugs 2021, 19, 424. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.K.; Polishook, J.D.; White, J.F. Endophytic Fungi: Biodiversity of Fungi; Elsevier: Burlington, VT, USA, 2004; pp. 241–270. [Google Scholar]
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites. Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef]
- Kharwar, R.N.; Mishra, A.; Gond, S.K.; Stierle, A.; Stierle, D. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011, 28, 1208–1228. [Google Scholar] [CrossRef]
- Rahman, S.-U.; Rasool, M.H.; Rafi, M. Penicillin production by wild isolates of Penicillium chrysogenum in Pakistan. Braz. J. Microbiol. 2012, 43, 476–481. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, I.C. Tribe 5. Ingeae. In Advances in Legume Systematics Part 1; Polhill, R.M., Raven, P.H., Eds.; The Royal Botanic Gardens: Kew, UK, 1981; pp. 173–190. [Google Scholar]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa, 2nd ed.; E. & S. Livingstone Ltd.: Edinburgh, UK, 1962; p. 553. [Google Scholar]
- Kokila, K.; Priyadharshini, S.D.; Sujatha, V. Phytopharmacological properties of Albizia species: A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 70–73. [Google Scholar]
- Malewska, T.; Kichu, M.; Barnes, E.C.; Vemulpad, S.; Jamie, J.F.; Brophy, J.J.; Imchen, I. Antimicrobial properties of plants of Chungtia village used customarily to treat skin related ailments: From antimicrobial screening to isolation of active compounds. Arch. Org. Inorg. Chem. Sci. 2018, 3, 285–303. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Rank, C.; Nielsen, K.F.; Larsen, T.O. Metabolomics of Aspergillus fumigatus. Med. Mycol. 2009, 47 (Suppl. 1), S53–S71. [Google Scholar] [CrossRef]
- Jiang, L.; Pu, H.; Xiang, J.; Su, M.; Yan, X.; Yang, D.; Zhu, X.; Shen, B.; Duan, Y.; Huang, Y. Huanglongmycin AC, cytotoxic polyketides biosynthesized by a putative type II polyketide synthase from Streptomyces sp. CB09001. Front. Chem. 2018, 6, 254. [Google Scholar] [CrossRef]
- Xu, Y.-M.; Madasu, C.; Liu, M.X.; Wijeratne, E.M.K.; Dierig, D.; White, B.; Molnár, I.; Gunatilaka, A.L. Cycloartane-and Lanostane-Type Triterpenoids from the Resin of Parthenium argentatum AZ-2, a Byproduct of Guayule Rubber Production. ACS Omega 2021, 6, 15486–15498. [Google Scholar] [CrossRef]
- Wang, Q.-X.; Bao, L.; Yang, X.-L.; Guo, H.; Yang, R.-N.; Ren, B.; Zhang, L.-X.; Dai, H.-Q.; Guo, L.-D.; Liu, H.-W. Polyketides with antimicrobial activity from the solid culture of an endolichenic fungus Ulocladium sp. Fitoterapia 2012, 83, 209–214. [Google Scholar] [CrossRef]
- Zhao, M.; Gödecke, T.; Gunn, J.; Duan, J.-A.; Che, C.-T. Protostane and fusidane triterpenes: A mini-review. Molecules 2013, 18, 4054–4080. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [Green Version]
- El-Hawary, S.S.; Sayed, A.M.; Rateb, M.E.; Bakeer, W.; AbouZid, S.F.; Mohammed, R. Secondary metabolites from fungal endophytes of Solanum nigrum. Nat. Prod. Res. 2017, 31, 2568–2571. [Google Scholar] [CrossRef]
- Ferreira, E.G.; Torres, M.D.C.M.; da Silva, A.B.; Colares, L.L.; Pires, K.; Lotufo, T.M.; Silveira, E.R.; Pessoa, O.D.; Costa-Lotufo, L.V.; Jimenez, P.C. Prospecting Anticancer Compounds in Actinomycetes Recovered from the Sediments of Saint Peter and Saint Paul’s Archipelago, Brazil. Chem. Biodivers. 2016, 13, 1149–1157. [Google Scholar] [CrossRef]
- Evidente, A.; Bruno, G.; Andolfi, A.; Sparapano, L. Two Naphthalenone Pentakides from Liquid Cultures of “Phaeoacremonium aleophilum”, a Fungus Associated with Esca of Grapevine. Phytopathol. Mediterr. 2000, 39, 162–168. [Google Scholar]
- Ge, H.M.; Yu, Z.G.; Zhang, J.; Wu, J.H.; Tan, R.X. Bioactive alkaloids from endophytic Aspergillus fumigatus. J. Nat. Prod. 2009, 72, 753–755. [Google Scholar] [CrossRef]
- Cano, P.M.; Jamin, E.L.; Tadrist, S.; Bourdaud’hui, P.; Péan, M.; Debrauwer, L.; Oswald, I.P.; Delaforge, M.; Puel, O. New untargeted metabolic profiling combining mass spectrometry and isotopic labeling: Application on Aspergillus fumigatus grown on wheat. Anal. Chem. 2013, 85, 8412–8420. [Google Scholar] [CrossRef]
