Applications of Carbon Dots for the Photocatalytic and Electrocatalytic Reduction of CO2
Abstract
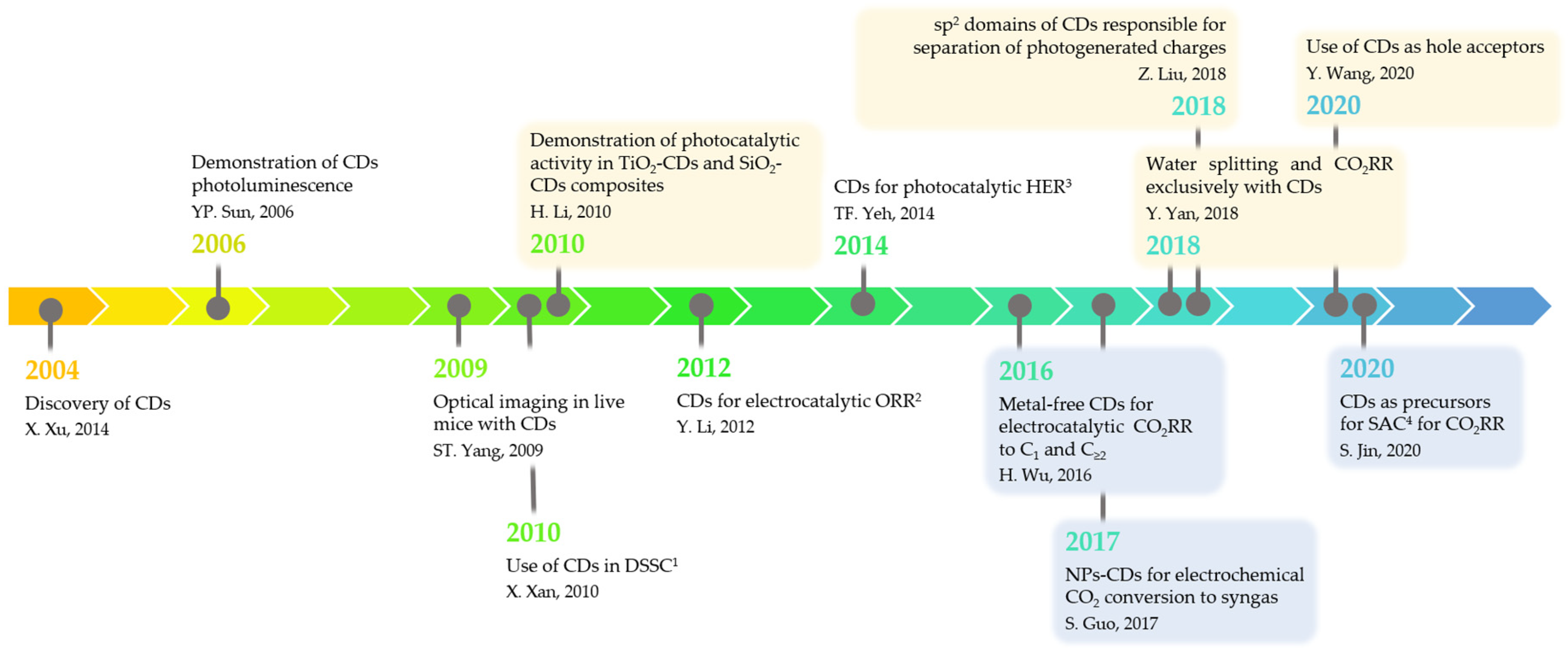
:1. Introduction
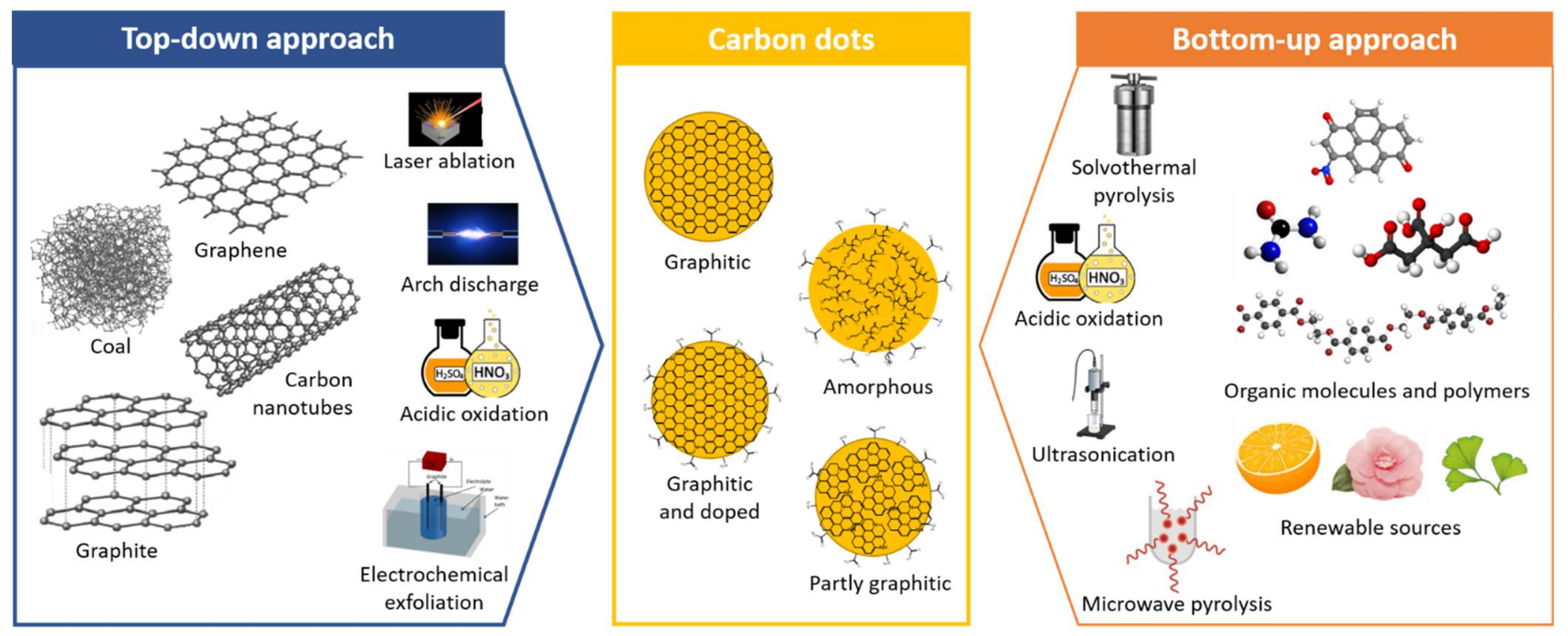
2. Strategies for CDs Synthesis
2.1. Synthesis Methods
2.1.1. Top–Down Approach
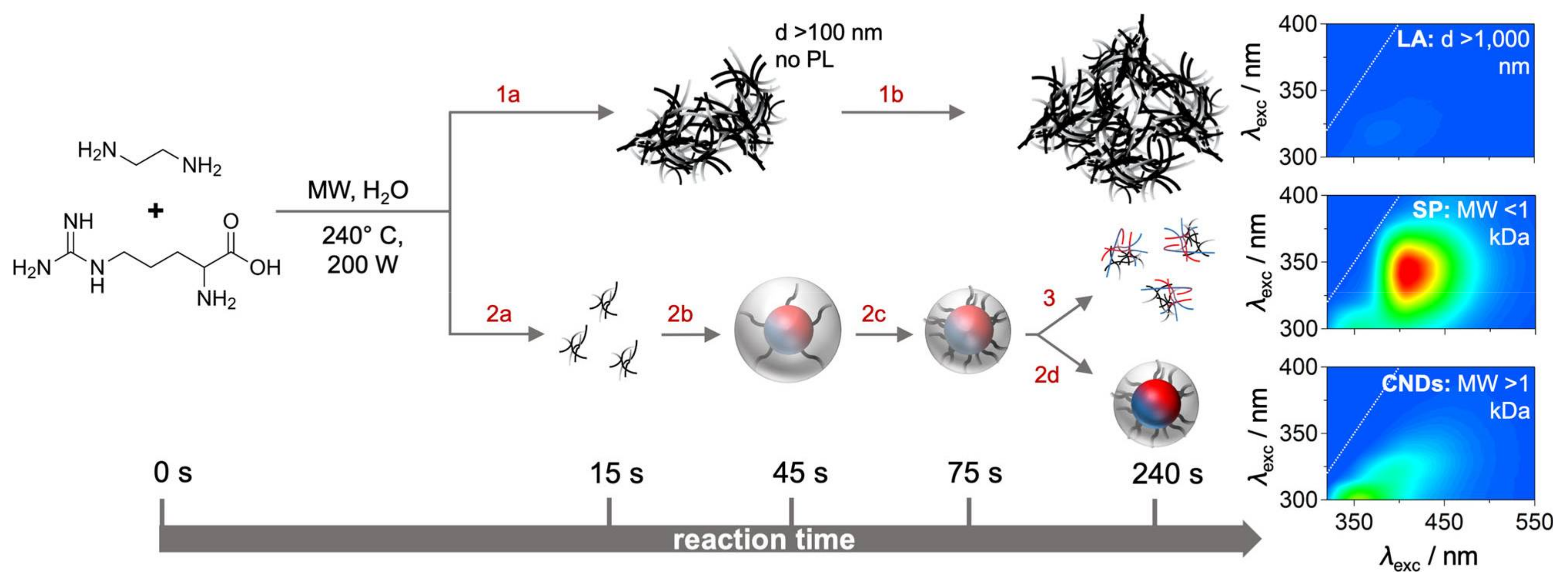
2.1.2. Bottom–Up Approach
2.2. Purification of Carbon Dots
2.3. Metal Doping, Modification, and Post-Processing of Carbon Dots
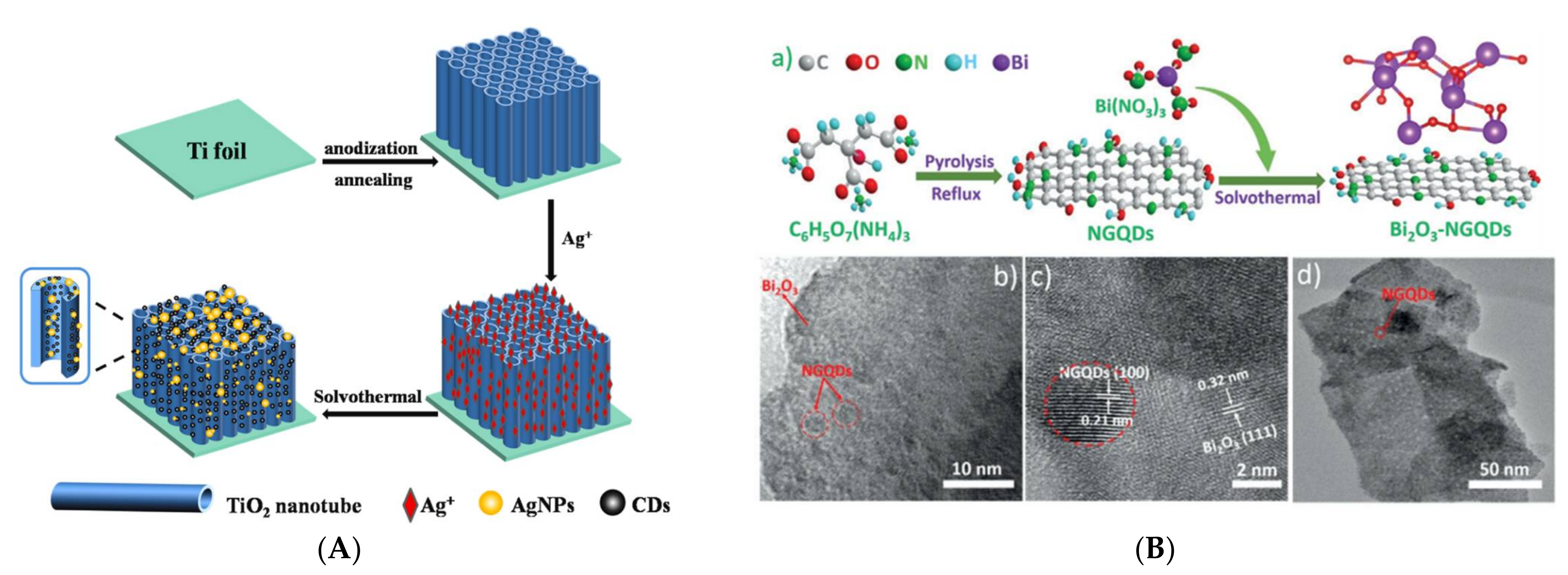
2.4. Synthesis of CDs-Composites for CO2 Reduction
3. Photocatalytic CO2 Reduction
3.1. Metal-Free Catalysts
3.2. Metal/Transition Metal-Based Hybrids and Composites
4. Electrocatalytic CO2 Reduction
4.1. Metal-Free Catalysts
4.2. Transition Metal-Based Single-Atom Catalysts
4.3. Metal/Metal-Oxide Composites
5. Summary and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritchie, H.; Roser, M. CO2 and Greenhouse Gas Emissions. Our World Data. 2020. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 30 January 2021).
