Construction of a Chemical Kinetic Model of Five-Component Gasoline Surrogates under Lean Conditions
Abstract
:1. Introduction
2. Construction of Chemical Kinetic Model
2.1. CHX Sub-Mechanism
2.1.1. Simplified DRGEP
2.1.2. Analysis of the Oxidation Path of CHX
2.2. Mechanism Merger and Modification
3. Results and Discussion
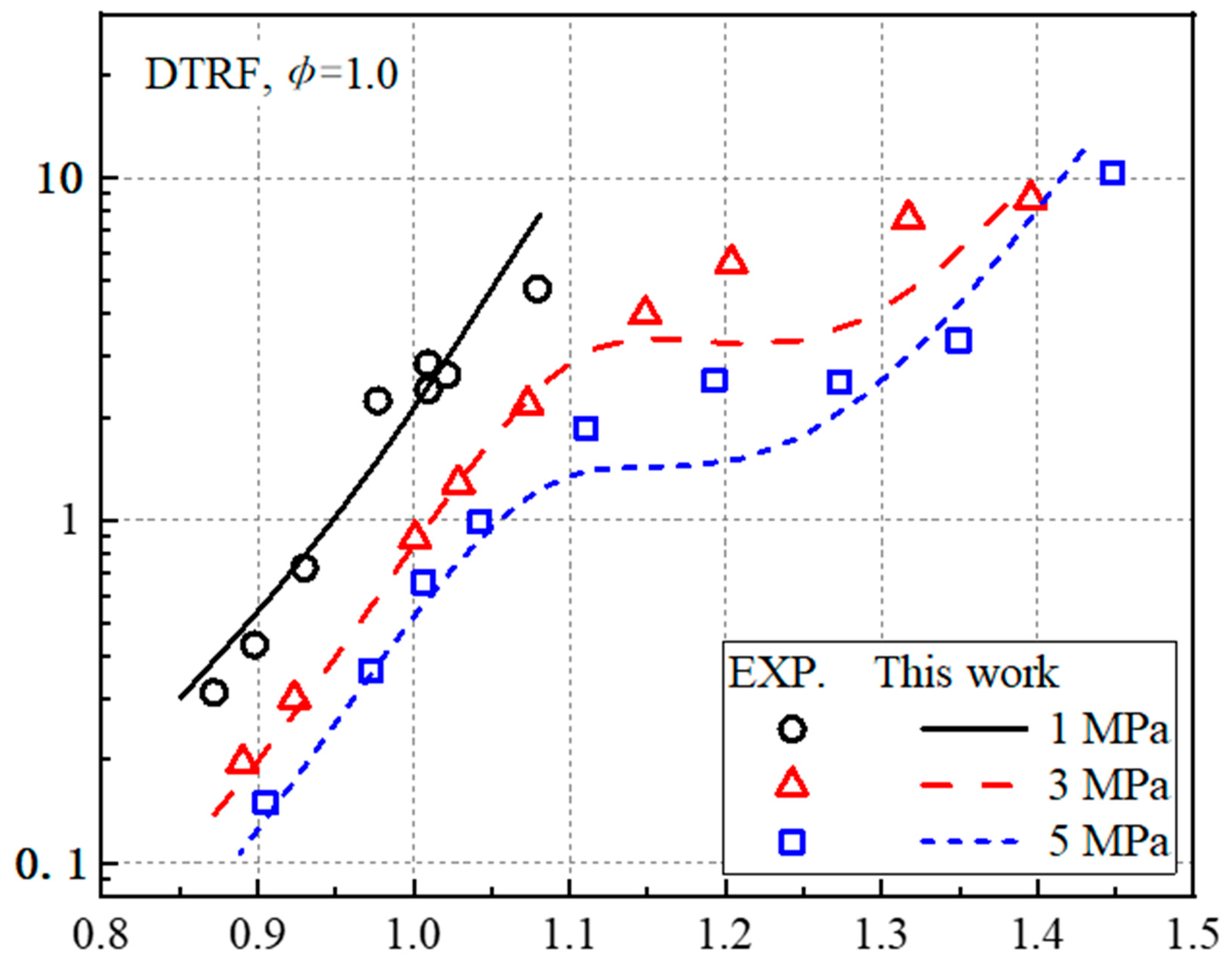
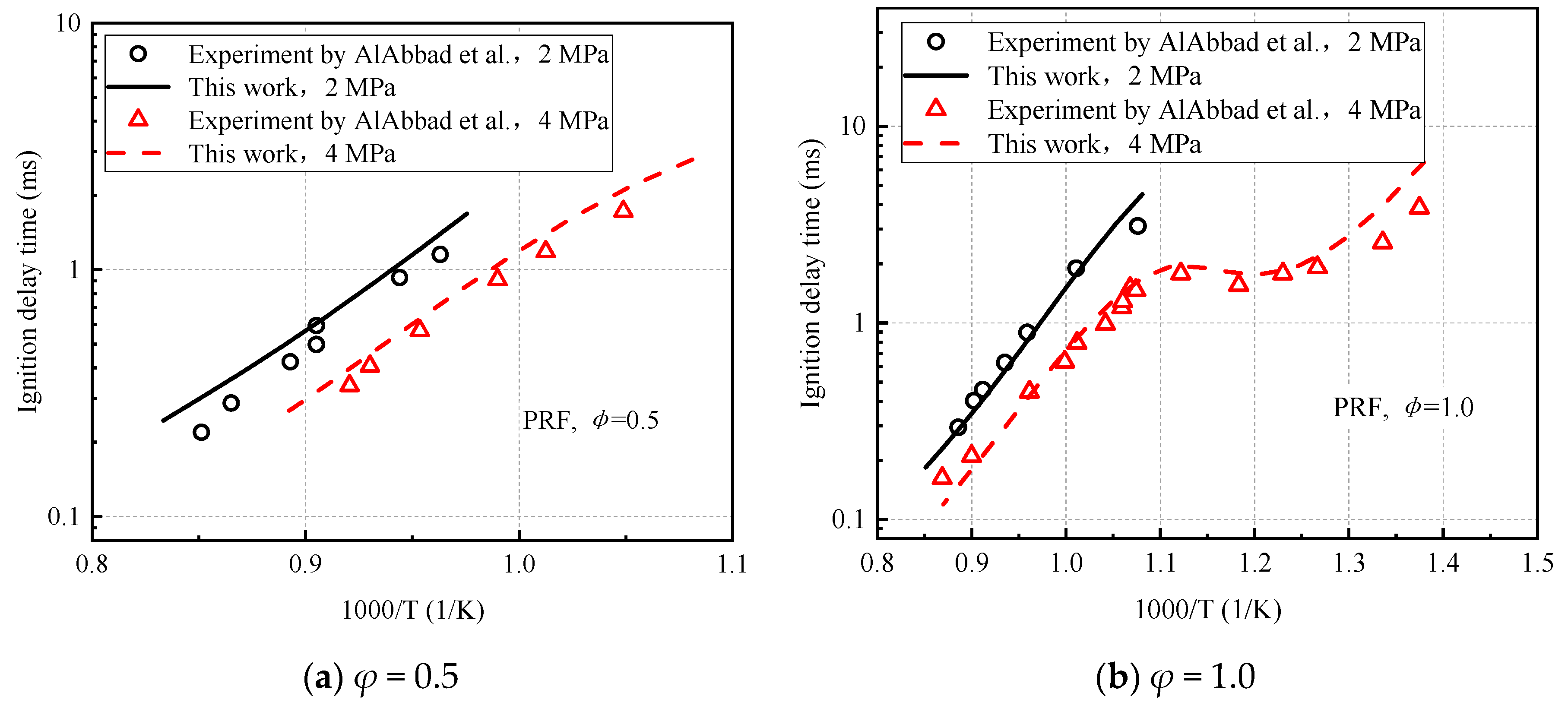
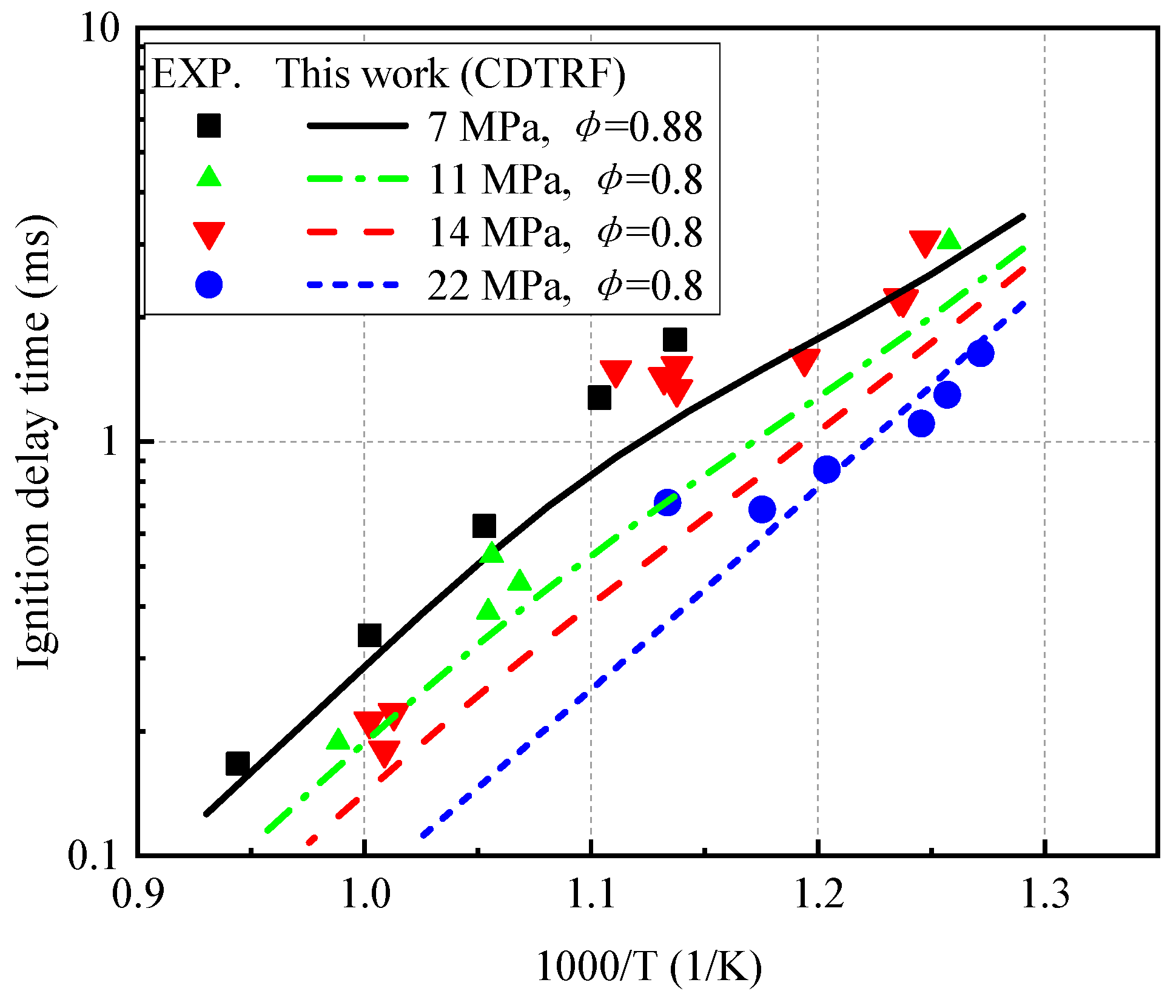
3.1. Ignition Delay Times
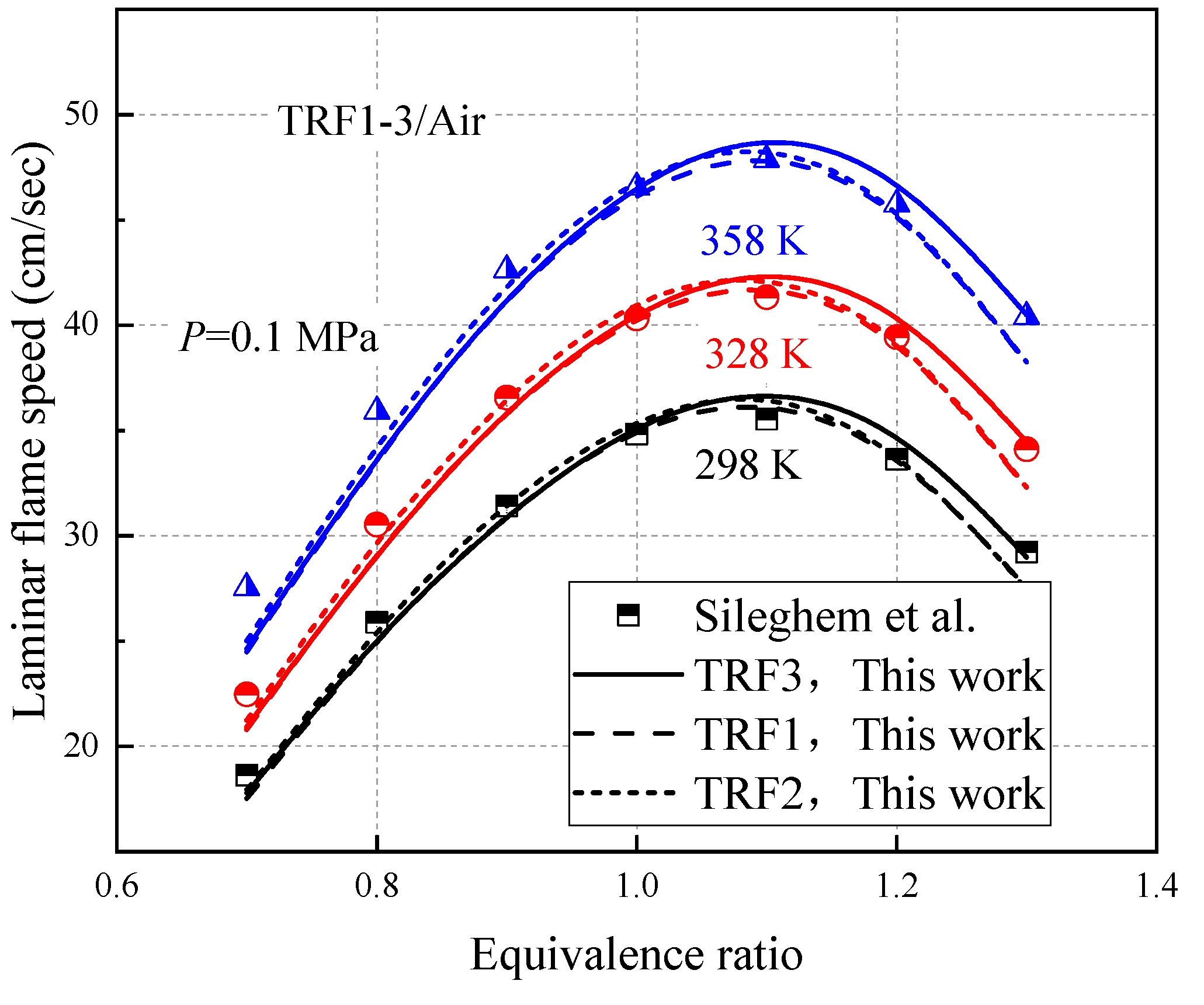
3.2. Laminar Flame Speeds
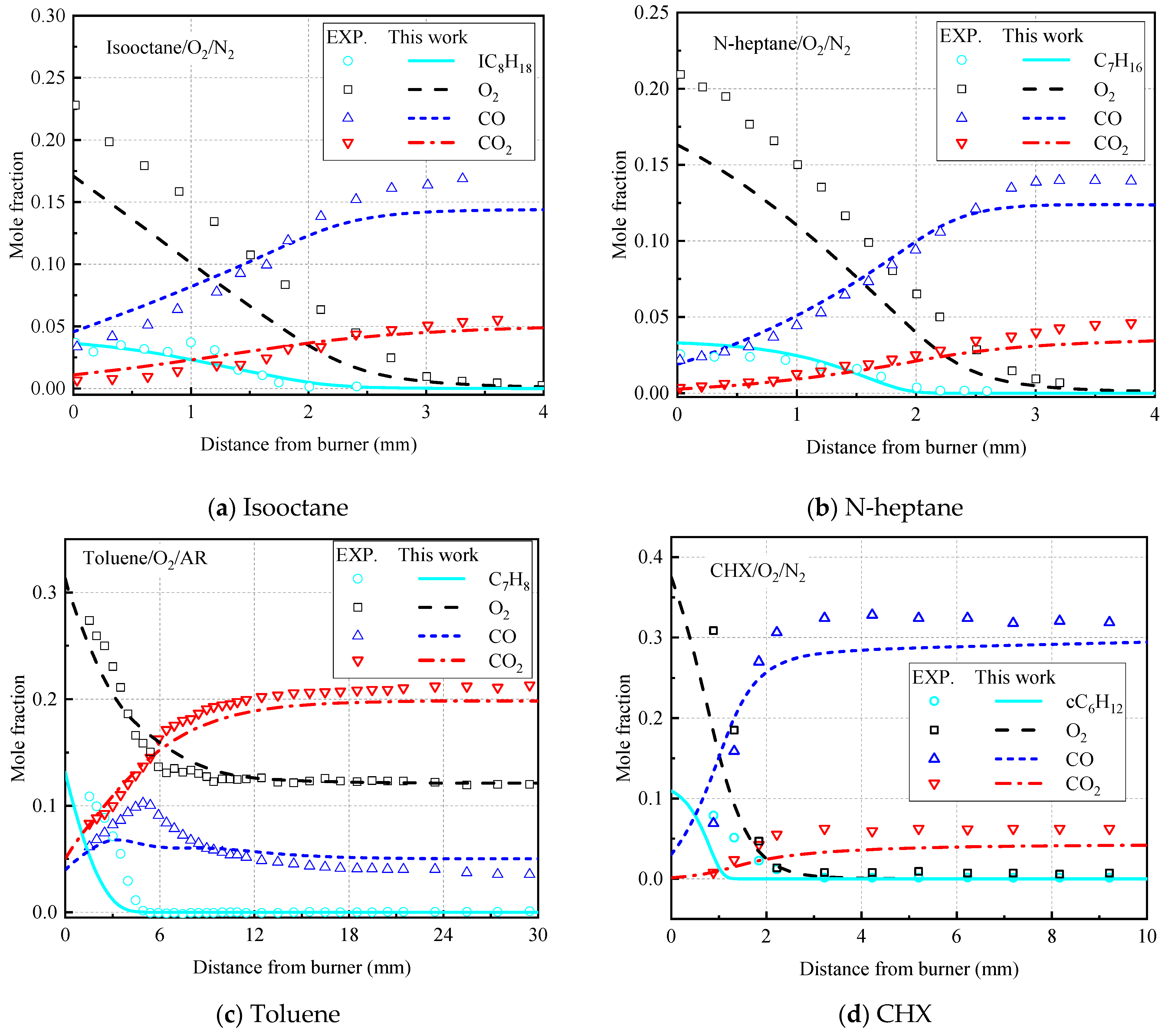
3.3. Vital Species Distributions in Premixed Flames
3.4. Validation of Real Gasoline
4. Materials and Methods
4.1. Source of Kinetic Mechanisms
4.2. Mechanism Simplification Method
4.2.1. Direct Relational Graph Considering Error Propagation
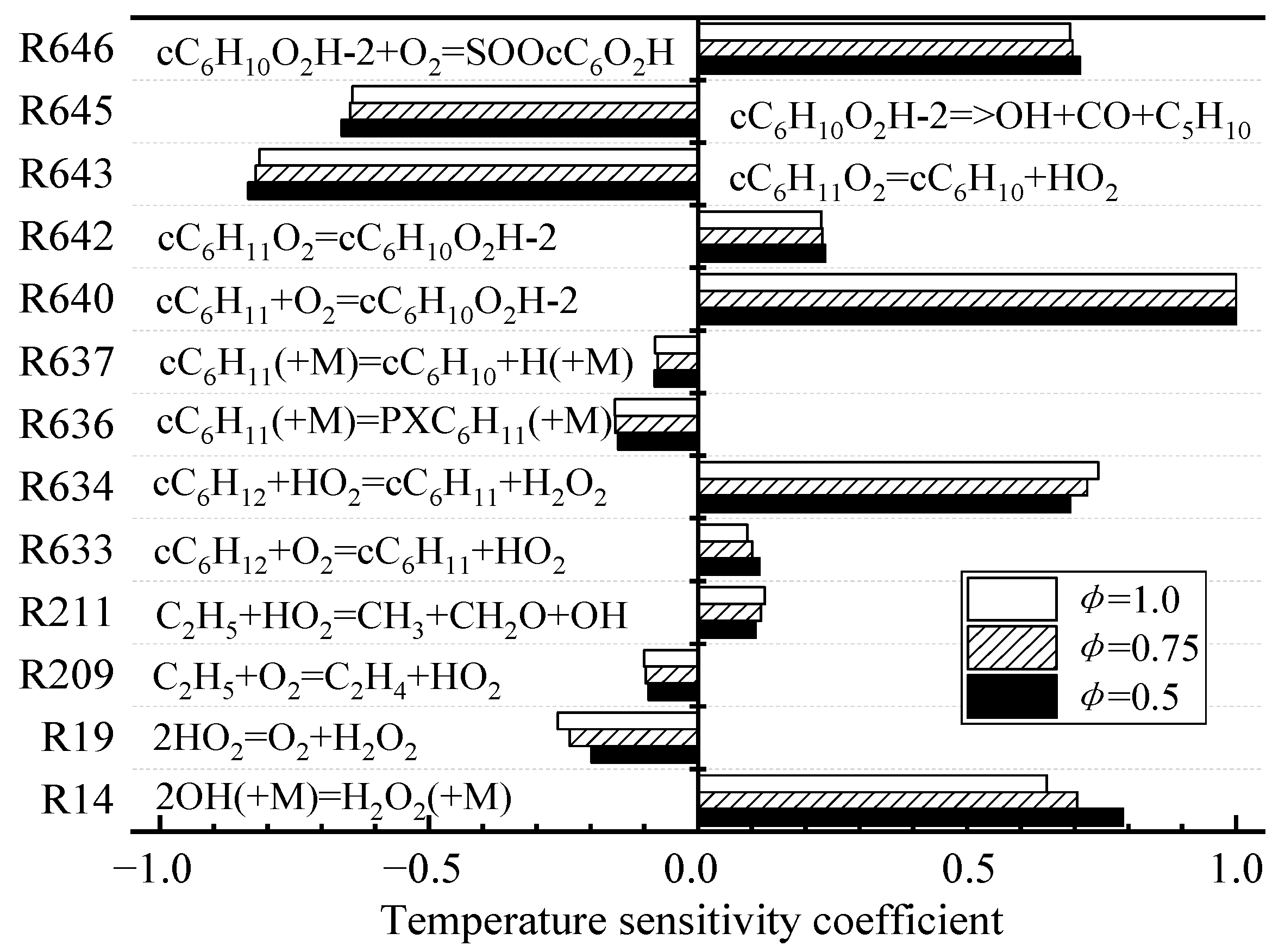
4.2.2. Rate-of-Production Analysis and Sensitivity Analysis
The simplification methods of the three mechanisms all have their own advantages. DRGEP simplification is based on the correlation between species and can greatly reduce the scale of complex mechanisms. Nevertheless, DRGEP can only simplify the detailed mechanism to the framework level. ROP can get a better simplified mechanism at the same degree of simplification, but it is easy to discard those elementary reactions that have a smaller reaction rate but have a greater impact on the system in the process of oxidation-path analysis. SA makes up for the shortcomings of ROP, and uses sensitivity coefficients to reflect the degree of influence of elementary reactions on system parameters and retrieve some of the forgotten elementary reactions.
4.3. Model Calculation Method
5. Conclusions
- (1)
- In this research, a reduced chemical kinetic model of five-component gasoline substitutes is developed to meet the prediction of gasoline ignition and combustion characteristics under new combustion methods. Based on the construction and validation of the model, the conclusions were obtained as follow: Under lean combustion conditions, the JetSurF 2.0 mechanism is simplified using DRGEP, ROP, and SA, and a CHX simplification mechanism consisting of 81 species and 280 elementary reactions is obtained, which can be accurate to describe the ignition and flame propagation characteristics of CHX.