- Li, H.; Xiao, J.; Gao, Y.-Q.; Tang, J.-J.; Zhang, A.-L.; Gao, J.-M. Chaetoglobosins from Chaetomium globosum, an endophytic fungus in Ginkgo biloba, and their phytotoxic and cytotoxic activities. J. Agric. Food Chem. 2014, 62, 3734–3741. [Google Scholar] [CrossRef]
- Limbadri, S.; Luo, X.; Lin, X.; Liao, S.; Wang, J.; Zhou, X.; Yang, B.; Liu, Y. Bioactive novel indole alkaloids and steroids from deep sea-derived fungus Aspergillus fumigatus SCSIO 41012. Molecules 2018, 23, 2379. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, K.F.; Månsson, M.; Rank, C.; Frisvad, J.C.; Larsen, T.O. Dereplication of microbial natural products by LC-DAD-TOFMS. J. Nat. Prod. 2011, 74, 2338–2348. [Google Scholar] [CrossRef]
- Isogai, A.; Washizu, M.; Kondo, K.; Murakoshi, S.; Suzuki, A. Isolation and identification of (+)-hexylitaconic acid as a plant growth regulator. Agric. Biol. Chem. 1984, 48, 2607–2609. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Ruan, C.; Bai, X.; Chen, J.; Wang, H. Heterocyclic alkaloids as antimicrobial agents of Aspergillus fumigatus D endophytic on Edgeworthia chrysantha. Chem. Nat. Compd. 2018, 54, 411–414. [Google Scholar] [CrossRef]
- Gauthier, T.; Wang, X.; Sifuentes, J.D.S.; Fysikopoulos, A.; Tadrist, S.; Canlet, C.; Artigot, M.P.; Loiseau, N.; Oswald, I.P.; Puel, O. Trypacidin, a spore-borne toxin from Aspergillus fumigatus, is cytotoxic to lung cells. PLoS ONE 2012, 7, e29906. [Google Scholar] [CrossRef]
- Jiao, R.H.; Xu, S.; Liu, J.Y.; Ge, H.M.; Ding, H.; Xu, C.; Zhu, H.L.; Tan, R.X. Chaetominine, a cytotoxic alkaloid produced by endophytic Chaetomium sp. IFB-E015. Org. Lett. 2006, 8, 5709–5712. [Google Scholar] [CrossRef]
- Ando, O.; Satake, H.; Nakajima, M.; Sato, A.; Nakamura, T.; Kinoshita, T.; Furuya, K.; Haneishi, T. Synerazol, a new antifungal antibiotic. J. Antibiot. 1991, 44, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Liu, R.; Chen, L.; Zhu, T.; Zhu, W.M.; Gu, Q.Q. Two new hetero-spirocyclic γ-lactam derivatives from marine sediment-derived fungus Aspergillus sydowi D2–6. Arch. Pharm. Res. 2010, 33, 499–502. [Google Scholar] [CrossRef]
- Han, J.; Liu, M.; Jenkins, I.D.; Liu, X.; Zhang, L.; Quinn, R.J.; Feng, Y. Genome-Inspired Chemical Exploration of Marine Fungus Aspergillus fumigatus MF071. Mar. Drugs 2020, 18, 352. [Google Scholar] [CrossRef]
- Jiao, W.; Blunt, J.W.; Cole, A.L.; Munro, M.H. Fumagiringillin, a New Fumagillin Derivative from a Strain of the Fungus Aspergillus fumigatus. J. Nat. Prod. 2004, 67, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, D.; Ohshiro, T.; Ohtawa, M.; Yamazaki, H.; Nagamitsu, T.; Tomoda, H. In vitro metabolism of pyripyropene A and ACAT inhibitory activity of its metabolites. J. Antibiot. 2015, 68, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Brakni, R.; Ali Ahmed, M.; Burger, P.; Schwing, A.; Michel, G.; Pomares, C.; Hasseine, L.; Boyer, L.; Fernandez, X.; Landreau, A.; et al. UHPLC-HRMS/MS Based Profiling of Algerian Lichens and Their Antimicrobial Activities. Chem. Biodivers. 2018, 15, e1800031. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Honda, C.; Kobayashi, I.; Katsuta, R.; Matsumura, S.; Wagatsuma, I.; Takehisa, M.; Shindo, H.; Hosaka, M.; Nukada, T.; et al. Transglycosylation forms novel glycoside ethyl α-maltoside and ethyl α-isomaltoside in sake during the brewing process by α-glucosidase A of Aspergillus oryzae. J. Agric. Food Chem. 2019, 68, 1419–1426. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Dong, J.; Liu, J.; Xu, W.; Wei, Z.; Li, Y.; Wu, H.; Xiao, H. Network pharmacology-based investigation of protective mechanism of Aster tataricus on lipopolysaccharide-induced acute lung injury. Int. J. Mol. Sci. 2019, 20, 543. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.-D.; Huang, X.-L.; Ma, Q.-Y.; Xie, Q.-Y.; Wang, P.; Chen, P.-W.; Zhou, L.-M.; Yuan, J.-Z.; Dai, H.-F.; Luo, D.-Q.; et al. Helvolic acid derivatives with antibacterial activities against Streptococcus agalactiae from the marine-derived fungus Aspergillus fumigatus HNMF0047. J. Nat. Prod. 2018, 81, 1869–1876. [Google Scholar] [CrossRef]