- Styring, P.; Jansen, D.; de Coninck, H.; Reith, H.; Armstrong, K. Carbon Capture and Utilisation in the Green Economy; Center for Low Carbon Futures: New York, NY, USA, 2011; ISBN 9780957258815. [Google Scholar]
- Fontecilla-Camps, J.C.; Amara, P.; Cavazza, C.; Nicolet, Y.; Volbeda, A. Structure–function relationships of anaerobic gas-processing metalloenzymes. Nature 2009, 460, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Franco, F.; Rettenmaier, C.; Jeon, H.S.; Roldan Cuenya, B. Transition metal-based catalysts for the electrochemical CO2 reduction: From atoms and molecules to nanostructured materials. Chem. Soc. Rev. 2020, 49, 6884–6946. [Google Scholar] [CrossRef] [PubMed]
- Kinzel, N.W.; Werlé, C.; Leitner, W. Transition Metal Complexes as Catalysts for the Electroconversion of CO2: An Organometallic Perspective. Angew. Chemie Int. Ed. 2021, 60, 11628–11686. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kassymova, M.; Cai, X.; Zang, S.-Q.; Jiang, H.-L. Photocatalytic CO2 reduction over metal-organic framework-based materials. Coord. Chem. Rev. 2020, 412, 213262. [Google Scholar] [CrossRef]
- Perazio, A.; Lowe, G.; Gobetto, R.; Bonin, J.; Robert, M. Light-driven catalytic conversion of CO2 with heterogenized molecular catalysts based on fourth period transition metals. Coord. Chem. Rev. 2021, 443, 214018. [Google Scholar] [CrossRef]
- Boutin, E.; Merakeb, L.; Ma, B.; Boudy, B.; Wang, M.; Bonin, J.; Anxolabéhère-Mallart, E.; Robert, M. Molecular catalysis of CO2 reduction: Recent advances and perspectives in electrochemical and light-driven processes with selected Fe, Ni and Co aza macrocyclic and polypyridine complexes. Chem. Soc. Rev. 2020, 49, 5772–5809. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 1, 1–12. [Google Scholar] [CrossRef]
- Yang, N.; Waldvogel, S.R.; Jiang, X. Electrochemistry of carbon dioxide on carbon electrodes. ACS Appl. Mater. Interfaces 2016, 8, 28357–28371. [Google Scholar] [CrossRef]
- Dai, L. Carbon-based catalysts for metal-free electrocatalysis. Curr. Opin. Electrochem. 2017, 4, 18–25. [Google Scholar] [CrossRef]
- Wu, J.; Sharifi, T.; Gao, Y.; Zhang, T.; Ajayan, P.M. Emerging Carbon-Based Heterogeneous Catalysts for Electrochemical Reduction of Carbon Dioxide into Value-Added Chemicals. Adv. Mater. 2019, 31, 1804257. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Dastafkan, K.; Ren, W.; Yang, W.; Zhao, C. Carbon-based catalysts for electrochemical CO2 reduction. Sustain. Energy Fuels 2019, 3, 2890–2906. [Google Scholar] [CrossRef]
- Messias, S.; Nunes da Ponte, M.; S Reis-Machado, A. Carbon Materials as Cathode Constituents for Electrochemical CO2 Reduction—A Review. C 2019, 5, 83. [Google Scholar] [CrossRef] [Green Version]
- Gawande, M.B.; Fornasiero, P.; Zbořil, R. Carbon-based single-atom catalysts for advanced applications. ACS Catal. 2020, 10, 2231–2259. [Google Scholar] [CrossRef]
- Wu, J.; Ma, S.; Sun, J.; Gold, J.I.; Tiwary, C.; Kim, B.; Zhu, L.; Chopra, N.; Odeh, I.N.; Vajtai, R.; et al. A metal-free electrocatalyst for carbon dioxide reduction to multi-carbon hydrocarbons and oxygenates. Nat. Commun. 2016, 7, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, K.; Quan, X. Carbon-based materials for electrochemical reduction of CO2 to C2+oxygenates: Recent progress and remaining challenges. ACS Catal. 2021, 11, 2076–2097. [Google Scholar] [CrossRef]
- Yeh, T.; Teng, C.; Chen, S.; Teng, H. Nitrogen-doped graphene oxide quantum dots as photocatalysts for overall water-splitting under visible light Illumination. Adv. Mater. 2014, 26, 3297–3303. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar] [CrossRef]
- Jana, J.; Ngo, Y.-L.T.; Chung, J.S.; Hur, S.H. Contribution of Carbon Dot Nanoparticles in Electrocatalysis: Development in Energy Conversion Process. J. Electrochem. Sci. Technol. 2020, 11, 220–237. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, G.; Li, P.; Zhang, X.; Fan, H.; Zhang, Y.; Wang, X.; Zhang, C. A minireview on doped carbon dots for photocatalytic and electrocatalytic applications. Nanoscale 2020, 12, 13899–13906. [Google Scholar] [CrossRef]
- Hu, C.; Li, M.; Qiu, J.; Sun, Y.-P. Design and fabrication of carbon dots for energy conversion and storage. Chem. Soc. Rev. 2019, 48, 2315–2337. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Shiral Fernando, K.A.; Liang, W.; Seilkop, A.; Monica Veca, L.; Sun, Y.-P.; Bunker, C.E. Carbon dots for energy conversion applications. J. Appl. Phys. 2019, 125, 220903. [Google Scholar] [CrossRef] [Green Version]
- Shaari, N.; Kamarudin, S.K.; Bahru, R. Carbon and graphene quantum dots in fuel cell application: An overview. Int. J. Energy Res. 2021, 45, 1396–1424. [Google Scholar] [CrossRef]
- He, C.; Xu, P.; Zhang, X.; Long, W. The synthetic strategies, photoluminescence mechanisms and promising applications of carbon dots: Current state and future perspective. Carbon N. Y. 2022, 186, 91–127. [Google Scholar] [CrossRef]
- Yan, X.; Cui, X.; Li, B.; Li, L. Large, solution-processable graphene quantum dots as light absorbers for photovoltaics. Nano Lett. 2010, 10, 1869–1873. [Google Scholar] [CrossRef]
- Yang, S.-T.; Cao, L.; Luo, P.G.; Lu, F.; Wang, X.; Wang, H.; Meziani, M.J.; Liu, Y.; Qi, G.; Sun, Y.-P. Carbon dots for optical imaging in vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, M.; Huang, H.; Liu, Y.; Kang, Z. Advances, challenges and promises of carbon dots. Inorg. Chem. Front. 2017, 4, 1963–1986. [Google Scholar] [CrossRef]
- Ge, L.; Pan, N.; Jin, J.; Wang, P.; LeCroy, G.E.; Liang, W.; Yang, L.; Teisl, L.R.; Tang, Y.; Sun, Y.-P. Systematic comparison of carbon dots from different preparations—consistent optical properties and photoinduced redox characteristics in visible spectrum and structural and mechanistic implications. J. Phys. Chem. C 2018, 122, 21667–21676. [Google Scholar] [CrossRef]
- Qin, X.; Zhu, S.; Xiao, F.; Zhang, L.; Shao, M. Active sites on heterogeneous single-iron-atom electrocatalysts in CO2 reduction reaction. ACS Energy Lett. 2019, 4, 1778–1783. [Google Scholar] [CrossRef]
- Hu, X.-M.; Hval, H.H.; Bjerglund, E.T.; Dalgaard, K.J.; Madsen, M.R.; Pohl, M.-M.; Welter, E.; Lamagni, P.; Buhl, K.B.; Bremholm, M. Selective CO2 reduction to CO in water using earth-abundant metal and nitrogen-doped carbon electrocatalysts. ACS Catal. 2018, 8, 6255–6264. [Google Scholar] [CrossRef]
- Pillar-Little, T.J.; Wanninayake, N.; Nease, L.; Heidary, D.K.; Glazer, E.C.; Kim, D.Y. Superior photodynamic effect of carbon quantum dots through both type I and type II pathways: Detailed comparison study of top-down-synthesized and bottom-up-synthesized carbon quantum dots. Carbon N. Y. 2018, 140, 616–623. [Google Scholar] [CrossRef]
- Chichkov, B.N.; Momma, C.; Nolte, S.; Von Alvensleben, F.; Tünnermann, A. Femtosecond, picosecond and nanosecond laser ablation of solids. Appl. Phys. A 1996, 63, 109–115. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Hoffman, J.; Morgiel, J.; Mościcki, T.; Stobiński, L.; Szymański, Z.; Małolepszy, A. Luminescent Carbon Dots Synthesized by the Laser Ablation of Graphite in Polyethylenimine and Ethylenediamine. Materials (Basel) 2021, 14, 729. [Google Scholar] [CrossRef]
- Orooji, Y.; Ghanbari Gol, H.; Jaleh, B.; Rashidian Vaziri, M.R.; Eslamipanah, M. Large Optical Nonlinearity of the Activated Carbon Nanoparticles Prepared by Laser Ablation. Nanomaterials 2021, 11, 737. [Google Scholar] [CrossRef] [PubMed]