- (2)
- The model is coupled and modified to form a five-component simplified mechanism of CDTRF consisting of 115 species and 414 elementary reactions.
- (3)
- Under a wide range of conditions, the mechanism’s ignition delay time, laminar flame speed, and the experimental and calculated results of sub-mechanism fuel, multi-component gasoline-surrogate fuel, and real gasoline are compared. The mechanism in this study can accurately reproduce the combustion and oxidation of each component of the gasoline-surrogate fuel mixture and real gasoline.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, Z.; Ye, J.; Lv, J.; Xu, W.; Tan, D.; Jiang, F.; Huang, H. Investigation on the effects of non-uniform porosity catalyst on SCR characteristic based on the field synergy analysis. J. Environ. Chem. Eng. 2022, 10, 107056. [Google Scholar] [CrossRef]
- Zhang, Z.; Ye, J.; Tan, D.; Feng, Z.; Luo, J.; Tan, Y.; Huang, Y. The effects of Fe2O3 based DOC and SCR catalyst on the combustion and emission characteristics of a diesel engine fueled with biodiesel. Fuel 2021, 290, 120039. [Google Scholar] [CrossRef]
- Tan, D.; Chen, Z.; Li, J.; Luo, J.; Yang, D.; Cui, S.; Zhang, Z. Effects of Swirl and Boiling Heat Transfer on the Performance Enhancement and Emission Reduction for a Medium Diesel Engine Fueled with Biodiesel. Processes 2021, 9, 568. [Google Scholar] [CrossRef]
- Duan, X.; Lai, M.-C.; Jansons, M.; Guo, G.; Liu, J. A review of controlling strategies of the ignition timing and combustion phase in homogeneous charge compression ignition (HCCI) engine. Fuel 2021, 285, 119142. [Google Scholar] [CrossRef]
- d’Ambrosio, S.; Iemmolo, D.; Mancarella, A.; Vitolo, R. Preliminary Optimization of the PCCI Combustion Mode in a Diesel Engine through a Design of Experiments. Energy Procedia 2016, 101, 909–916. [Google Scholar] [CrossRef] [Green Version]