- Tomoda, H.; Tabata, N.; Yang, D.-J.; Takayanagi, H.; Nishida, H.; Omura, S.; Kaneko, T. Pyripyropenes, Novel ACAT Inhibitors Produced by Aspergillus fumigatus III. Structure Elucidation of Pyripyropenes E to L. J. Antibiot. 1995, 48, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Tamiya, H.; Ochiai, E.; Kikuchi, K.; Yahiro, M.; Toyotome, T.; Watanabe, A.; Yaguchi, T.; Kamei, K. Secondary metabolite profiles and antifungal drug susceptibility of Aspergillus fumigatus and closely related species, Aspergillus lentulus, Aspergillus udagawae, and Aspergillus viridinutans. J. Infect. Chemother. 2015, 21, 385–391. [Google Scholar] [CrossRef]
- Yang, S.; Shan, L.; Luo, H.; Sheng, X.; Du, J.; Li, Y. Rapid classification and identification of chemical components of Schisandra chinensis by UPLC-Q-TOF/MS combined with data post-processing. Molecules 2017, 22, 1778. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.C.; Dunn, S.R.; Altvater, J.; Dove, S.G.; Nette, G.W. Rapid identification of long-chain polyunsaturated fatty acids in a marine extract by HPLC-MS using data-dependent acquisition. Anal. Chem. 2012, 84, 5976–5983. [Google Scholar] [CrossRef]
- El-Elimat, T.; Figueroa, M.; Ehrmann, B.M.; Cech, N.B.; Pearce, C.J.; Oberlies, N.H. High-resolution MS, MS/MS, and UV database of fungal secondary metabolites as a dereplication protocol for bioactive natural products. J. Nat. Prod. 2013, 76, 1709–1716. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Wang, K.L.; Liu, M.; She, Z.-G.; Wang, C.-Y. Bioactive steroid derivatives and butyrolactone derivatives from a Gorgonian-derived Aspergillus sp. fungus. Chem. Biodivers. 2015, 12, 1398–1406. [Google Scholar] [CrossRef]
- Takaishi, Y.; Murakami, Y.; Ohashi, T.; Nakano, K.; Tomimatsu, T. Three triterpenes from Astraeus hygrometricus. Phytochemistry 1987, 26, 2341–2344. [Google Scholar] [CrossRef]
- Kaaniche, F.; Hamed, A.; Abdel-Razek, A.S.; Wibberg, D.; Abdissa, N.; El Euch, I.Z.; Allouche, N.; Mellouli, L.; Shaaban, M.; Sewald, N. Bioactive secondary metabolites from new endophytic fungus Curvularia. sp isolated from Rauwolfia macrophylla. PLoS ONE 2019, 14, e0217627. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xu, L.; Ren, W.; Zhao, D.; Zhu, Y.; Wu, X. Bioactive metabolites from Chaetomium globosum L18, an endophytic fungus in the medicinal plant Curcuma wenyujin. Phytomedicine 2012, 19, 364–368. [Google Scholar] [CrossRef]
- Fujimoto, H.; Negishi, E.; Yamaguchi, K.; Nishi, N.; Yamazaki, M. Isolation of new tremorgenic metabolites from an Ascomycete, Corynascus setosus. Chem. Pharm. Bull. 1996, 44, 1843–1848. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.-H.; Zhang, Y.; Liang, G.-Y.; Liu, R.-M.; Li, X.-G.; Zhang, L.-T.; Chen, D.-X.; Zhong, J.-J. Synergistic antitumor efficacy of antibacterial helvolic acid from Cordyceps taii and cyclophosphamide in a tumor mouse model. Exp. Biol. Med. 2017, 242, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Mehedi, M.A.U.; Molla, A.H.; Khondkar, P.; Sultana, S.; Islam, M.A.; Rashid, M.A.; Chowdhury, R. Pseurotin A: An antibacterial secondary metabolite from Aspergillus fumigatus. Asian J. Chem. 2010, 22, 2611–2614. [Google Scholar]
- Yamada, T.; Ohshima, M.; Yuasa, K.; Kikuchi, T.; Tanaka, R. Assignment of the CD cotton effect to the chiral center in pseurotins, and the stereochemical revision of pseurotin A2. Mar. Drugs 2016, 14, 74. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.M.; Li, Y.; Liu, J.Y.; Song, Y.C.; Tan, R.X. Anti-Helicobacter pylori metabolites from Rhizoctonia sp. Cy064, an endophytic fungus in Cynodon dactylon. Fitoterapia 2004, 75, 451–456. [Google Scholar] [CrossRef]