- Reyes, D.; Camacho, M.; Camacho, M.; Mayorga, M.; Weathers, D.; Salamo, G.; Wang, Z.; Neogi, A. Laser ablated carbon nanodots for light emission. Nanoscale Res. Lett. 2016, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushwaha, N.; Mittal, J.; Pandey, S.; Kumar, R. High temperature acidic oxidation of multiwalled Carbon nanotubes and synthesis of Graphene quantum dots. Int. J. Nano Dimens. 2018, 9, 191–197. [Google Scholar]
- Iannazzo, D.; Pistone, A.; Salamò, M.; Galvagno, S.; Romeo, R.; Giofré, S.V.; Branca, C.; Visalli, G.; Di Pietro, A. Graphene quantum dots for cancer targeted drug delivery. Int. J. Pharm. 2017, 518, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Saheeda, P.; Sabira, K.; Dhaneesha, M.; Jayaleksmi, S. Investigation on the pH-independent photoluminescence emission from carbon dots impregnated on polymer matrix. Luminescence 2018, 33, 22–28. [Google Scholar] [CrossRef]
- Hu, C.; Yu, C.; Li, M.; Wang, X.; Yang, J.; Zhao, Z.; Eychmüller, A.; Sun, Y.; Qiu, J. Chemically tailoring coal to fluorescent carbon dots with tuned size and their capacity for Cu (II) detection. Small 2014, 10, 4926–4933. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, J.; Li, N.; Tian, J.; Li, K.; Jiang, J.; Liu, J.; Tian, Q.; Chen, P. Systematic Bandgap Engineering of Graphene Quantum Dots and Applications for Photocatalytic Water Splitting and CO2 Reduction. ACS Nano 2018, 12, 3523–3532. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xia, J.; Zhou, C.; Via, B.; Xia, Y.; Zhang, F.; Li, Y.; Xia, L.; Tang, J. Synthesis of strongly green-photoluminescent graphene quantum dots for drug carrier. Colloids Surf. B Biointerfaces 2013, 112, 192–196. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Z.; Qing, S.; Xue, N.; Jia, S.; Zhang, L.; Li, L.; Li, N.; Shi, L.; Chen, J. Improving methane selectivity of photo-induced CO2 reduction on carbon dots through modification of nitrogen-containing groups and graphitization. Appl. Catal. B Environ. 2018, 232, 86–92. [Google Scholar] [CrossRef]
- Jin, S.; Ni, Y.; Hao, Z.; Zhang, K.; Lu, Y.; Yan, Z.; Wei, Y.; Lu, Y.; Chan, T.; Chen, J. A Universal Graphene Quantum Dot Tethering Design Strategy to Synthesize Single-Atom Catalysts. Angew. Chemie Int. Ed. 2020, 59, 21885–21889. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Booker, C.; Li, R.; Zhou, X.; Sham, T.-K.; Sun, X.; Ding, Z. An electrochemical avenue to blue luminescent nanocrystals from multiwalled carbon nanotubes (MWCNTs). J. Am. Chem. Soc. 2007, 129, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.-T. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chemie 2010, 122, 4532–4536. [Google Scholar] [CrossRef]
- Zhao, S.; Tang, Z.; Guo, S.; Han, M.; Zhu, C.; Zhou, Y.; Bai, L.; Gao, J.; Huang, H.; Li, Y.; et al. Enhanced Activity for CO2 Electroreduction on a Highly Active and Stable Ternary Au-CDots-C3N4 Electrocatalyst. ACS Catal. 2018, 8, 188–197. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, S.; Wu, X.; Li, H.; Zhou, Y.; Zhu, C.; Yang, N.; Jiang, X.; Gao, J.; Bai, L.; et al. A Co3O4-CDots-C3N4 three component electrocatalyst design concept for efficient and tunable CO2 reduction to syngas. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, S.; Gao, J.; Zhu, C.; Wu, X.; Fu, Y.; Huang, H.; Liu, Y.; Kang, Z. Cu-CDots nanocorals as electrocatalyst for highly efficient CO2 reduction to formate. Nanoscale 2017, 9, 298–304. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Zhao, Y.; Shi, G.; Deng, L.; Hou, Y.; Qu, L. An electrochemical avenue to green-luminescent graphene quantum dots as potential electron-acceptors for photovoltaics. Adv. Mater. 2011, 23, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yuan, G.; Lin, M.; Wang, P.; Li, S.; Tang, J.; Lin, J.; Huang, Y.; Zhang, Y. Large-scale electrochemical fabrication of nitrogen-doped carbon quantum dots and their application as corrosion inhibitor for copper. J. Mater. Sci. 2021, 56, 12909–12919. [Google Scholar] [CrossRef]
- Zheng, X.; Ren, S.; Wang, L.; Gai, Q.; Dong, Q.; Liu, W. Controllable functionalization of carbon dots as fluorescent sensors for independent Cr (VI), Fe (III) and Cu (II) ions detection. J. Photochem. Photobiol. A Chem. 2021, 417, 113359. [Google Scholar] [CrossRef]
- Li, L.; Ji, J.; Fei, R.; Wang, C.; Lu, Q.; Zhang, J.; Jiang, L.; Zhu, J. A facile microwave avenue to electrochemiluminescent two-color graphene quantum dots. Adv. Funct. Mater. 2012, 22, 2971–2979. [Google Scholar] [CrossRef]
- Zhuo, S.; Shao, M.; Lee, S.-T. Upconversion and downconversion fluorescent graphene quantum dots: Ultrasonic preparation and photocatalysis. ACS Nano 2012, 6, 1059–1064. [Google Scholar] [CrossRef]
- Prasad, K.P.; Chen, Y.; Sk, M.A.; Than, A.; Wang, Y.; Sun, H.; Lim, K.-H.; Dong, X.; Chen, P. Fluorescent quantum dots derived from PEDOT and their applications in optical imaging and sensing. Mater. Horizons 2014, 1, 529–534. [Google Scholar] [CrossRef]
- Chen, C.; Yan, X.; Liu, S.; Wu, Y.; Wan, Q.; Sun, X.; Zhu, Q.; Liu, H.; Ma, J.; Zheng, L.; et al. Highly Efficient Electroreduction of CO2 to C2+ Alcohols on Heterogeneous Dual Active Sites. Angew. Chemie Int. Ed. 2020, 59, 16459–16464. [Google Scholar] [CrossRef]
- Pizzetti, M.; Petricci, E.; Tecnologico, C. Heterogeneous catalysis under microwave heating. La Chim. l’Industria 2012, 78–81. [Google Scholar]
- Baluta, S.; Lesiak, A.; Cabaj, J. Simple and cost-effective electrochemical method for norepinephrine determination based on carbon dots and tyrosinase. Sensors (Switzerland) 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Hsieh, C.-W.; Kung, M.-L.; Chu, L.-Y.; Huang, H.-J.; Chen, H.-T.; Wu, D.-C.; Kuo, C.-H.; Hsieh, S.-L.; Hsieh, S. Eco-friendly synthesis of shrimp egg-derived carbon dots for fluorescent bioimaging. J. Biotechnol. 2014, 189, 114–119. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Wu, M.; Feng, X.; Redfern, S.A.T.; Shang, Y.; Yong, X.; Feng, T.; Wu, K.; Liu, Z.; et al. Carbon-Quantum-Dots-Loaded Ruthenium Nanoparticles as an Efficient Electrocatalyst for Hydrogen Production in Alkaline Media. Adv. Mater. 2018, 30, 1800676. [Google Scholar] [CrossRef] [PubMed]
- Qing, W.; Chen, K.; Yang, Y.; Wang, Y.; Liu, X. Cu2+-doped carbon dots as fluorescence probe for specific recognition of Cr(VI) and its antimicrobial activity. Microchem. J. 2020, 152, 104262. [Google Scholar] [CrossRef]
- Hong, W.T.; Yang, H.K. Luminescent properties of carbon dots originated from pine pollen foranti-counterfeiting application. Opt. LASER Technol. 2022, 145, 107452. [Google Scholar] [CrossRef]
- Kim, D.; Jo, G.; Chae, Y.; Subramani, S.; Lee, B.Y.; Kim, E.J.; Ji, M.-K.; Sim, U.; Hyun, H. Bioinspired Camellia japonica carbon dots with high near-infrared absorbance for efficient photothermal cancer therapy. Nanoscale 2021, 13, 14426–14434. [Google Scholar] [CrossRef]
- Shaik, S.A.; Sengupta, S.; Varma, R.S.; Gawande, M.B.; Goswami, A. Syntheses of N-Doped Carbon Quantum Dots (NCQDs) from Bioderived Precursors: A Timely Update. ACS Sustain. Chem. Eng. 2021, 9, 3–49. [Google Scholar] [CrossRef]
- Sendão, R.; de Yuso, M.D.V.M.; Algarra, M.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Comparative life cycle assessment of bottom-up synthesis routes for carbon dots derived from citric acid and urea. J. Clean. Prod. 2020, 254, 1–10. [Google Scholar] [CrossRef]