- Raza, M.; Wang, H.; Yao, M. Numerical investigation of reactivity controlled compression ignition (RCCI) using different multi-component surrogate combinations of diesel and gasoline. Appl. Energy 2019, 242, 462–479. [Google Scholar] [CrossRef]
- Tan, J.Y.; Bonatesta, F.; Ng, H.K.; Gan, S. Developments in computational fluid dynamics modelling of gasoline direct injection engine combustion and soot emission with chemical kinetic modelling. Appl. Therm. Eng. 2016, 107, 936–959. [Google Scholar] [CrossRef] [Green Version]
- Atef, N.; Kukkadapu, G.; Mohamed, S.Y.; Rashidi, M.A.; Banyon, C.; Mehl, M.; Heufer, K.A.; Nasir, E.F.; Alfazazi, A.; Das, A.K.; et al. A comprehensive iso-octane combustion model with improved thermochemistry and chemical kinetics. Combust. Flame 2017, 178, 111–134. [Google Scholar] [CrossRef] [Green Version]
- AlAbbad, M.; Badra, J.; Djebbi, K.; Farooq, A. Ignition delay measurements of a low-octane gasoline blend, designed for gasoline compression ignition (GCI) engines. Proc. Combust. Inst. 2019, 37, 171–178. [Google Scholar] [CrossRef]
- Zheng, Z.L.; Liang, Z.L. R educed Chemical Kinetic Model of a Gasoline Surrogate Fuel for HCCI Combustion. Acta Phys.-Chim. Sin. 2015, 31, 1265–1274. (In Chinese) [Google Scholar] [CrossRef]
- Liu, Y.D.; Jia, M.; Xie, M.Z.; Pang, B. Development of a New Skeletal Chemical Kinetic Model of Toluene Reference Fuel with Application to Gasoline Surrogate Fuels for Computational Fluid Dynamics Engine Simulation. Energy Fuels 2013, 27, 4899–4909. [Google Scholar] [CrossRef]
- Mannaa, O.; Mansour, M.S.; Roberts, W.L.; Chung, S.H. Laminar burning velocities at elevated pressures for gasoline and gasoline surrogates associated with RON. Combust. Flame 2015, 162, 2311–2321. [Google Scholar] [CrossRef]
- Andrae, J.C.G.; Kovács, T. Evaluation of Adding an Olefin to Mixtures of Primary Reference Fuels and Toluene To Model the Oxidation of a Fully Blended Gasoline. Energy Fuels 2016, 30, 7721–7730. [Google Scholar] [CrossRef]
- Park, S.; Wang, Y.; Chung, S.H.; Sarathy, S.M. Compositional effects on PAH and soot formation in counterflow diffusion flames of gasoline surrogate fuels. Combust. Flame 2017, 178, 46–60. [Google Scholar] [CrossRef] [Green Version]