- Couche, E.; Fkyerat, A.; Tabacchi, R. Asymmetric Synthesis of the cis-and trans-3,4-Dihydro-2,4,8-trihydroxynaphthalen-1(2H)-ones. Helv. Chim. Acta 2003, 86, 210–221. [Google Scholar] [CrossRef]
- Klaiklay, S.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Buatong, J.; Bussaban, B. Metabolites from the mangrove-derived fungus Xylaria cubensis PSU-MA34. Arch. Pharm. Res. 2012, 35, 1127–1131. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kiriyama, N.; Arahata, S.S. Studies on the metabolic products of Aspergillus fumigatus (J-4). Chemical structure of metabolic products. Chem. Pharm. Bull. 1968, 16, 304–310. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-X.; Kang, K.-H.; Kim, H.J.; Kim, S.-K. In vitro induction of apoptosis by isosclerone from marine-derived fungus Aspergillus fumigatus. Bioorg. Med. Chem. Lett. 2014, 24, 3923–3927. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, T.; Zhang, X.; Zhang, J.; Zhao, C. An alkaloid and a steroid from the endophytic fungus Aspergillus fumigatus. Molecules 2015, 20, 1424–1433. [Google Scholar] [CrossRef] [Green Version]
- Barron, D.; Varin, L.; Ibrahim, R.K.; Harborne, J.B.; Williams, C.A. Sulphated flavonoids—An update. Phytochemistry 1988, 27, 2375–2395. [Google Scholar] [CrossRef]
- Rana, K.L.; Kour, D.; Kaur, T.; Devi, R.; Negi, C.; Yadav, A.N.; Yadav, N.; Singh, K.; Saxena, A.K. Endophytic fungi from medicinal plants: Biodiversity and biotechnological applications. In Microbial Endophytes: Functional Biology and Applications, 1st ed.; Kumar, A., Radhakrishnan, E.K., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 273–305. [Google Scholar]
- Gioia, L.; d’Errico, G.; Sinno, M.; Ranesi, M.; Woo, S.L.; Vinale, F. A Survey of Endophytic Fungi Associated with High-Risk Plants Imported for Ornamental Purposes. Agriculture 2020, 10, 643. [Google Scholar] [CrossRef]
- Nasimiyu, V.W.; Wagara, I.N.; Obonyo, M.A.; Matasyoh, J.C. Isolation, identification and bioactivity of fungal endophytes from selected Kenyan medicinal plants. Afr. J. Microbiol. Res. 2018, 12, 405–412. [Google Scholar]
- Sharma, N.; Kushwaha, M.; Arora, D.; Jain, S.; Singamaneni, V.; Sharma, S.; Shankar, R.; Bhushan, S.; Gupta, P.; Jaglan, S. New Cytochalasin from Rosellinia sanctae-cruciana, an Endophytic Fungus of Albizia lebbeck. J. Appl. Microbiol. 2018, 125, 111–120. [Google Scholar] [CrossRef]
- Ibrahim, M.; Oyebanji, E.; Fowora, M.; Aiyeolemi, A.; Orabuchi, C.; Akinnawo, B.; Adekunle, A.A. Extracts of endophytic fungi from leaves of selected Nigerian ethnomedicinal plants exhibited antioxidant activity. BMC Complement. Med. Ther. 2021, 21, 98. [Google Scholar] [CrossRef]
- Nagia, M.M.; El-Metwally, M.M.; Shaaban, M.; El-Zalabani, S.M.; Hanna, A.G. Four butyrolactones and diverse bioactive secondary metabolites from terrestrial Aspergillus flavipes MM2: Isolation and structure determination. Org. Med. Chem. Lett. 2012, 2, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shameem, N.; Kamili, A.N.; Ahmad, M.; Masoodi, F.A.; Parray, J.A. Antimicrobial activity of crude fractions and morel compounds from wild edible mushrooms of North western Himalaya. Microb. Pathog. 2017, 105, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Oh, Y.S.; Kwak, M.-K.; Chong, K.P. Biophysical characterization of antibacterial compounds derived from pathogenic fungi Ganoderma boninense. J. Microbiol. 2021, 59, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Tomasic, T.; Katsamakas, S.; Hodnik, Z.; Ilaš, J.; Brvar, M.; Solmajer, T.J.; Montalvao, S.; Tammela, P.; Banjanac, M.; Ergović, G.; et al. Discovery of 4,5,6,7-tetrahydrobenzo [1,2-d] thiazoles as novel DNA gyrase inhibitors targeting the ATP-binding site. J. Med. Chem. 2015, 58, 5501–5521. [Google Scholar] [CrossRef]