- Ren, J.; Malfatti, L.; Innocenzi, P. Citric Acid Derived Carbon Dots, the Challenge of Understanding the Synthesis-Structure Relationship. C 2020, 7, 2. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, S.; Zhang, S.; Fu, Y.; Wang, L.; Zhao, X.; Yang, B. Investigation from chemical structure to photoluminescent mechanism: A type of carbon dots from the pyrolysis of citric acid and an amine. J. Mater. Chem. C 2015, 3, 5976–5984. [Google Scholar] [CrossRef]
- Duan, P.; Zhi, B.; Coburn, L.; Haynes, C.L.; Schmidt-Rohr, K. A molecular fluorophore in citric acid/ethylenediamine carbon dots identified and quantified by multinuclear solid-state nuclear magnetic resonance. Magn. Reson. Chem. 2020, 58, 1130–1138. [Google Scholar] [CrossRef]
- Li, M.; Wang, M.; Zhu, L.; Li, Y.; Yan, Z.; Shen, Z.; Cao, X. Facile microwave assisted synthesis of N-rich carbon quantum dots/dual-phase TiO2 heterostructured nanocomposites with high activity in CO2 photoreduction. Appl. Catal. B Environ. 2018, 231, 269–276. [Google Scholar] [CrossRef]
- Christé, S.; da Silva, J.C.G.E.; da Silva, L.P. Evaluation of the environmental impact and efficiency of N-doping strategies in the synthesis of carbon dots. Materials 2020, 13, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinterberger, V.; Damm, C.; Haines, P.; Guldi, D.M.; Peukert, W. Purification and structural elucidation of carbon dots by column chromatography. Nanoscale 2019, 11, 8464–8474. [Google Scholar] [CrossRef] [PubMed]
- LeCroy, G.E.; Yang, S.T.; Yang, F.; Liu, Y.; Fernando, K.A.S.; Bunker, C.E.; Hu, Y.; Luo, P.G.; Sun, Y.P. Functionalized carbon nanoparticles: Syntheses and applications in optical bioimaging and energy conversion. Coord. Chem. Rev. 2016, 320–321, 66–81. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Ye, Y.; Guo, R.; Wang, A.; Zou, G.; Hou, H.; Ji, X. Kilogram-Scale Synthesis and Functionalization of Carbon Dots for Superior Electrochemical Potassium Storage. ACS Nano 2021, 15, 6872–6885. [Google Scholar] [CrossRef]
- Dong, W.; Zhou, S.; Dong, Y.; Wang, J.; Ge, X.; Sui, L. The preparation of ethylenediamine-modified fluorescent carbon dots and their use in imaging of cells. Luminescence 2015, 30, 867–871. [Google Scholar] [CrossRef]
- Zhong, H.; Sa, R.; Lv, H.; Yang, S.; Yuan, D.; Wang, X.; Wang, R. Covalent Organic Framework Hosting Metalloporphyrin-Based Carbon Dots for Visible-Light-Driven Selective CO2 Reduction. Adv. Funct. Mater. 2020, 30, 2–9. [Google Scholar] [CrossRef]
- Hao, A.; Guo, X.; Wu, Q.; Sun, Y.; Cong, C.; Liu, W. Exploring the interactions between polyethyleneimine modified fluorescent carbon dots and bovine serum albumin by spectroscopic methods. J. Lumin. 2016, 170, 90–96. [Google Scholar] [CrossRef]
- Cailotto, S.; Amadio, E.; Facchin, M.; Selva, M.; Pontoglio, E.; Rizzolio, F.; Riello, P.; Toffoli, G.; Benedetti, A.; Perosa, A. Carbon dots from sugars and ascorbic acid: Role of the precursors on morphology, properties, toxicity, and drug uptake. ACS Med. Chem. Lett. 2018, 9, 832–837. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, W.; Qiu, L.; Jiang, X.; Zuo, D.; Wang, D.; Yang, L. One pot synthesis of highly luminescent polyethylene glycol anchored carbon dots functionalized with a nuclear localization signal peptide for cell nucleus imaging. Nanoscale 2015, 7, 6104–6113. [Google Scholar] [CrossRef]
- Peng, Z.; Ji, C.; Zhou, Y.; Zhao, T.; Leblanc, R.M. Polyethylene glycol (PEG) derived carbon dots: Preparation and applications. Appl. Mater. Today 2020, 20, 100677. [Google Scholar] [CrossRef]
- Wei, X.; Wang, C.; Ding, S.; Yang, K.; Tian, F.; Li, F. One-step synthesis of Ag nanoparticles/carbon dots/TiO2 nanotube arrays composite photocatalyst with enhanced photocatalytic activity. J. Environ. Chem. Eng. 2021, 9, 104729. [Google Scholar] [CrossRef]
- Strauss, V.; Wang, H.; Delacroix, S.; Ledendecker, M.; Wessig, P. Carbon nanodots revised: The thermal citric acid/urea reaction. Chem. Sci. 2020, 11, 8256–8266. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk, W.; Świergosz, T.; Bednarz, S.; Walas, K.; Bashmakova, N.V.; Bogdał, D. Luminescence phenomena of carbon dots derived from citric acid and urea-a molecular insight. Nanoscale 2018, 10, 13889–13894. [Google Scholar] [CrossRef] [PubMed]
- Sendão, R.M.S.; Crista, D.M.A.; Afonso, A.C.P.; Martínez De Yuso, M.D.V.; Algarra, M.; Esteves Da Silva, J.C.G.; Pinto Da Silva, L. Insight into the hybrid luminescence showed by carbon dots and molecular fluorophores in solution. Phys. Chem. Chem. Phys. 2019, 21, 20919–20926. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Qu, D.; Yang, D.; Nie, B.; Zhao, Y.; Fan, H.; Sun, Z. Synthesis of Carbon Dots with Multiple Color Emission by Controlled Graphitization and Surface Functionalization. Adv. Mater. 2018, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.K.; Kumar, S.; Tonda, S. N-doped C dot/CoAl-layered double hydroxide/g-C3N4 hybrid composites for efficient and selective solar-driven conversion of CO2 into CH4. Compos. Part B Eng. 2019, 176, 107212. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Han, X.; Godin, R.; Chen, J.; Zhou, W.; Jiang, C.; Thompson, J.F.; Mustafa, K.B.; Shevlin, S.A.; et al. Unique hole-accepting carbon-dots promoting selective carbon dioxide reduction nearly 100% to methanol by pure water. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Yadav, R.M.; Li, Z.; Zhang, T.; Sahin, O.; Roy, S.; Gao, G.; Guo, H.; Vajtai, R.; Wang, L.; Ajayan, P.M. Amine Functionalized Carbon Nanodots Electrocatalysts Converting Carbon Dioxide to Methane. Adv. Mater. 2021, 2105690. [Google Scholar] [CrossRef]
- Chowdhury, D.; Gogoi, N.; Majumdar, G. Fluorescent carbon dots obtained from chitosan gel. RSC Adv. 2012, 2, 12156–12159. [Google Scholar] [CrossRef]
- Sonsin, A.F.; Nascimento, S.M.S.; Albuquerque, I.M.B.; Silva, E.C.O.; Rocha, J.C.A.; Oliveira, R.S.; Barbosa, C.D.E.S.; Souza, S.T.; Fonseca, E.J.S. Temperature-dependence on the optical properties of chitosan carbon dots in the solid state. RSC Adv. 2021, 11, 2767–2773. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, H.; Wang, Y.; Xiong, Z.; Zhao, X.; Xia, Y. Chitosan-derived N-doped carbon dots for fluorescent determination of nitrite and bacteria imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 251, 119468. [Google Scholar] [CrossRef] [PubMed]
- Konwar, A.; Gogoi, N.; Majumdar, G.; Chowdhury, D. Green chitosan–carbon dots nanocomposite hydrogel film with superior properties. Carbohydr. Polym. 2015, 115, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Rong, M.; Liang, Y.; Zhao, D.; Chen, B.; Pan, C.; Deng, X.; Chen, Y.; He, J. A ratiometric fluorescence visual test paper for an anthrax biomarker based on functionalized manganese-doped carbon dots. Sensors Actuators, B Chem. 2018, 265, 498–505. [Google Scholar] [CrossRef]
- Pajewska-Szmyt, M.; Buszewski, B.; Gadzała-Kopciuch, R. Sulphur and nitrogen doped carbon dots synthesis by microwave assisted method as quantitative analytical nano-tool for mercury ion sensing. Mater. Chem. Phys. 2020, 242, 122484. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Y.; Liu, J.; Huang, K.; Chen, Y.; Li, Y.; Zhu, J.J. Low Overpotential for Electrochemically Reducing CO2 to CO on Nitrogen-Doped Graphene Quantum Dots-Wrapped Single-Crystalline Gold Nanoparticles. ACS Energy Lett. 2018, 3, 946–951. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, Green, and Blue Luminescence by Carbon Dots: Full-Color Emission Tuning and Multicolor Cellular Imaging. Angew. Chemie 2015, 127, 5450–5453. [Google Scholar] [CrossRef]