- Fikri, M.; Herzler, J.; Starke, R.; Schulz, C.; Roth, P.; Kalghatgi, G.T. Autoignition of gasoline surrogates mixtures at intermediate temperatures and high pressures. Combust. Flame 2008, 152, 276–281. [Google Scholar] [CrossRef]
- Andrae, J.C.G. Development of a detailed kinetic model for gasoline surrogate fuels. Fuel 2008, 87, 2013–2022. [Google Scholar] [CrossRef]
- Zhu, Z.; Zheng, Z.; Kong, J. Construction of reduced mechanism and prediction of the RON of toluene primary reference fuel/ethanol/diisobutylene. Renew. Energy 2021, 172, 862–881. [Google Scholar] [CrossRef]
- Andrae, J.C.G. Kinetic Modeling of the Influence of Cyclohexane on the Homogeneous Ignition of a Gasoline Surrogate Fuel. Energy Fuels 2018, 32, 3975–3984. [Google Scholar] [CrossRef]
- vom Lehn, F.; Cai, L.; Pitsch, H. Sensitivity analysis, uncertainty quantification, and optimization for thermochemical properties in chemical kinetic combustion models. Proc. Combust. Inst. 2019, 37, 771–779. [Google Scholar] [CrossRef]
- Chu, H.; Ya, Y.; Nie, X.; Qiao, F.; Jiaqiang, E. Effects of adding cyclohexane, n-hexane, ethanol, and 2,5-dimethylfuran to fuel on soot formation in laminar coflow n-heptane/iso-octane diffusion flame. Combust. Flame 2021, 225, 120–135. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Y.; Wu, Z.; Wang, S.; Lu, X.; Huang, Z. Ignition delay of diisobutylene-containing multicomponent gasoline surrogates: Shock tube measurements and modeling study. Fuel 2019, 235, 1387–1399. [Google Scholar] [CrossRef]
- Ren, S.; Kokjohn, S.L.; Wang, Z.; Liu, H.; Wang, B.; Wang, J. A multi-component wide distillation fuel (covering gasoline, jet fuel and diesel fuel) mechanism for combustion and PAH prediction. Fuel 2017, 208, 447–468. [Google Scholar] [CrossRef]
- Voisin, D.; Marchal, A.; Reuillon, M.; Boettner, J.C.; Cathonnet, M. Experimental and Kinetic Modeling Study of Cyclohexane Oxidation in a JSR at High Pressure. Combust. Sci. Technol. 2007, 138, 137–158. [Google Scholar] [CrossRef]