- Lv, J.-M.; Hu, D.; Gao, H.; Kushiro, T.; Awakawa, T.; Chen, G.-D.; Wang, C.-X.; Abe, I.; Yao, X.-S. Biosynthesis of helvolic acid and identification of an unusual C-4-demethylation process distinct from sterol biosynthesis. Nat. Commun. 2017, 8, 1644. [Google Scholar] [CrossRef] [Green Version]
- Zaman, K.A.U.; Hu, Z.; Wu, X.; Hou, S.; Saito, J.; Kondratyuk, T.P.; Pezzuto, J.M.; Cao, S. NF-κB inhibitory and antibacterial helvolic and fumagillin derivatives from Aspergillus terreus. J. Nat. Prod. 2020, 83, 730–737. [Google Scholar] [CrossRef]
- Von Daehne, W.; Godtfredsen, W.O.; Rasmussen, P.R. Structure-activity relationships in fusidic acid-type antibiotics. Adv. Appl. Microbiol. 1979, 25, 95–146. [Google Scholar]
- Bodley, J.W.; Godtfredsen, W.O. Studies on translocation XI: Structure-function relationships of the fusidane-type antibiotics. Biochem. Biophys. Res. Commun. 1972, 46, 871–877. [Google Scholar] [CrossRef]
- Cai, S.; King, J.B.; Du, L.; Powell, D.R.; Cichewicz, R.H. Bioactive sulfur-containing sulochrin dimers and other metabolites from an Alternaria sp. isolate from a Hawaiian soil sample. J. Nat. Prod. 2014, 77, 2280–2287. [Google Scholar] [CrossRef] [Green Version]
- Correia-da-Silva, M.; Sousa, E.; Pinto, M.M. Emerging sulfated flavonoids and other polyphenols as drugs: Nature as an inspiration. Med. Res. Rev. 2014, 34, 223–279. [Google Scholar] [CrossRef]
- Carvalhal, F.; Correia-da-Silva, M.; Sousa, E.; Pinto, M.; Kijjoa, A. Sulfation pathways: Sources and biological activities of marine sulfated steroids. J. Mol. Endocrinol. 2018, 61, T211–T231. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [Green Version]
- Sissi, C.; Palumbo, M. In front of and behind the replication fork: Bacterial type IIA topoisomerases. Cell. Mol. Life Sci. 2010, 67, 2001–2024. [Google Scholar] [CrossRef]
- Alt, S.; Mitchenall, L.A.; Maxwell, A.; Heide, L. Inhibition of DNA gyrase and DNA topoisomerase IV of Staphylococcus aureus and Escherichia coli by aminocoumarin antibiotics. J. Antimicrob. Chemother. 2011, 66, 2061–2069. [Google Scholar] [CrossRef] [Green Version]
- Dilika, F.; Bremner, P.D.; Meyer, J.J.M. Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: A plant used during circumcision rites. Fitoterapia 2000, 71, 450–452. [Google Scholar] [CrossRef]
- Zhang, D.; Noviendri, D.; Nursid, M.; Yang, X.-D.; Son, B.-W. 12,13-Dihydroxyfumitremorgin C, fumitremorgin C, and brevianamide F, antibacterial diketopiperazine alkaloids from the marine-derived fungus Pseudallescheria sp. Nat. Prod. Sci. 2007, 13, 251–254. [Google Scholar]
- Chukwujekwu, J.C.; Coombes, P.H.; Mulholland, D.A.; Van Staden, J. Emodin, an antibacterial anthraquinone from the roots of Cassia occidentalis. S. Afr. J. Bot. 2006, 72, 295–297. [Google Scholar] [CrossRef] [Green Version]
- Eble, T.; Hanson, F. Fumaglllin, an Antibiotic from Aspergillus fumigatus H-3. Antibiot. Chemother. 1951, 1, 54–58. [Google Scholar]
- Pinheiro, E.A.A.; Carvalho, J.M.; dos Santos, D.C.P.; Feitosa, A.D.O.; Marinho, P.S.B.; Guilhon, G.M.S.P.; de Souza, A.D.L.; da Silva, F.M.A.; Marinho, A.M.D.R. Antibacterial activity of alkaloids produced by endophytic fungus Aspergillus sp. EJC08 isolated from medical plant Bauhinia guianensis. Nat. Prod. Res. 2013, 27, 1633–1638. [Google Scholar] [CrossRef]
- Khedr, A.I.; Kouno, I.; Tanaka, T.; Yamada, K. New diketopiperazine derivatives from culture broth of Staphylococcus sp. isolated from Corallina officinalis Lineaus. Heterocycles 2013, 87, 1029–1037. [Google Scholar] [CrossRef]
- Schulz, B.; Wanke, U.; Draeger, S.; Aust, H.-J. Endophytes from herbaceous plants and shrubs: Effectiveness of surface sterilization methods. Mycol. Res. 1993, 97, 1447–1450. [Google Scholar] [CrossRef]
- Fisher, F.; Cook, N.B. Fundamentals of Diagnostic Mycology, 1st ed.; W.B. Saunders: Philadelphia, PA, USA, 1998. [Google Scholar]