- Chen, Z.; Mou, K.; Wang, X.; Liu, L. Nitrogen-Doped Graphene Quantum Dots Enhance the Activity of Bi 2 O 3 Nanosheets for Electrochemical Reduction of CO2 in a Wide Negative Potential Region. Angew. Chemie 2018, 130, 12972–12976. [Google Scholar] [CrossRef]
- Zhang, T.; Li, W.; Huang, K.; Guo, H.; Li, Z.; Fang, Y.; Yadav, R.M.; Shanov, V.; Ajayan, P.M.; Wang, L. Regulation of functional groups on graphene quantum dots directs selective CO2 to CH4 conversion. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Xia, C.; Qiu, Y.; Xia, Y.; Zhu, P.; King, G.; Zhang, X.; Wu, Z.; Kim, J.Y.T.; Cullen, D.A.; Zheng, D. General synthesis of single-atom catalysts with high metal loading using graphene quantum dots. Nat. Chem. 2021, 13, 887–894. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonokemija. Znan. Američko udruženje za Napred. Znan. 1991, 247, 1439–1445. [Google Scholar]
- Ong, W.J.; Putri, L.K.; Tan, Y.C.; Tan, L.L.; Li, N.; Ng, Y.H.; Wen, X.; Chai, S.P. Unravelling charge carrier dynamics in protonated g-C3N4 interfaced with carbon nanodots as co-catalysts toward enhanced photocatalytic CO2 reduction: A combined experimental and first-principles DFT study. Nano Res. 2017, 10, 1673–1696. [Google Scholar] [CrossRef]
- Li, H.; Deng, Y.; Liu, Y.; Zeng, X.; Wiley, D.; Huang, J. Carbon quantum dots and carbon layer double protected cuprous oxide for efficient visible light CO2 reduction. Chem. Commun. 2019, 55, 4419–4422. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, X.; MacFarlane, D.R. Carbon quantum dots/Cu2O heterostructures for solar-light-driven conversion of CO2 to methanol. Adv. Energy Mater. 2015, 5, 1401077. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, S.; Guo, S.; Wang, H.; Sun, Y.; Yao, B.; Liu, Y.; Huang, H.; Kang, Z. Carbon quantum dot-covered porous Ag with enhanced activity for selective electroreduction of CO2 to CO. Inorg. Chem. Front. 2019, 6, 1453–1460. [Google Scholar] [CrossRef]
- Cao, L.; Sahu, S.; Anilkumar, P.; Bunker, C.E.; Xu, J.; Fernando, K.A.S.; Wang, P.; Guliants, E.A.; Tackett, K.N.; Sun, Y.P. Carbon nanoparticles as visible-light photocatalysts for efficient CO2 conversion and beyond. J. Am. Chem. Soc. 2011, 133, 4754–4757. [Google Scholar] [CrossRef]
- Zhou, Y.; Mintz, K.J.; Oztan, C.Y.; Hettiarachchi, S.D.; Peng, Z.; Seven, E.S.; Liyanage, P.Y.; De La Torre, S.; Celik, E.; Leblanc, R.M. Embedding carbon dots in superabsorbent polymers for additive manufacturing. Polymers 2018, 10, 921. [Google Scholar] [CrossRef] [Green Version]
- Dehvari, K.; Liu, K.Y.; Tseng, P.-J.; Gedda, G.; Girma, W.M.; Chang, J.-Y. Sonochemical-assisted green synthesis of nitrogen-doped carbon dots from crab shell as targeted nanoprobes for cell imaging. J. Taiwan Inst. Chem. Eng. 2019, 95, 495–503. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Yang, L.Y.; Fan, X.Y.; Jin, J.C.; Mei, J.; Peng, W.; Jiang, F.L.; Xiao, Q.; Liu, Y. Low temperature synthesis of highly stable phosphate functionalized two color carbon nanodots and their application in cell imaging. Carbon N. Y. 2014, 66, 351–360. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Zhang, Z. Graphitized carbon dots emitting strong green photoluminescence. J. Mater. Chem. C 2013, 1, 4902–4907. [Google Scholar] [CrossRef]
- Gong, X.; Lu, W.; Liu, Y.; Li, Z.; Shuang, S.; Dong, C.; Choi, M.M.F. Low temperature synthesis of phosphorous and nitrogen co-doped yellow fluorescent carbon dots for sensing and bioimaging. J. Mater. Chem. B 2015, 3, 6813–6819. [Google Scholar] [CrossRef]
- Qin, X.; Liu, J.; Zhang, Q.; Chen, W.; Zhong, X.; He, J. Synthesis of Yellow-Fluorescent Carbon Nano-dots by Microplasma for Imaging and Photocatalytic Inactivation of Cancer Cells. Nanoscale Res. Lett. 2021, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shi, Y.; Pan, Y.; Cheng, X.; Zhang, L.; Chen, J.; Li, M.J.; Yi, C. Quinoline derivative-functionalized carbon dots as a fluorescent nanosensor for sensing and intracellular imaging of Zn2+. J. Mater. Chem. B 2014, 2, 5020–5027. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Pang, S.; Wang, L.; Li, Q.; Kreiter, M.; Liu, C.Y. One-step synthesis of highly luminescent carbon dots in noncoordinating solvents. Chem. Mater. 2010, 22, 4528–4530. [Google Scholar] [CrossRef]
- Guo, C.X.; Zhao, D.; Zhao, Q.; Wang, P.; Lu, X. Na+-functionalized carbon quantum dots: A new draw solute in forward osmosis for seawater desalination. Chem. Commun. 2014, 50, 7318–7321. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Arefina, I.A.; Danilov, D.V.; Koroleva, A.V.; Zhizhin, E.V.; Parfenov, P.S.; Kuznetsova, V.A.; Ismagilov, A.O.; Litvin, A.P.; Fedorov, A. V Chiral carbon dots based on L/D-cysteine produced via room temperature surface modification and one-pot carbonization. Nanoscale 2021, 13, 8058–8066. [Google Scholar] [CrossRef]
- Yuan, Y.H.; Liu, Z.X.; Li, R.S.; Zou, H.Y.; Lin, M.; Liu, H.; Huang, C.Z. Synthesis of nitrogen-doping carbon dots with different photoluminescence properties by controlling the surface states. Nanoscale 2016, 8, 6770–6776. [Google Scholar] [CrossRef]
- Khavlyuk, P.D.; Stepanidenko, E.A.; Bondarenko, D.P.; Danilov, D.V.; Koroleva, A.V.; Baranov, A.V.; Maslov, V.G.; Kasak, P.; Fedorov, A.V.; Ushakova, E.V. The influence of thermal treatment conditions (solvothermal versus microwave) and solvent polarity on the morphology and emission of phloroglucinol-based nitrogen-doped carbon dots. Nanoscale 2021, 13, 3070–3078. [Google Scholar] [CrossRef]
- Crista, D.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Evaluation of different bottom-up routes for the fabrication of carbon dots. Nanomaterials 2020, 10, 1316. [Google Scholar] [CrossRef]
- Rigodanza, F.; Burian, M.; Arcudi, F.; Đorđević, L.; Amenitsch, H.; Prato, M. Snapshots into carbon dots formation through a combined spectroscopic approach. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Liu, M.; Yang, B.; Feng, L.; Ji, Y.; Tao, L.; Wei, Y. Size tunable fluorescent nano-graphite oxides: Preparation and cell imaging applications. Phys. Chem. Chem. Phys. 2013, 15, 19013–19018. [Google Scholar] [CrossRef]
- Mohammed, L.J.; Omer, K.M. Dual functional highly luminescence B, N Co-doped carbon nanodots as nanothermometer and Fe3+/Fe2+ sensor. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, I.J.; Arnaiz, B.; Cacioppo, M.; Arcudi, F.; Prato, M. Nitrogen-doped carbon nanodots for bioimaging and delivery of paclitaxel. J. Mater. Chem. B 2018, 6, 5540–5548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Liu, J.; Ren, J.; Tang, F.; Meng, X. One-pot synthesis of active copper-containing carbon dots with laccase-like activities. Nanoscale 2015, 7, 19641–19646. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Godin, R.; Durrant, J.R.; Tang, J. Efficient Hole Trapping in Carbon Dot/Oxygen-Modified Carbon Nitride Heterojunction Photocatalysts for Enhanced Methanol Production from CO2 under Neutral Conditions. Angew. Chemie Int. Ed. 2021, 60, 20811–20816. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhou, N.; Lin, X.; Lin, J.; Chi, Y.; Chen, G. Extraction of electrochemiluminescent oxidized carbon quantum dots from activated carbon. Chem. Mater. 2010, 22, 5895–5899. [Google Scholar] [CrossRef]