- El-Bakali, A.; Braun-Unkhoff, M.; Dagaut, P.; Frank, P.; Cathonnet, M. Detailed kinetic reaction mechanism for cyclohexane oxidation at pressure up to ten atmospheres. Proc. Combust. Inst. 2000, 28, 1631–1638. [Google Scholar] [CrossRef]
- Buda, F.; Heyberger, B.; Fournet, R.; Glaude, P.; Warth, V.; Battin-Leclerc, F. Modeling of the gas-phase oxidation of cyclohexane. Energy Fuels 2006, 20, 1450–1459. [Google Scholar] [CrossRef]
- Wang, H.; Dames, E.; Sirjean, B.; Sheen, D.A.; Tango, R.; Violi, A.; Lai, J.Y.W.; Egolfopoulos, F.N.; Davidson, D.F.; Hanson, R.K.; et al. A High-Temperature Chemical Kinetic Model of N-Alkane (Up to N-Dodecane), Cyclohexane, and Methyl-, Ethyl-, N-Propyl and N-Butyl-Cyclohexane Oxidation at High Temperatures, JetSurF Version 2.0. 19 September 2010. Available online: http://web.stanford.edu/group/haiwanglab/JetSurF/JetSurF2.0/index.html (accessed on 16 March 2021).
- Zou, J.; Jin, H.; Liu, D.; Zhang, X.; Su, H.; Yang, J.; Farooq, A.; Li, Y. A comprehensive study on low-temperature oxidation chemistry of cyclohexane. II. Experimental and kinetic modeling investigation. Combust. Flame 2022, 235, 111500. [Google Scholar] [CrossRef]
- He, Z.; Zhao, W.; Liu, G.; Qian, Y.; Lu, X. Effects of short chain aromatics in gasoline on GDI engine combustion and emissions. Fuel 2021, 297, 120725. [Google Scholar] [CrossRef]
- He, Z.; Zhang, L.; Liu, G.; Qian, Y.; Lu, X. Evaluating the effects of olefin components in gasoline on GDI engine combustion and emissions. Fuel 2021, 291, 120131. [Google Scholar] [CrossRef]
- Zheng, D.; Zhong, B.-J.; Xiong, P.-F. Experimental study on laminar flame speeds and chemical kinetic model of 2,4,4-trimethyl-1-pentene. Fuel 2018, 229, 95–104. [Google Scholar] [CrossRef]
- Sileghem, L.; Alekseev, V.A.; Vancoillie, J.; Van Geem, K.M.; Nilsson, E.J.K.; Verhelst, S.; Konnov, A.A. Laminar burning velocity of gasoline and the gasoline surrogate components iso-octane, n-heptane and toluene. Fuel 2013, 112, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Fieweger, K.; Blumenthal, R.; Adomeit, G. Self-ignition of S.I. engine model fuels: A shock tube investigation at high pressure. Combust. Flame 1997, 109, 599–619. [Google Scholar] [CrossRef]