- Ellis, M.B.; Ellis, J.P. Microfungi on Land Plants: An Identification Handbook, 1st ed.; Croom Helm Ltd.: Kent, UK, 1985. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White., T.J., Eds.; Academic Press Inc.: New York, NY, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Hamed, A.A.; Abdel-Aziz, M.S.; Fadel, M.; Ghali, M.F. Antimicrobial, antidermatophytic, and cytotoxic activities from Streptomyces sp. MER4 isolated from Egyptian local environment. Bull. Natl. Res. Cent. 2018, 42, 22. [Google Scholar] [CrossRef]
- Hamed, A.A.; Soldatou, S.; Qader, M.M.; Arjunan, S.; Miranda, K.J.; Casolari, F.; Pavesi, C.; Diyaolu, O.A.; Thissera, B.; Eshelli, M.; et al. Screening Fungal Endophytes Derived from Under-Explored Egyptian Marine Habitats for Antimicrobial and Antioxidant Properties in Factionalised Textiles. Microorganisms 2020, 8, 1617. [Google Scholar] [CrossRef] [PubMed]
- Mohi El-Deen, E.M.; El-Meguid, A.; Eman, A.; Hasabelnaby, S.; Karam, E.A.; Nossier, E.S. Synthesis, Docking Studies, and In Vitro Evaluation of Some Novel Thienopyridines and Fused Thienopyridine-Quinolines as Antibacterial Agents and DNA Gyrase Inhibitors. Molecule 2019, 24, 3650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Naggar, M.; Sallam, H.A.; Shaban, S.S.; Abdel-Wahab, S.S.; Amr, A.E.-G.E.; Azab, M.E.; Nossier, E.S.; Al-Omar, M.A. Design, synthesis, and molecular docking study of novel heterocycles incorporating 1,3,4-thiadiazole moiety as potential antimicrobial and anticancer agents. Molecules 2019, 24, 1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, A.S.; Askar, A.A.; Nossier, E.S.; Naglah, A.M.; Moustafa, G.O.; Al-Omar, M.A. Antibacterial evaluation, in silico characters and molecular docking of Schiff bases derived from 5-aminopyrazoles. Molecules 2019, 24, 3130. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Patel, S.; Sharma, N.; Soisson, S.M.; Kishii, R.; Takei, M.; Fukuda, Y.; Lumb, K.J.; Singh, S.B. Structures of kibdelomycin bound to Staphylococcus aureus GyrB and ParE showed a novel U-shaped binding mode. ACS Chem. Biol. 2014, 9, 2023–2031. [Google Scholar] [CrossRef]
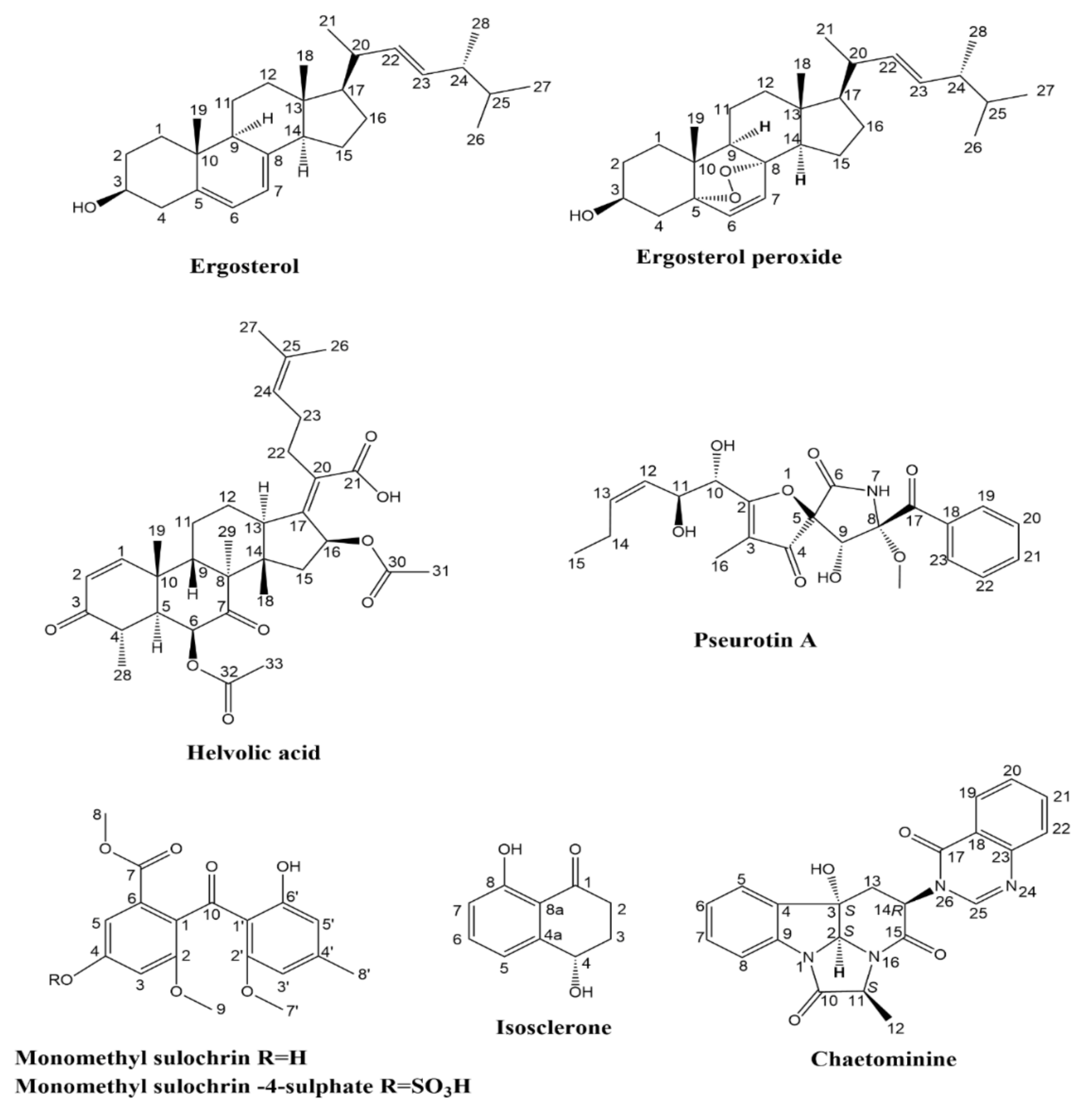
| No. | Rt (min) | Name | Ion m/z ppm | Molecular Formula | Fragmentation | Ref | |
|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||
| 1 | 1.92 | Cyclo-(Leu-Pro) | 211.1411 | C11H19N2O2 [M + H]+ | 197.1263 183.1469 | [19] | |
| 2 | 2.41 | Cyclo-(Phe-Pro) | 245.1266 | C14H17N2O2 [M + H]+ | 217.1298 154.0699 120.0771 | [20] | |
| 3 | 2.49 | Isosclerone | 357.2144 | C20H21O6 [2M + H]+ | 339.2029 297.1932 243.1441 | [21] | |
| 4 | 3.05 | 9-Deacetylfumigaclavine C | 325.2252 | C21H29N2O [M + H]+ | 307.2153 238.1444 | [22] | |
| 5 | 3.08 | Cyclotryprostatin A | 410.1730 | C22H24N3O5 [M − H]− | 308.1407 293.1173 | [23] | |
| 6 | 3.30 | Fumigaclavine B | 255.0293 | C16H19N2O [M − H]− | 237.0181 227.0344 211.0392 | [24] | |
| 7 | 3.32 | Fumigatoside F |
421.1488 | C22H21N4O5 [M + H]+ | 403.1451 286.0980 199.0497 120.0442 | [25] | |
| 8 | 3.35 | Pseurotin A | 454.1460 | C22H25NO8Na [M + Na]+ | 421.1453 316.0744 273.0687 | [26] | |
| 9 | 3.52 | Spirotryprostatin A | 396.3100 | C22H26N3O4 [M + H]+ | 340.1292 215.0815 | [23] | |
| 10 | 3.60 | Hexylitaconic Acid | 213.1128 | C11H17O4 [M − H]− | 195.1015 169.1230 151.1132 | [27] | |
| 11 | 3.61 | Fumigaclavine C | 367.2376 | C23H31O2N2 [M + H]+ | 307.2177 276.1752 238.1470 | [26] | |
| 12 | 3.64 | 6-Methoxyspirotryprostatin B (Spirotryprostatin G) | 394.1744 | C22H24N3O4 [M + H]+ | 269.1280 241.0602 213.0659 | [28] | |
| 13 | 3.71 | Tryptoquivaline F | 403.1399 | C22H19N4O4 [M + H]+ | 239.0816 211.0865 199.0502 171.0553 147.0551 | [29] | |
| 14 | 3.71 | Chaetominine | 401.1257 | C22H17N4O4 [M − H]− | 383.1139 237.0649 145.0400 | [30] | |
| 15 | 3.74 | 9-Deacetoxyfumigaclavine C | 309.2312 | C21H29N2 [M + H]+ | 278.1869 208.1018 | [22] | |
| 16 | 3.86 | Synerazol | 414.1528 | C22H24NO7 [M + H]+ | 221.0789 105.0323 77.0368 | [31] | |