- Zhao, Q.L.; Zhang, Z.L.; Huang, B.H.; Peng, J.; Zhang, M.; Pang, D.W. Facile preparation of low cytotoxicity fluorescent carbon nanocrystals by electrooxidation of graphite. Chem. Commun. 2008, 5116–5118. [Google Scholar] [CrossRef]
- Liu, H.; Ye, T.; Mao, C. Fluorescent carbon nanoparticles derived from candle soot. Angew. chemie 2007, 119, 6593–6595. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Sun, Y.; Geng, X.; Hu, Y.; Meng, H.; Ge, J.; Qu, L. Synthesis of Luminescent Carbon Dots with Ultrahigh Quantum Yield and Inherent Folate Receptor-Positive Cancer Cell Targetability. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Michaud, V.; Pracht, J.; Schilfarth, F.; Damm, C.; Platzer, B.; Haines, P.; Harreiß, C.; Guldi, D.M.; Spiecker, E.; Peukert, W. Well-separated water-soluble carbon dots via gradient chromatography. Nanoscale 2021, 13, 13116–13128. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, S.; Sun, J.; He, P.; Yuan, N.; Ding, J.; Mo, R.; Wang, G.; Ding, G.; Xie, X. Deep ultraviolet emission photoluminescence and high luminescece efficiency of ferric passivated graphene quantum dots: Strong negative inductive effect of Fe. Synth. Met. 2015, 209, 468–472. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Rathi, A.K.; Gawande, M.B.; Hola, K.; Goswami, A.; Kalytchuk, S.; Karakassides, M.A.; Kouloumpis, A.; Gournis, D.; Deligiannakis, Y.; et al. Fe(III)-functionalized carbon dots—Highly efficient photoluminescence redox catalyst for hydrogenations of olefins and decomposition of hydrogen peroxide. Appl. Mater. Today 2017, 7, 179–184. [Google Scholar] [CrossRef]
- Yang, W.; Huang, T.T.; Zhao, M.; Luo, F.; Weng, W.; Wei, Q.; Lin, Z.; Chen, G. High peroxidase-like activity of iron and nitrogen co-doped carbon dots and its application in immunosorbent assay. Talanta 2017, 164, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, T.; Li, Q.; Yan, Y.; Wang, F.; Cui, X.; Yang, Z.; Wang, Y.; Yang, Z.; Chang, K.; Liu, G. N,fe-doped carbon dot decorated gear-shaped wo3 for highly efficient UV-VIS-NIR-driven photocatalytic performance. Catalysts 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Feng, T.; Chen, Y.; Zhao, X.; Yang, B. Ionic-State Cobalt and Iron Co-doped Carbon Dots with Superior Electrocatalytic Activity for the Oxygen Evolution Reaction. ChemElectroChem 2019, 6, 2088–2094. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Y.; Yang, J.; Hu, Y.; Yang, R.; Li, Z.; Qu, L.; Lin, Y. High performance fluorescence biosensing of cysteine in human serum with superior specificity based on carbon dots and cobalt-derived recognition. Sensors Actuators, B Chem. 2019, 280, 62–68. [Google Scholar] [CrossRef]
- Guo, X.L.; Ding, Z.Y.; Deng, S.M.; Wen, C.C.; Shen, X.C.; Jiang, B.P.; Liang, H. A novel strategy of transition-metal doping to engineer absorption of carbon dots for near-infrared photothermal/photodynamic therapies. Carbon N. Y. 2018, 134, 519–530. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, P.; Wu, X.; Ma, C.; Luo, S.; Xu, M.; Li, W.; Liu, S. Nitrogen and copper (II) co-doped carbon dots for applications in ascorbic acid determination by non-oxidation reduction strategy and cellular imaging. Talanta 2020, 210, 120649. [Google Scholar] [CrossRef]
- Wu, W.; Zhan, L.; Fan, W.; Song, J.; Li, X.; Li, Z.; Wang, R.; Zhang, J.; Zheng, J.; Wu, M.; et al. Cu-N Dopants Boost Electron Transfer and Photooxidation Reactions of Carbon Dots. Angew. Chemie 2015, 127, 6640–6644. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, Z.; Zhou, Y.; Tammina, S.K.; Yang, Y. A double carbon dot system composed of N, Cl-doped carbon dots and N, Cu-doped carbon dots as peroxidase mimics and as fluorescent probes for the determination of hydroquinone by fluorescence. Microchim. Acta 2020, 187, 1–8. [Google Scholar] [CrossRef]
- Yang, M.; Tang, Q.; Meng, Y.; Liu, J.; Feng, T.; Zhao, X.; Zhu, S.; Yu, W.; Yang, B. Reversible “off-On” Fluorescence of Zn2+-Passivated Carbon Dots: Mechanism and Potential for the Detection of EDTA and Zn2+. Langmuir 2018, 34, 7767–7775. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Jia, M.; Meng, H.; Li, H.; Guan, Y.; Feng, L. Functionalization of Carbonaceous Nanodots from MnII-Coordinating Functional Knots. Chem. - A Eur. J. 2015, 21, 14843–14850. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Fu, J.; Zhou, Y.; Chang, Y.-C.; Min, Q.; Zhu, J.-J.; Lin, Y.; Zhu, W. Insights on forming N, O-coordinated Cu single-atom catalysts for electrochemical reduction CO2 to methane. Nat. Commun. 2021, 12, 1–9. [Google Scholar]
- Qu, J.; Zhang, X.; Zhang, S.; Wang, Z.; Yu, Y.; Ding, H.; Tang, Z.; Heng, X.; Wang, R.; Jing, S. A facile co-crystallization approach to fabricate two-component carbon dot composites showing time-dependent evolutive room temperature phosphorescence colors. Nanoscale Adv. 2021, 3, 5053–5061. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, S.; Sun, L.; Wu, S.; Hu, G.; Pang, X.; Smith, A.T.; Hu, C.; Zeng, S.; Wang, W. Ultralong lifetime and efficient room temperature phosphorescent carbon dots through multi-confinement structure design. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Guo, X.; Pan, Q.; Song, X.; Guo, Q.; Zhou, S.; Qiu, J.; Dong, G. Embedding carbon dots in Eu3+-doped metal-organic framework for label-free ratiometric fluorescence detection of Fe3+ ions. J. Am. Ceram. Soc. 2021, 104, 886–895. [Google Scholar] [CrossRef]
- Sun, M.; Han, Y.; Yuan, X.; Jing, P.; Zhang, L.; Zhao, J.; Zheng, Y. Efficient full-color emitting carbon-dot-based composite phosphors by chemical dispersion. Nanoscale 2020, 12, 15823–15831. [Google Scholar] [CrossRef]
- Zhou, Z.; Ushakova, E.V.; Liu, E.; Bao, X.; Li, D.; Zhou, D.; Tan, Z.; Qu, S.; Rogach, A.L. A co-crystallization induced surface modification strategy with cyanuric acid modulates the bandgap emission of carbon dots. Nanoscale 2020, 12, 10987–10993. [Google Scholar] [CrossRef]
- Li, S.; Ji, K.; Zhang, M.; He, C.; Wang, J.; Li, Z. Boosting the photocatalytic CO2 reduction of metal–organic frameworks by encapsulating carbon dots. Nanoscale 2020, 12, 9533–9540. [Google Scholar] [CrossRef]
- Cheng, S.; Ye, T.; Mao, H.; Wu, Y.; Jiang, W.; Ban, C.; Yin, Y.; Liu, J.; Xiu, F.; Huang, W. Electrostatically assembled carbon dots/boron nitride nanosheet hybrid nanostructures for thermal quenching-resistant white phosphors. Nanoscale 2020, 12, 524–529. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Y.; Zhang, P.; Li, F.; Liu, J.; Zhao, J.-F.; Huo, F.; Zhao, Q.; Huang, W. Green-Synthesized Phosphorescent Carbon Dot Composite for Multilevel Anti-Counterfeiting. Nanoscale Adv. 2021, 3, 4536–4540. [Google Scholar]
- Li, Q.; Wang, S.; Sun, Z.; Tang, Q.; Liu, Y.; Wang, L.; Wang, H.; Wu, Z. Enhanced CH4 selectivity in CO2 photocatalytic reduction over carbon quantum dots decorated and oxygen doping g-C3N4. Nano Res. 2019, 12, 2749–2759. [Google Scholar] [CrossRef]
- Lv, K.; Suo, W.; Shao, M.; Zhu, Y.; Wang, X.; Feng, J.; Fang, M. Nitrogen doped MoS2 and nitrogen doped carbon dots composite catalyst for electroreduction CO2 to CO with high Faradaic efficiency. Nano Energy 2019, 63, 103834. [Google Scholar] [CrossRef]