- Ciezki, H.; Adomeit, G. Shock-tube investigation of self-ignition of n-heptane-air mixtures under engine relevant conditions. Combust. Flame 1993, 93, 421–433. [Google Scholar] [CrossRef]
- Shen, H.-P.S.; Vanderover, J.; Oehlschlaeger, M.A. A shock tube study of the auto-ignition of toluene/air mixtures at high pressures. Proc. Combust. Inst. 2009, 32, 165–172. [Google Scholar] [CrossRef]
- Metcalfe, W.K.; Pitz, W.J.; Curran, H.J.; Simmie, J.M.; Westbrook, C.K. The development of a detailed chemical kinetic mechanism for diisobutylene and comparison to shock tube ignition times. Proc. Combust. Inst. 2007, 31, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, O.; Ribaucour, M.; Carlier, M.; Minetti, R. The production of benzene in the low-temperature oxidation of cyclohexane, cyclohexene, and cyclohexa-1,3-diene. Combust. Flame 2001, 127, 1971–1980. [Google Scholar] [CrossRef]
- Daley, S.M.; Berkowitz, A.M.; Oehlschlaeger, M.A. A shock tube study of cyclopentane and cyclohexane ignition at elevated pressures. Int. J. Chem. Kinet. 2008, 40, 624–634. [Google Scholar] [CrossRef]
- van Lipzig, J.P.J.; Nilsson, E.J.K.; de Goey, L.P.H.; Konnov, A.A. Laminar burning velocities of n-heptane, iso-octane, ethanol and their binary and tertiary mixtures. Fuel 2011, 90, 2773–2781. [Google Scholar] [CrossRef]
- Zhong, B.-J.; Peng, H.-S.; Zheng, D. The effect of different class of hydrocarbons on laminar flame speeds of three C7 fuels. Fuel 2018, 225, 225–229. [Google Scholar] [CrossRef]
- Davis, S.G.; Wang, H.; Breinsky, K.; Law, C.K. Laminar flame speeds and oxidation kinetics of benene-air and toluene-air flames. Symp. Combust. 1996, 26, 1025–1033. [Google Scholar] [CrossRef]
- Johnston, R.J.; Farrell, J.T. Laminar burning velocities and Markstein lengths of aromatics at elevated temperature and pressure. Proc. Combust. Inst. 2005, 30, 217–224. [Google Scholar] [CrossRef]
- Wu, F.; Kelley, A.P.; Law, C.K. Laminar flame speeds of cyclohexane and mono-alkylated cyclohexanes at elevated pressures. Combust. Flame 2012, 159, 1417–1425. [Google Scholar] [CrossRef]