| 17 | 4.10 | Azaspirofurans B | 398.1227 | C21H20NO7 [M + H]+ | 219.0641 105.0320 | [32] | |
| 18 | 4.15 | Monomethylsulochrin-4-sulphate | 425.0542 | C18H17O10S [M − H]− | 345.0978 313.0711 181.0503 | [33] | |
| 19 | 4.27 | Fumagiringillin | 475.2341 | C26H35O8 [M − H]− | 149.0601 131.0498 105.0708 97.0657 | [34] | |
| 20 | 4.32 | Questin | 283.0607 | C16H11O5 [M − H]− | 283.0613 268.0373 240.0431 | [26] | |
| 21 | 4.35 | Fumitremorgin B | 462.2388 | C27H31N3O4 [M−H2O + H]+ | 394.1750 319.1750 277.1282 | [26] | |
| 22 | 4.38 | Pyripyropene A | 584.2496 | C31H38NO10 [M + H]+ | 506.2165 202.0495 148.0389 | [35] | |
| 23 | 4.43 | Azaspirofurans A | 412.1392 | C22H22NO7 [M + H]+ | 219.0587 105.0273 | [32] | |
| 24 | 4.44 | Monomethyl sulochrin | 345.0978 | C18H17O7 [M − H]− | 313.0654 181.0446 166.0209 | [29] | |
| 25 | 4.45 | Methylorsilinate | 181.0503 | C9H9O4 [M − H]− | 138.0318 122.0369 123.0083 | [36] | |
| 26 | 4.55 | Fumitremorgin C | 380.1122 | C22H26N3O3 [M + H]+ | 412.1327 380.1072 324.2825 | [26] | |
| 27 | 4.65 | Ethylα-D-glucopyranoside | 209.0408 | C8H17O6 [M + H]+ | 181.0446 165.0503 | [37] | |
| 28 | 4.84 | Emodin | 269.0450 | C15H9O5 [M − H]− | 241.0508 225.0558 197.0603 | [38] | |
| 29 | 4.96 | 6,16-O-Dideacetyl helvolic acid 21,16-lactone | 467.2767 | C29H39O5 [M + H]+ | 468.2348 449.2682 421.2722 135.0777 | [39] | |
| 30 | 5.26 | 16-O-Deacetylhelvolic acid 21,16-lactone | 509.2873 | C31H41O6 [M + H]+ | 449.2567 322.2983 268.2873 135.0684 | [39] | |
| 31 | 5.26 | Helvolic acid | 567.2966 | C33H43O8 [M − H]− | 525.2847 463.2842 403.2631 217.1227 | [26] | |
| 32 | 5.44 | 16-O-propionyl-16-O-deacetylhelvolic acid/ 6-O-propionyl-6-O-deacetylhelvolic acid | 581.3124 | C34H45O8 [M − H]− | 567.2964 441.2527 397.2271 311.1687 293.2122 | [39] | |
| 33 | 5.45 | 6-O-propionyl-6,16-O-dideacetylhelvolic acid21,16-lactone |
523.3043 | C32H43O6 [M + H]+ | 449.2689 403.2631 135.0807 | [39] | |
| 34 | 5.51 | Pyripyropene F | 466.2596 | C28H36NO5 [M + H]+ | 392.2209 202.0500 148.0390 | [40] | |
| 35 | 5.65 | Pyripyropene O | 508.3419 | C29H34NO7 [M − H]− | 464.3519 377.3212 115.0031 73.0296 | [41] | |
| 36 | 6.09 | Linoleic acid | 279.2324 | C18H31O2 [M − H]− | 279.2325 261.2223 | [42] | |
| 37 | 6.34 | Oleic acid | 281.2484 | C18H33O2 [M − H]− | 263.2366 240.9990 | [43] | |
| 38 | 6.37 | 5,8-Epidioxyergosta-6,9(11),22-trien-3-ol | 427.2474 | C28H43O3 [M + H]+ | 409.3046 381.3149 363.2885 267.1719 147.0028 | [44] | |
| 39 | 6.82 | Ergosterol peroxide | 429.3734 | C28H45O3 [M + H]+ | 411.3615 393.3512 341.0154 | [44] | |
| 40 | 6.90 | (22E)-Ergosta4,6,8(14), 22,24(28)-pentaen-3-one | 391.3007 | C28H39O [M + H]+ | 267.1748 149.0235 69.0703 | [45] | |
| 41 | 7.29 | Ergosta4,6,8(14),22-tetraen-3-one | 393.3165 | C28H41O [M + H]+ | 268.1830 253.1595 | [46] | |
| 42 | 7.54 | Ergosterol | 397.3822 | C28H45O [M + H]+ | 395.3319 377.3214 | [26] | |
| Test Microrganisms | Zone of Inhibition (ZI, mm) and Minimum Inhibitory Concentration (MIC, µg/mL) | |||||
|---|---|---|---|---|---|---|
| Ethyl Acetate Extract | Ciprofloxacin | Nystatin | ||||
| ZI | MIC | ZI | MIC | ZI | MIC | |
| S. aureus | 23.07 ± 0.51 | 7.81 | 36.90 ± 0.36 | 0.63 | - | - |
| B. subtilis | 10.63 ± 0.25 | 31.25 | 41.63 ± 0.93 | 0.31 | - | - |
| E. coli | 6.37 ± 0.32 | 62.50 | 35.40 ± 0.53 | 1.25 | - | - |
| P. aeruginosa | NA | 125.00 | 30.87 ± 0.35 | 2.50 | - | - |
| P. vulgaris | 14.37 ± 0.47 | 15.63 | 34.50 ± 0.40 | 1.25 | - | - |
| C. albicans | 17.13 ± 0.55 | 15.63 | - | - | 30.27 ± 0.25 | 2.50 |
| A. niger | NA | 125.00 | - | - | 22.27 ± 0.25 | 5.00 |
| Compounds | Zone of Inhibition (ZI, mm) | Minimum Inhibitory Concentration (MIC, µg/mL) |
|---|---|---|
| Ergosterol | 14.10 ± 0.30 | 15.63 |
| Ergosterol Peroxide | NA | NA |
| Helvolic acid | 33.00 ± 0.95 | 1.95 |
| Pseurotin A | 10.83 ± 0.21 | 31.25 |
| Monomethyl sulochrin | 9.90 ± 0.20 | 31.25 |
| Isosclerone | NA | NA |