- Shen, H.; Peppel, T.; Strunk, J.; Sun, Z. Photocatalytic Reduction of CO2 by Metal-Free-Based Materials: Recent Advances and Future Perspective. Sol. RRL 2020, 4, 1900546. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Jiang, Z.; Wong, P.K.; Lee, C. Recent Progress on Carbon Nitride and Its Hybrid Photocatalysts for CO2 Reduction. Sol. RRL 2021, 2000478, 1–25. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Wageh, S.; Al-ghamdi, A.A.; Xie, J. Graphene in Photocatalysis: A Review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Q.; Bai, X.; Ge, Z.; Yang, Q.; Yin, C. Carbothermal activation synthesis of 3D porous g-C3N4/carbon nanosheets composite with superior performance for CO2 photoreduction. Appl. Catal. B Environ. 2018, 239, 196–203. [Google Scholar] [CrossRef]
- Hsu, H.; Shown, I.; Wei, H.; Chang, Y.; Du, H.; Lin, Y.; Tseng, C.; Wang, C.; Chen, L.; Lin, Y.; et al. Graphene oxide as a promising photocatalyst for CO2 to methanol conversion. Nanoscale 2013, 5, 262–268. [Google Scholar] [CrossRef]
- Yadav, D.; Yadav, R.K.; Kumar, A.; Park, N.J.; Baeg, J.O. Functionalized Graphene Quantum Dots as Efficient Visible-Light Photocatalysts for Selective Solar Fuel Production from CO2. ChemCatChem 2016, 8, 3389–3393. [Google Scholar] [CrossRef]
- Sahu, S.; Liu, Y.; Wang, P.; Bunker, C.E.; Fernando, K.A.S.; Lewis, W.K.; Guliants, E.A.; Yang, F.; Wang, J.; Sun, Y.P. Visible-light photoconversion of carbon dioxide into organic acids in an aqueous solution of carbon dots. Langmuir 2014, 30, 8631–8636. [Google Scholar] [CrossRef]
- Duan, X.; Xu, J.; Wei, Z.; Ma, J.; Guo, S.; Wang, S.; Liu, H.; Dou, S. Metal-free carbon materials for CO2 electrochemical reduction. Adv. Mater. 2017, 29, 1701784. [Google Scholar] [CrossRef]
- Varela, A.S.; Ju, W.; Bagger, A.; Franco, P.; Rossmeisl, J.; Strasser, P. Electrochemical reduction of CO2 on metal-nitrogen-doped carbon catalysts. ACS Catal. 2019, 9, 7270–7284. [Google Scholar] [CrossRef]
- Kumar, B.; Asadi, M.; Pisasale, D.; Sinha-Ray, S.; Rosen, B.A.; Haasch, R.; Abiade, J.; Yarin, A.L.; Salehi-Khojin, A. Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Wu, J.; Yadav, R.M.; Liu, M.; Sharma, P.P.; Tiwary, C.S.; Ma, L.; Zou, X.; Zhou, X.-D.; Yakobson, B.I.; Lou, J. Achieving highly efficient, selective, and stable CO2 reduction on nitrogen-doped carbon nanotubes. ACS Nano 2015, 9, 5364–5371. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.P.; Wu, J.; Yadav, R.M.; Liu, M.; Wright, C.J.; Tiwary, C.S.; Yakobson, B.I.; Lou, J.; Ajayan, P.M.; Zhou, X. Nitrogen-doped carbon nanotube arrays for high-efficiency electrochemical reduction of CO2: On the understanding of defects, defect density, and selectivity. Angew. Chemie 2015, 127, 13905–13909. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, P.; Ubnoske, S.; Brennaman, M.K.; Song, N.; House, R.L.; Glass, J.T.; Meyer, T.J. Polyethylenimine-enhanced electrocatalytic reduction of CO2 to formate at nitrogen-doped carbon nanomaterials. J. Am. Chem. Soc. 2014, 136, 7845–7848. [Google Scholar] [CrossRef]
- Li, J.; Zan, W.-Y.; Kang, H.; Dong, Z.; Zhang, X.; Lin, Y.; Mu, Y.-W.; Zhang, F.; Zhang, X.-M.; Gu, J. Graphitic-N highly doped graphene-like carbon: A superior metal-free catalyst for efficient reduction of CO2. Appl. Catal. B Environ. 2021, 298, 120510. [Google Scholar] [CrossRef]
- Wu, J.; Liu, M.; Sharma, P.P.; Yadav, R.M.; Ma, L.; Yang, Y.; Zou, X.; Zhou, X.-D.; Vajtai, R.; Yakobson, B.I. Incorporation of nitrogen defects for efficient reduction of CO2 via two-electron pathway on three-dimensional graphene foam. Nano Lett. 2016, 16, 466–470. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Hou, X.; Ma, C.; Tan, T. Nitrogen-doped graphenes as efficient electrocatalysts for the selective reduction of carbon dioxide to formate in aqueous solution. Green Chem. 2016, 18, 3250–3256. [Google Scholar] [CrossRef]
- Sreekanth, N.; Nazrulla, M.A.; Vineesh, T.V.; Sailaja, K.; Phani, K.L. Metal-free boron-doped graphene for selective electroreduction of carbon dioxide to formic acid/formate. Chem. Commun. 2015, 51, 16061–16064. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Quan, X.; Yu, H. Efficient electrochemical reduction of carbon dioxide to acetate on nitrogen-doped nanodiamond. J. Am. Chem. Soc. 2015, 137, 11631–11636. [Google Scholar] [CrossRef]
- Song, Y.; Chen, W.; Zhao, C.; Li, S.; Wei, W.; Sun, Y. Metal-free nitrogen-doped mesoporous carbon for electroreduction of CO2 to ethanol. Angew. Chemie 2017, 129, 10980–10984. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Cheng, K.; Quan, X.; Fan, X.; Su, Y.; Chen, S.; Zhao, H.; Zhang, Y.; Yu, H. Selective electrochemical reduction of carbon dioxide to ethanol on a boron-and nitrogen-Co-doped nanodiamond. Angew. Chemie 2017, 129, 15813–15817. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Wang, G.; Li, Y.; Wen, Z. Perfluorinated covalent triazine framework derived hybrids for the highly selective electroconversion of carbon dioxide into methane. Angew. Chemie Int. Ed. 2018, 57, 13120–13124. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Sadakiyo, M.; Luo, R.; Heima, M.; Yamauchi, M.; Kenis, P.J.A. One-step electrosynthesis of ethylene and ethanol from CO2 in an alkaline electrolyzer. J. Power Sources 2016, 301, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Liu, M.; Wu, J.; Ajayan, P.M.; Li, J.; Liu, B.; Yakobson, B.I. How nitrogen-doped graphene quantum dots catalyze electroreduction of CO2 to hydrocarbons and oxygenates. ACS Catal. 2017, 7, 6245–6250. [Google Scholar] [CrossRef]
- Montoya, J.H.; Shi, C.; Chan, K.; Nørskov, J.K. Theoretical insights into a CO dimerization mechanism in CO2 electroreduction. J. Phys. Chem. Lett. 2015, 6, 2032–2037. [Google Scholar] [CrossRef]
- Ji, S.; Chen, Y.; Wang, X.; Zhang, Z.; Wang, D.; Li, Y. Chemical synthesis of single atomic site catalysts. Chem. Rev. 2020, 120, 11900–11955. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Y.; Huang, L.-B.; Liu, X.-Z.; Zhang, Q.-H.; He, C.; Wu, Z.-Y.; Zhang, L.-J.; Wu, J.; Yang, W. Cascade anchoring strategy for general mass production of high-loading single-atomic metal-nitrogen catalysts. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.B.; Hung, S.-F.; Liu, S.; Yuan, K.; Miao, S.; Zhang, L.; Huang, X.; Wang, H.-Y.; Cai, W.; Chen, R. Atomically dispersed Ni (I) as the active site for electrochemical CO2 reduction. Nat. Energy 2018, 3, 140–147. [Google Scholar] [CrossRef]
- Liu, S.; Tao, H.; Zeng, L.; Liu, Q.; Xu, Z.; Liu, Q.; Luo, J.-L. Shape-dependent electrocatalytic reduction of CO2 to CO on triangular silver nanoplates. J. Am. Chem. Soc. 2017, 139, 2160–2163. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Senanayake, S.D.; Zhang, Y.; Xu, W.; Polyansky, D.E. Effect of chloride anions on the synthesis and enhanced catalytic activity of silver nanocoral electrodes for CO2 electroreduction. Acs Catal. 2015, 5, 5349–5356. [Google Scholar] [CrossRef]
- Lu, Q.; Rosen, J.; Zhou, Y.; Hutchings, G.S.; Kimmel, Y.C.; Chen, J.G.; Jiao, F. A selective and efficient electrocatalyst for carbon dioxide reduction. Nat. Commun. 2014, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.I. Electrochemical CO2 reduction on metal electrodes. In Modern Aspects of Electrochemistry; Springer: New York, NY, USA, 2008; pp. 89–189. [Google Scholar]