- Gauthier, B.M.; Davidson, D.F.; Hanson, R.K. Shock tube determination of ignition delay times in full-blend and surrogate fuel mixtures. Combust. Flame 2004, 139, 300–311. [Google Scholar] [CrossRef]
- El-Bakali, A.; Delfau, J.L.; Vovelle, C. Experimental Study of 1 Atmosphere, Rich, Premixedn-heptane and iso-octane Flames. Combust. Sci. Technol. 1998, 140, 69–91. [Google Scholar] [CrossRef]
- Li, Y.; Cai, J.; Zhang, L.; Yuan, T.; Zhang, K.; Qi, F. Investigation on chemical structures of premixed toluene flames at low pressure. Proc. Combust. Inst. 2011, 33, 593–600. [Google Scholar] [CrossRef]
- Ciajolo, A.; Tregrossi, A.; Mallardo, M.; Faravelli, T.; Ranzi, E. Experimental and kinetic modeling study of sooting atmospheric-pressure cyclohexane flame. Proc. Combust. Inst. 2009, 32, 585–591. [Google Scholar] [CrossRef]
- Kukkadapu, G.; Kumar, K.; Sung, C.-J.; Mehl, M.; Pitz, W.J. Experimental and surrogate modeling study of gasoline ignition in a rapid compression machine. Combust. Flame 2012, 159, 3066–3078. [Google Scholar] [CrossRef]
- Zheng, D.; Zeng, D.; Zeng, Q. Characteristics and Kinetic Analysis of Ignition for Different Gasoline Surrogate Fuel Models. J. Energy Resour. Technol. 2020, 142, 082302. [Google Scholar] [CrossRef]
- Davidson, D.F.; Shao, J.K.; Choudhary, R.; Mehl, M.; Obrecht, N.; Hanson, R.K. Ignition delay time measurements and modeling for gasoline at very high pressures. Proc. Combust. Inst. 2019, 37, 4885–4892. [Google Scholar] [CrossRef]
- Zhao, Z.; Conley, J.; Kazakov, A.; Dryer, F. Burning Velocities of Real Gasoline Fuel at 353 K and 500 K. J. Fuels Lubr. 2003, 112, 2624–2629. Available online: https://www.jstor.org/stable/44742478 (accessed on 23 August 2021).
- Tian, G.; Daniel, R.; Li, H.; Xu, H.; Shuai, S.; Richards, P. Laminar Burning Velocities of 2,5-Dimethylfuran Compared with Ethanol and Gasoline. Energy Fuels 2010, 24, 3898–3905. [Google Scholar] [CrossRef]
- Andrae, J.; Johansson, D.; Björnbom, P.; Risberg, P.; Kalghatgi, G. Co-oxidation in the auto-ignition of primary reference fuels and n-heptane/toluene blends. Combust. Flame 2005, 140, 267–286. [Google Scholar] [CrossRef]