| Monomethyl sulochrin-4-sulphate | 26.56 ± 0.51 | 3.91 |
| Chaetominine | NA | NA |
| Ciprofloxacin | 36.90 ± 0.36 | 0.63 |
| Sample | IC50 (M ± S.D.) (µg/mL) | |
|---|---|---|
| DNA Gyrase | Topoisomerase IV | |
| Ethyl acetate extract | 0.86 ± 0.05 | 1.23 ± 0.07 |
| Ciprofloxacin | 0.51 ± 0.03 | 2.15 ± 0.12 |
| S. aureus DNA Gyrase | ||||
|---|---|---|---|---|
| Compounds | Docking Score (Kcal/mol) | Amino Acid Residues (Bond Length A°) | Atoms of Compound | Type of Bond |
| Ciprofloxacin | −7.22 | Ser1084(2.79); DG9; DG9 | O(OH)(COOH); N(quinoline); Pyridine(quinoline) | H-acc Arene-cation Arene-arene |
| Ergosterol | −7.40 | Asp1083(2.76); Asp1083(2.78); Ser1084(2.82); Ser1084(3.13) | O(OH); O(OH); O(OH); O(OH) | H-acc H-acc H-acc H-acc |
| Helvolic acid | −8.83 | Asp508(3.38); Leu583(2.47); Gly584(2.89); His1081(2.70); Ser1085(2.72) | H(OH); O(OH); O(OH); O(CO at p-7); O(CO)(acetoxy at p-6) | H-don H-acc H-acc H-acc H-acc |
| Monomethyl sulochrin-4- sulphate | −7.79 | Asp437(2.65); Ser438(2.48); Asp1083(2.99); Asp1083(3.46); Ser1084(3.02); Ser1084(2.54) Ser1084(2.85) DG9(2.38) | O(OH)(phenol); O(OH)(phenol); O(S=O)(OSO3H); H(OSO3H); O(S=O)(OSO3H); O(OH)(OSO3H); O(OH)(OSO3H); O(CO)(benzoyl) | H-acc H-acc H-don H-don H-acc H-acc H-acc H-acc |
| S. aureus Topoisomerase IV | ||||
|---|---|---|---|---|
| Compounds | Docking Score (Kcal/mol) | Amino Acid Residues (Bond Length A°) | Atoms of Compound | Type of Bond |
| Novobiocin | −7.60 | Asn49(3.30); Asp76(2.04); Arg138(3.11); Pro82 | H(OH)(oxan-4-yl); H(OCONH2); O(CO)(coumarin); C6H2(coumarin) | H-don H-don H-acc Arene-cation |
| Ergosterol | −7.92 | Arg79(2.62); Arg138(3.11) | O(OH); O(OH) | H-acc H-acc |
| Helvolic acid | −8.43 | Asn49(1.57); Asn49(2.86); Asn49(3.05); Asp52(3.01) | H(OH)(COOH); O(CO)(COOH); O(CO)(COOH); O(CO)(COOH) | H-don H-don H-don H-don |
| Monomethyl sulochrin-4-sulphate | −8.25 | Asn49(3.59); Asp52(2.47); Arg79(2.64); Gly80(2.61); Arg138(2.93); Arg138(2.99) | H(OH)(phenol); H(OH)(phenol); O(OSO3H); O(S=O)(OSO3H); O(S=O)(OSO3H); O(S=O)(OSO3H) | H-don H-don H-acc H-don H-acc H-acc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, M.E.; Mohamed, O.G.; El-Fishawy, A.M.; El-Askary, H.I.; El-Senousy, A.S.; El-Beih, A.A.; Nossier, E.S.; Naglah, A.M.; Almehizia, A.A.; Tripathi, A.; et al. Identification of Antibacterial Metabolites from Endophytic Fungus Aspergillus fumigatus, Isolated from Albizia lucidior Leaves (Fabaceae), Utilizing Metabolomic and Molecular Docking Techniques. Molecules 2022, 27, 1117. https://doi.org/10.3390/molecules27031117
Hussein ME, Mohamed OG, El-Fishawy AM, El-Askary HI, El-Senousy AS, El-Beih AA, Nossier ES, Naglah AM, Almehizia AA, Tripathi A, et al. Identification of Antibacterial Metabolites from Endophytic Fungus Aspergillus fumigatus, Isolated from Albizia lucidior Leaves (Fabaceae), Utilizing Metabolomic and Molecular Docking Techniques. Molecules. 2022; 27(3):1117. https://doi.org/10.3390/molecules27031117
Chicago/Turabian StyleHussein, Mai E., Osama G. Mohamed, Ahlam M. El-Fishawy, Hesham I. El-Askary, Amira S. El-Senousy, Ahmed A. El-Beih, Eman S. Nossier, Ahmed M. Naglah, Abdulrahman A. Almehizia, Ashootosh Tripathi, and et al. 2022. "Identification of Antibacterial Metabolites from Endophytic Fungus Aspergillus fumigatus, Isolated from Albizia lucidior Leaves (Fabaceae), Utilizing Metabolomic and Molecular Docking Techniques" Molecules 27, no. 3: 1117. https://doi.org/10.3390/molecules27031117
APA StyleHussein, M. E., Mohamed, O. G., El-Fishawy, A. M., El-Askary, H. I., El-Senousy, A. S., El-Beih, A. A., Nossier, E. S., Naglah, A. M., Almehizia, A. A., Tripathi, A., & Hamed, A. A. (2022). Identification of Antibacterial Metabolites from Endophytic Fungus Aspergillus fumigatus, Isolated from Albizia lucidior Leaves (Fabaceae), Utilizing Metabolomic and Molecular Docking Techniques. Molecules, 27(3), 1117. https://doi.org/10.3390/molecules27031117