- Zhu, W.; Zhang, Y.-J.; Zhang, H.; Lv, H.; Li, Q.; Michalsky, R.; Peterson, A.A.; Sun, S. Active and selective conversion of CO2 to CO on ultrathin Au nanowires. J. Am. Chem. Soc. 2014, 136, 16132–16135. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Reske, R.; Zeng, Z.; Zhao, Z.-J.; Greeley, J.; Strasser, P.; Cuenya, B.R. Exceptional size-dependent activity enhancement in the electroreduction of CO2 over Au nanoparticles. J. Am. Chem. Soc. 2014, 136, 16473–16476. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.B.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [Green Version]
- Hansen, H.A.; Varley, J.B.; Peterson, A.A.; Nørskov, J.K. Understanding trends in the electrocatalytic activity of metals and enzymes for CO2 reduction to CO. J. Phys. Chem. Lett. 2013, 4, 388–392. [Google Scholar] [CrossRef]
- Innocent, B.; Liaigre, D.; Pasquier, D.; Ropital, F.; Léger, J.-M.; Kokoh, K.B. Electro-reduction of carbon dioxide to formate on lead electrode in aqueous medium. J. Appl. Electrochem. 2009, 39, 227. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, P.; Meyer, T.J. Nanostructured tin catalysts for selective electrochemical reduction of carbon dioxide to formate. J. Am. Chem. Soc. 2014, 136, 1734–1737. [Google Scholar] [CrossRef]
- Koh, J.H.; Won, D.H.; Eom, T.; Kim, N.-K.; Jung, K.D.; Kim, H.; Hwang, Y.J.; Min, B.K. Facile CO2 electro-reduction to formate via oxygen bidentate intermediate stabilized by high-index planes of Bi dendrite catalyst. ACS Catal. 2017, 7, 5071–5077. [Google Scholar] [CrossRef]
- Li, F.; Zhao, S.-F.; Chen, L.; Khan, A.; MacFarlane, D.R.; Zhang, J. Polyethylenimine promoted electrocatalytic reduction of CO2 to CO in aqueous medium by graphene-supported amorphous molybdenum sulphide. Energy Environ. Sci. 2016, 9, 216–223. [Google Scholar] [CrossRef]
- Gao, D.; Sinev, I.; Scholten, F.; Arán-Ais, R.M.; Divins, N.J.; Kvashnina, K.; Timoshenko, J.; Roldan Cuenya, B. Selective CO2 Electroreduction to Ethylene and Multicarbon Alcohols via Electrolyte-Driven Nanostructuring. Angew. Chemie 2019, 131, 17203–17209. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.; Cheng, T.; Flores Espinosa, M.; Fei, H.; Duan, X.; Goddard III, W.A.; Huang, Y. A highly active star decahedron Cu nanocatalyst for hydrocarbon production at low overpotentials. Adv. Mater. 2019, 31, 1805405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosse, P.; Gao, D.; Scholten, F.; Sinev, I.; Mistry, H.; Roldan Cuenya, B. Dynamic changes in the structure, chemical state and catalytic selectivity of Cu nanocubes during CO2 electroreduction: Size and support effects. Angew. Chemie Int. Ed. 2018, 57, 6192–6197. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Ke, L.; Ding, Y.; Zhang, Y.; Rui, K.; Lin, H.; Zhu, J. Recent advances in Cu-based catalysts for electroreduction of carbon dioxide. Mater. Chem. Front. 2021, 5, 2668–2683. [Google Scholar] [CrossRef]
- Li, Y.; Kim, D.; Louisia, S.; Xie, C.; Kong, Q.; Yu, S.; Lin, T.; Aloni, S.; Fakra, S.C.; Yang, P. Electrochemically scrambled nanocrystals are catalytically active for CO2-to-multicarbons. Proc. Natl. Acad. Sci. USA 2020, 117, 9194–9201. [Google Scholar] [CrossRef]
- Wang, S.; Kou, T.; Baker, S.E.; Duoss, E.B.; Li, Y. Recent progress in electrochemical reduction of CO2 by oxide-derived copper catalysts. Mater. Today Nano 2020, 100096. [Google Scholar] [CrossRef]
- Lv, J.; Jouny, M.; Luc, W.; Zhu, W.; Zhu, J.; Jiao, F. A highly porous copper electrocatalyst for carbon dioxide reduction. Adv. Mater. 2018, 30, 1803111. [Google Scholar] [CrossRef]
- Kim, J.; Choi, W.; Park, J.W.; Kim, C.; Kim, M.; Song, H. Branched copper oxide nanoparticles induce highly selective ethylene production by electrochemical carbon dioxide reduction. J. Am. Chem. Soc. 2019, 141, 6986–6994. [Google Scholar] [CrossRef]

| Catalyst | Major Products | Yield | Reaction Conditions |
|---|---|---|---|
| g-C3N4/carbon nanosheets [158] | CO, CH4 | 229 µmol CO/g 112 µmol CH4/g (in 7 h) | H2O |
| Graphene Oxide [159] | CH3OH | 0.172 µmol/g·h | H2O |
| Ni-PCD@TD-COF 1 [78] | CO, H2 | 956 µmol CO/g (in 2 h) 19 µmol H2/g (in 2 h) | ACN:H2O:TEOA 3:1:1 2 |
| CDs-CN 3 [89] | CH3OH, CO | 13.9 µmol CH3OH/g·h 0.05 µmol CO/g·h | H2O |
| CDs-FAT 4 [126] | CH3OH | 24.2 µmol CH3OH/g·h | H2O |
| CDs-g-C3N4 3% wt. [103] | CH4, CO | 37.06 µmol CH4/g 68.8 µmol CO/g (in 10 h) | H2O |
| CL@CDs/Cu2O [104] | CH4, CH3OH | 99.6 µmol CH3OH/g·h 8 µmol CH4/g·h | H2O, TEOA |
| CDs/Cu2O [105] | CH3OH | 55.7 µmol CH3OH/g·h | H2O and dry ice |
| N-CDs/LDH/g-C3N4 [88] | CH4 | 25.4 µmol CH4/g·h | H2O |
| CDs/oxygen doped C3N4 [153] | CH4 | 1.2 µmol CH4/g·(in 8 h) | H2O |
| GQDs-BNPTL [44] | CH3OH | 0.695 µmol CH3OH/g·h | H2O |
| GQDs vs CDs [46] | CDs: CO | 400 µmol CO/g·h, | H2O |
| GQDs: CH4 | 983 µmol CH4/g·h, | ||
| N,S-CDs/TiO2 [72] | CO, CH4 | 1.838 µmol CO 1.195 µmol CH4 (in 6 h) | H2O |
| GQDs-ANP 5/biocatalyst [160] | HCOOH | 198 µmol HCOOH (in 2 h) | H2O, TEOA |
| CDs@PEG-Au [161] | HCOOH CH3COOH | 1.2 µmol HCOOH/g·h 0.06 µmol CH3COOH/g·h | H2O |
| Catalyst | Electrolyte | Major Product(s) (FE, %) | Applied Potential (vs. RHE) |
|---|---|---|---|
| N-doped 3D graphene foam [169] | 0.1 M KHCO3 | CO (85%) | −0.58 V |
| N-doped graphene [170] | 0.5 M KHCO3 | HCOO− (73%) | −0.84 V |
| N-doped nanodiamond/Si rod array (NDD/Si RA) [172] | 0.5 M NaHCO3 | CH3COO− (77.3−77.6%) | −0.8 V/−1.0 V |
| Perfluorinated covalent triazine framework (CTF) [175] | 0.1 M KHCO3 | CH4 (99.3%) | −0.7 V/−0.9 V |
| N-doped cylindrical mesoporous carbon [173] | 0.1 M KHCO3 | CH3CH2OH (77%) | −0.56 V |
| B-/N-co-doped nanodiamond [174] | 0.1 M NaHCO3 | CH3CH2OH (93.2%) | −1.0 V |
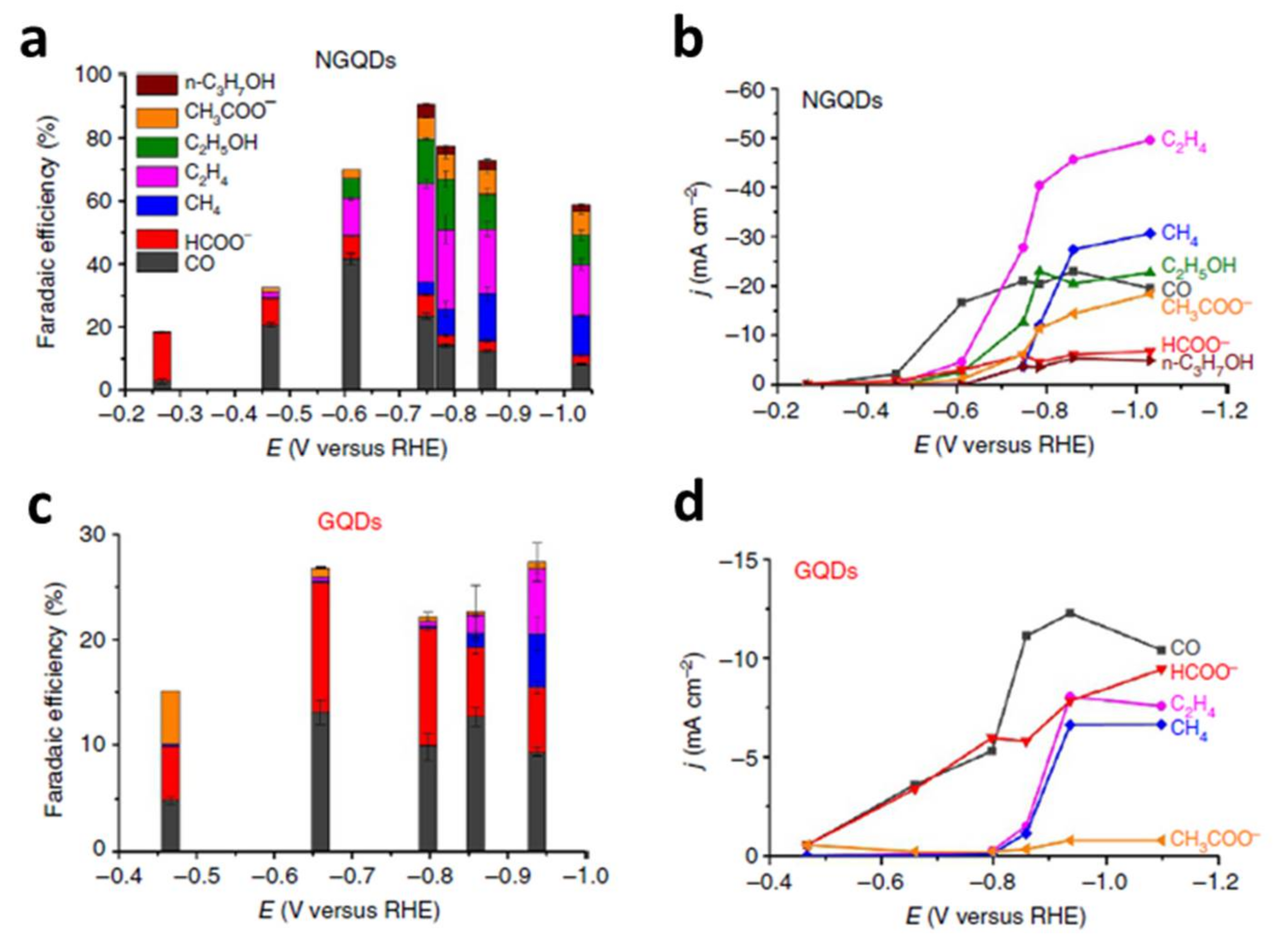
| N-GQDs [17] | 1 M KOH | CH4 (15%) | –0.86 V |
| C2H4 (31%) | –0.75 V | ||
| NH2-functionalized GQDs [100] | 1 M KOH | CH4 (70.0%) | −0.95 V |
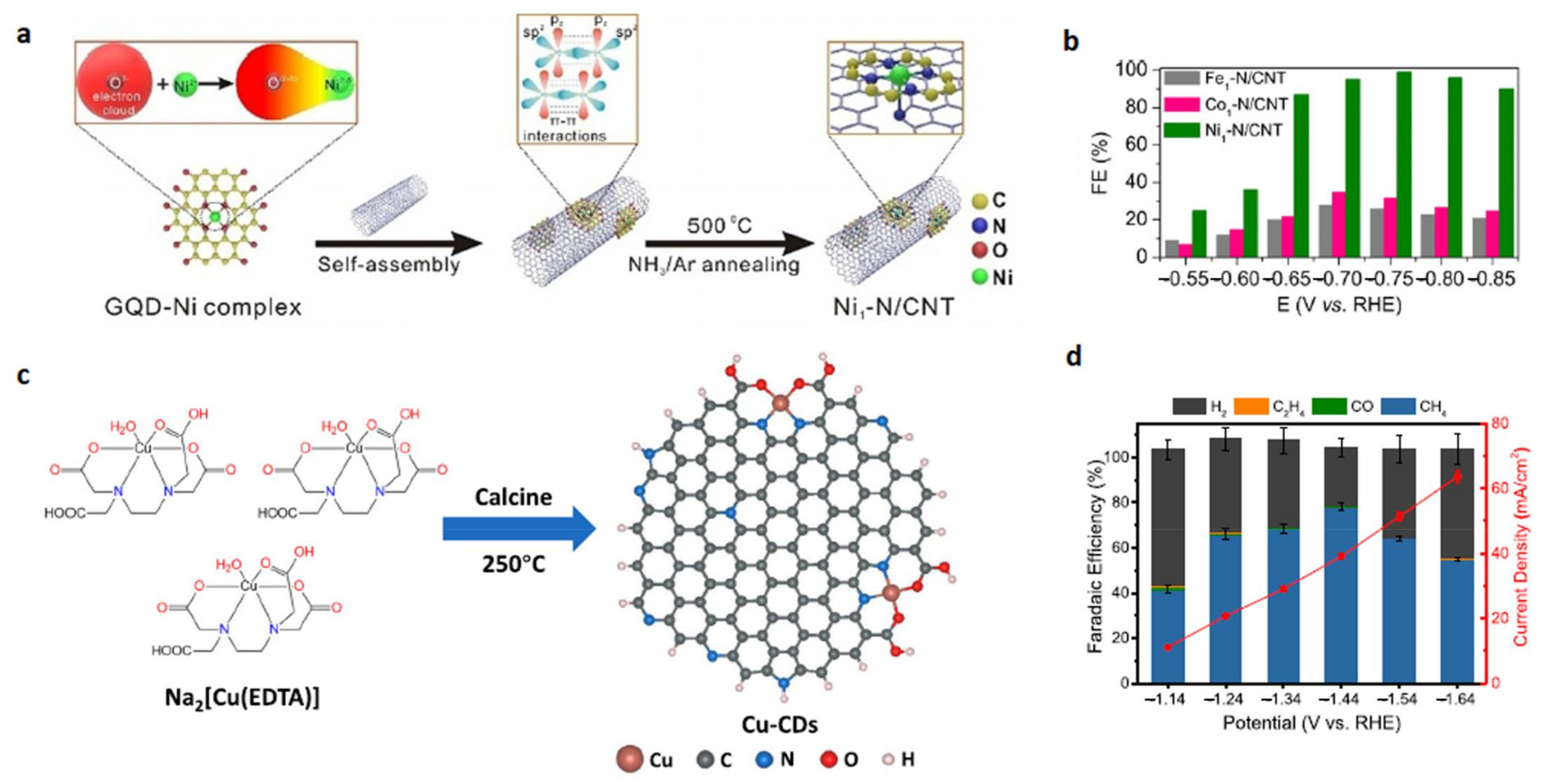
| Ni1-N/CNT [47] | 0.1 M KHCO3 | CO (99%) | –0.75 V |
| Cu-CDs [144] | 0.5 M KHCO3 | CH4 (78%) | −1.44 V |
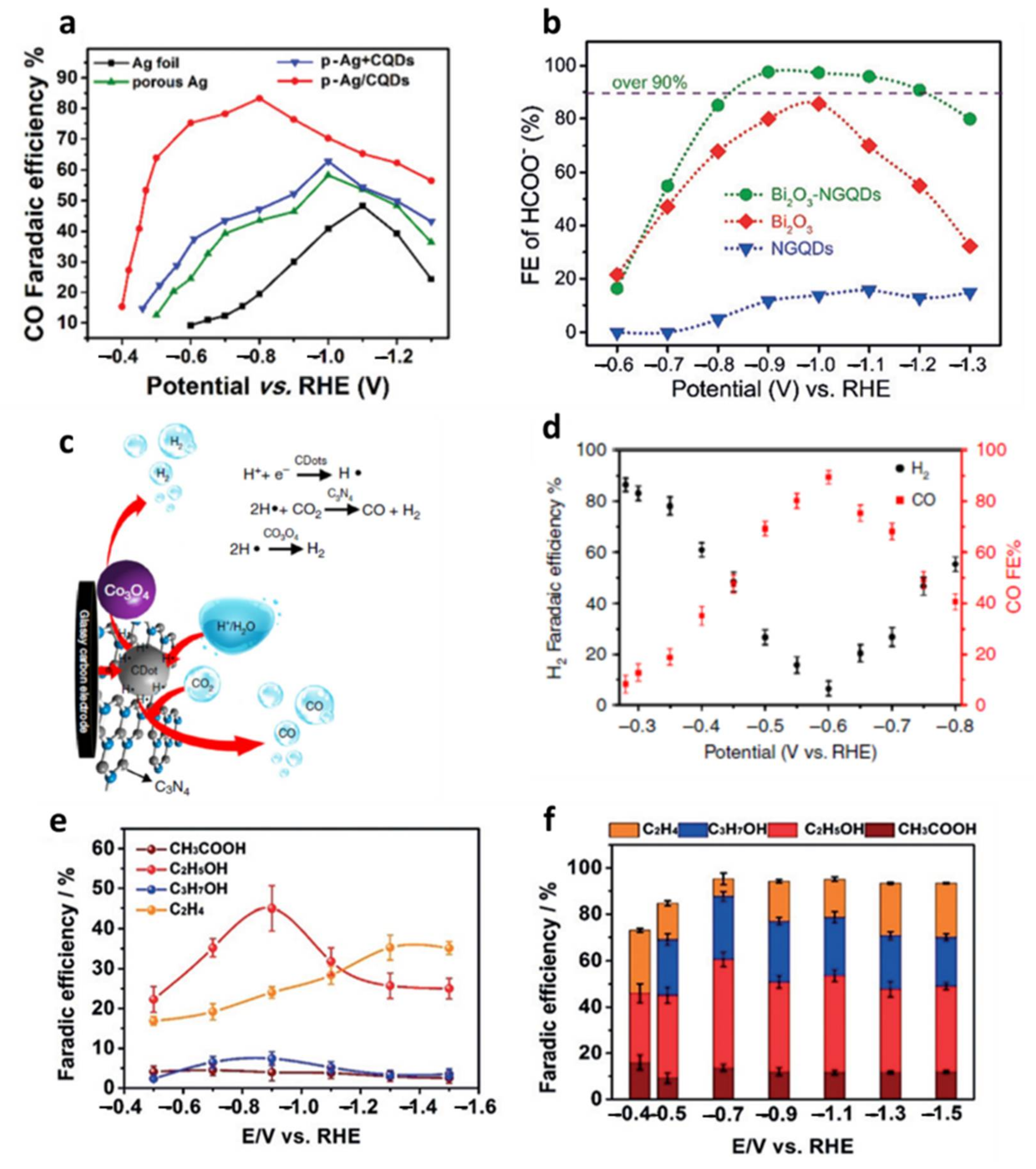
| porous Ag/CDs [106] | 0.5 M KHCO3 | CO (83.2%) | −0.8 V |
| Au NPs/N-GQDs [97] | 0.5 M KHCO3 | CO (93%) | −0.25 V |
| Bi2O3-N-GQDs [99] | 0.5 M KHCO3 | HCOO− (98.1%) | −0.9 V |
| MoS2/N-CDs [154] | EMIM-BF4 (94 mol% water) 1 | CO (90.2%) | −0.9 V |
| Co3O4-CDs-C3N4 [51] | 0.5 M KHCO3 | CO (89%) | −0.6 V |
| Au-CDs-C3N4 [50] | 0.5 M KHCO3 | CO (79.8%) | −0.5 V |
| Cu-GQDs nanocorals [52] | 0.5 M KHCO3 | HCOO− (68%) CH3OH (11%) | −0.7 V |
| N-GQDs/Cu-nr [59] | 1 M KOH | C≥2 products (80.4%) C≥2 alcohols (52.4%) | −0.9 V |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingo-Tafalla, B.; Martínez-Ferrero, E.; Franco, F.; Palomares-Gil, E. Applications of Carbon Dots for the Photocatalytic and Electrocatalytic Reduction of CO2. Molecules 2022, 27, 1081. https://doi.org/10.3390/molecules27031081
Domingo-Tafalla B, Martínez-Ferrero E, Franco F, Palomares-Gil E. Applications of Carbon Dots for the Photocatalytic and Electrocatalytic Reduction of CO2. Molecules. 2022; 27(3):1081. https://doi.org/10.3390/molecules27031081
Chicago/Turabian StyleDomingo-Tafalla, Beatriu, Eugenia Martínez-Ferrero, Federico Franco, and Emilio Palomares-Gil. 2022. "Applications of Carbon Dots for the Photocatalytic and Electrocatalytic Reduction of CO2" Molecules 27, no. 3: 1081. https://doi.org/10.3390/molecules27031081
APA StyleDomingo-Tafalla, B., Martínez-Ferrero, E., Franco, F., & Palomares-Gil, E. (2022). Applications of Carbon Dots for the Photocatalytic and Electrocatalytic Reduction of CO2. Molecules, 27(3), 1081. https://doi.org/10.3390/molecules27031081