- Machrafi, H.; Cavadias, S.; Amouroux, J. The development and experimental validation of a reduced ternary kinetic mechanism for the auto-ignition at HCCI conditions, proposing a global reaction path for ternary gasoline surrogates. Fuel Processing Technol. 2009, 90, 247–263. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Law, C.K. A directed relation graph method for mechanism reduction. Proc. Combust. Inst. 2005, 30, 1333–1341. [Google Scholar] [CrossRef]
- Pepiot-Desjardins, P.; Pitsch, H. An efficient error-propagation-based reduction method for large chemical kinetic mechanisms. Combust. Flame 2008, 154, 67–81. [Google Scholar] [CrossRef]
- Yang, C.; Zheng, Z. Chemical Kinetic Model of Multicomponent Gasoline Surrogate Fuel with Nitric Oxide in HCCI Combustion. Molecules 2020, 25, 2273. [Google Scholar] [CrossRef]
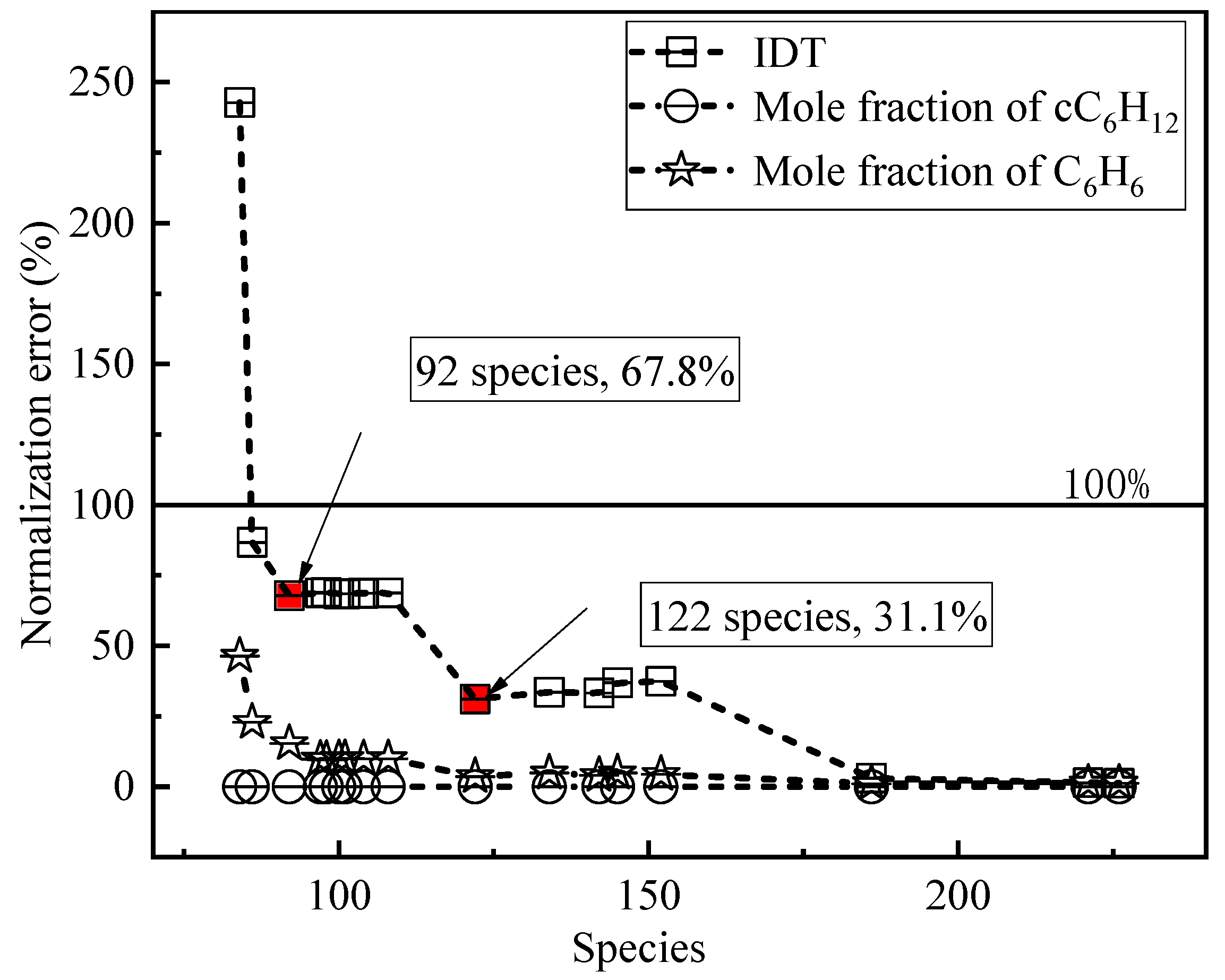
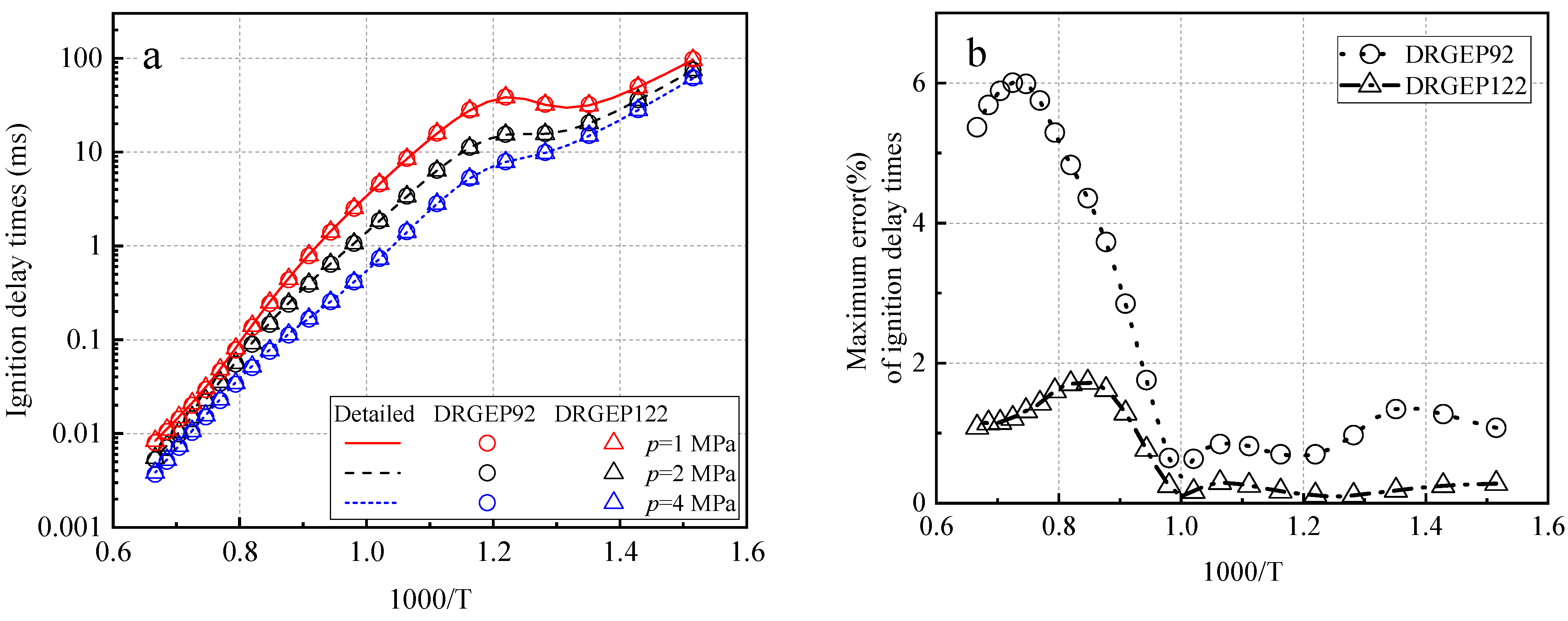
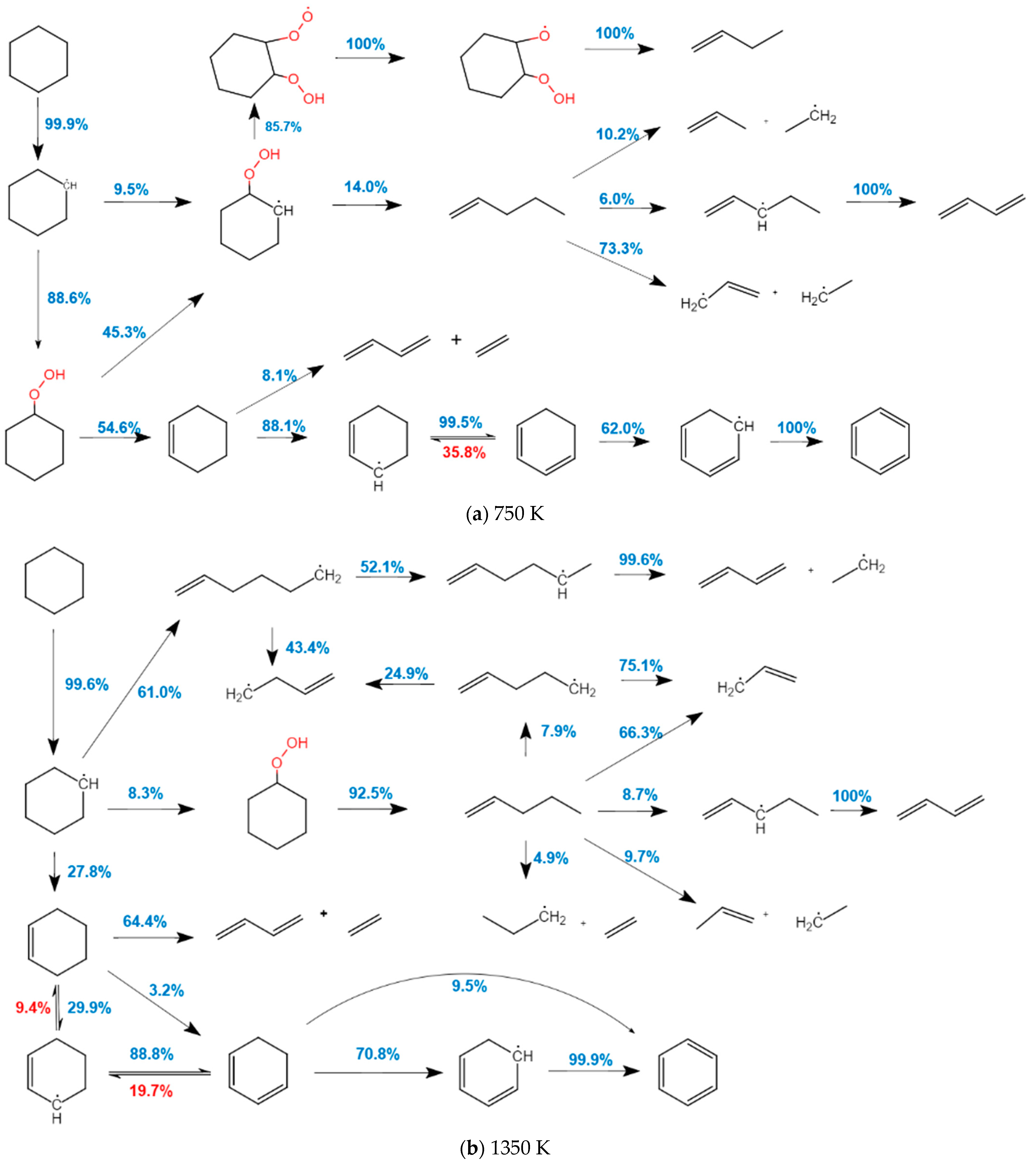
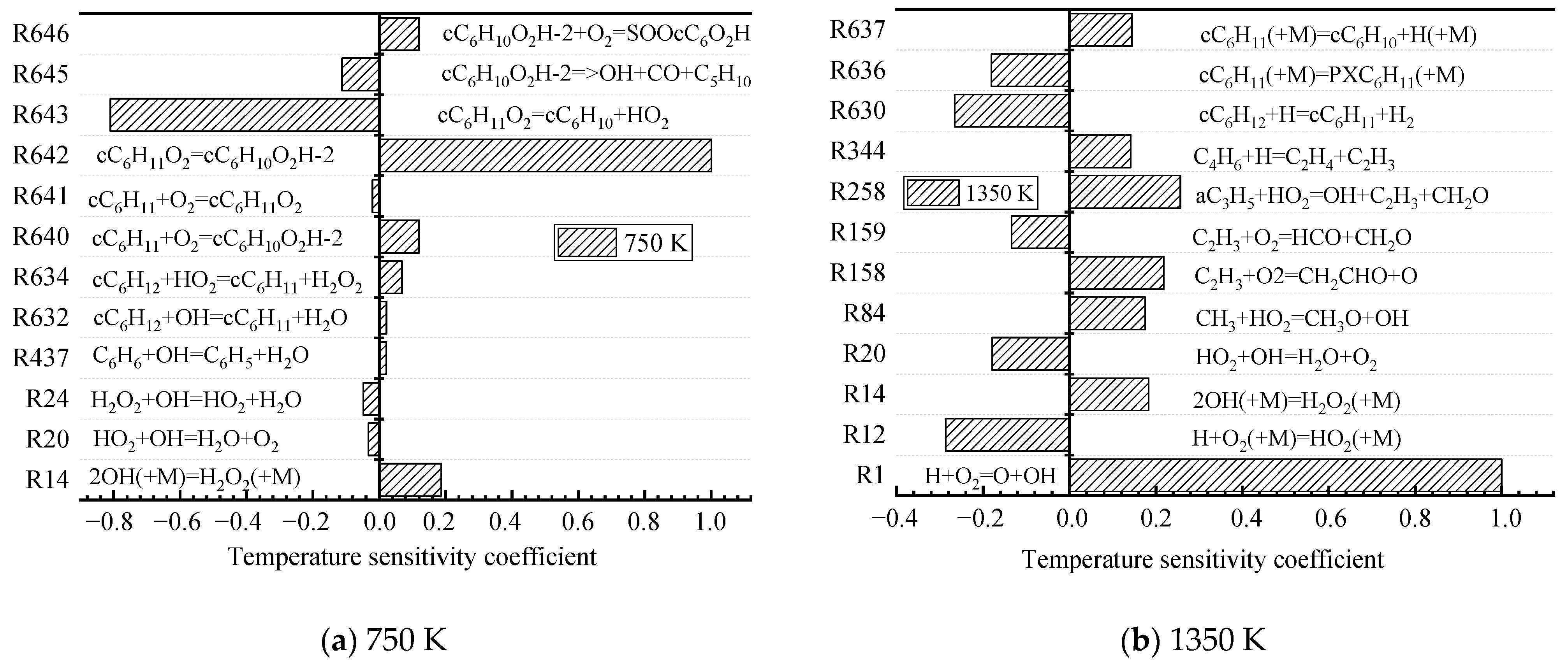






| Target Parameters | Absolute Tolerance | Relative Tolerance |
|---|---|---|
| Mole fraction of cC6H12 | 1 × 10−4 | 20 |
| Mole fraction of C6H6 | 1 × 10−4 | 20 |
| IDT | 1 × 10−6 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Zheng, Z. Construction of a Chemical Kinetic Model of Five-Component Gasoline Surrogates under Lean Conditions. Molecules 2022, 27, 1080. https://doi.org/10.3390/molecules27031080
Yang C, Zheng Z. Construction of a Chemical Kinetic Model of Five-Component Gasoline Surrogates under Lean Conditions. Molecules. 2022; 27(3):1080. https://doi.org/10.3390/molecules27031080
Chicago/Turabian StyleYang, Chao, and Zhaolei Zheng. 2022. "Construction of a Chemical Kinetic Model of Five-Component Gasoline Surrogates under Lean Conditions" Molecules 27, no. 3: 1080. https://doi.org/10.3390/molecules27031080
APA StyleYang, C., & Zheng, Z. (2022). Construction of a Chemical Kinetic Model of Five-Component Gasoline Surrogates under Lean Conditions. Molecules, 27(3), 1080. https://doi.org/10.3390/molecules27031080





