Nanoparticles of Costus speciosus Ameliorate Diabetes-Induced Structural Changes in Rat Prostate through Mediating the Pro-Inflammatory Cytokines IL 6, IL1β and TNF-α
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of C. speciosus Extract (CSE)
2.2. Preparation of C. speciosus Extract-Loaded Nanostructured Lipid Carriers (CSE-NLCs)
2.3. Characterization of CSE-NLCs
2.3.1. Particle Size and Zeta-Potential Measurements
2.3.2. Encapsulation Efficiency (EE) and Loading Capacity (DL)
2.3.3. The Particle Morphology Using Scanning Electron Microscopy
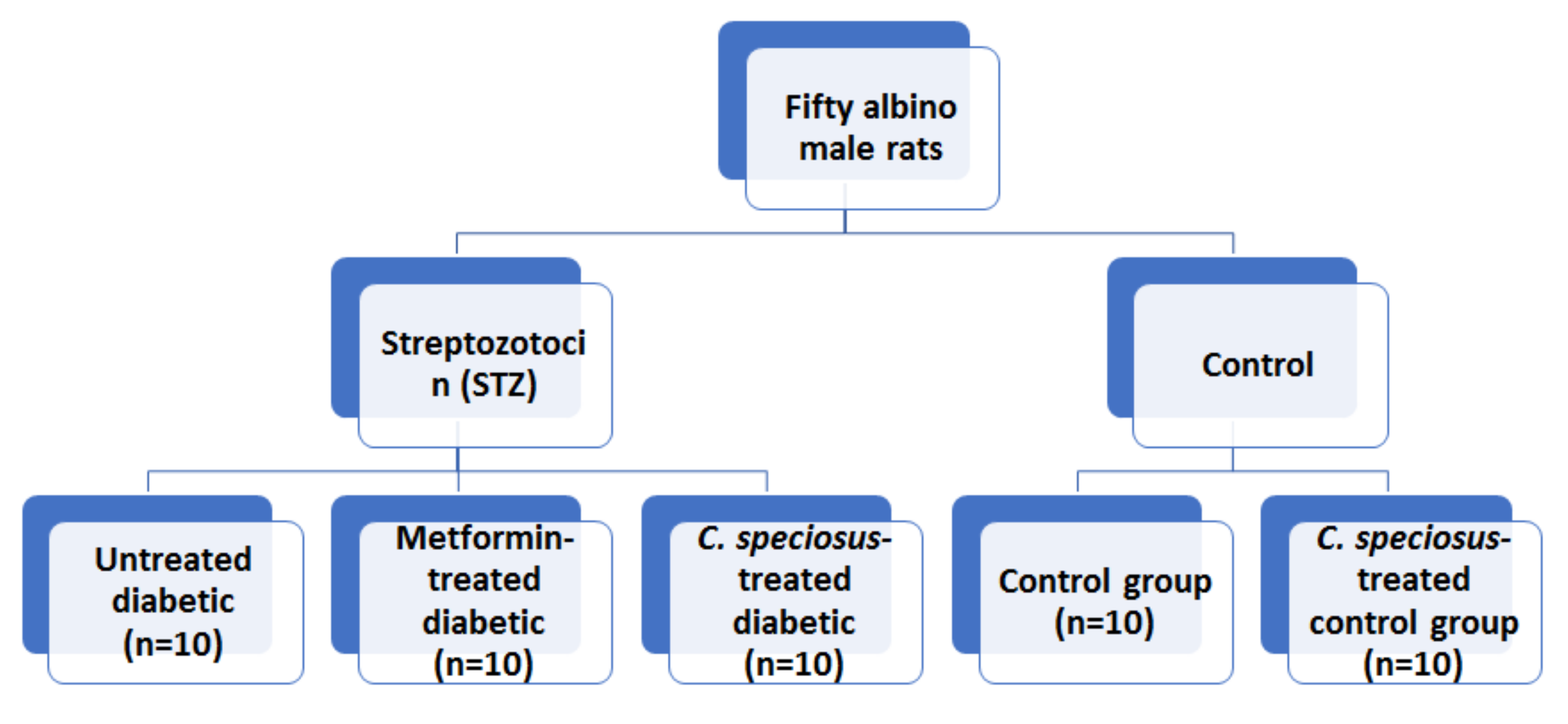
2.4. Animals and Experimental Design
2.5. Biochemical Assessment
2.6. Assessment of Gene Expression Using Quantitative Real-Time Polymerase Reaction (qRT-PCR)
2.7. Histopathological Assessment
2.8. Statistical Analysis
3. Results
3.1. Preparation and Characterization of CSE-NLCs
3.2. Effect of C. speciosus on Body and Prostate Weight
3.3. Effect of C. speciosus on BGL and Insulin
3.4. Effect of C. speciosus on Oxidants/Antioxidants Profile
3.5. Effect of C. speciosus on Testosterone Level
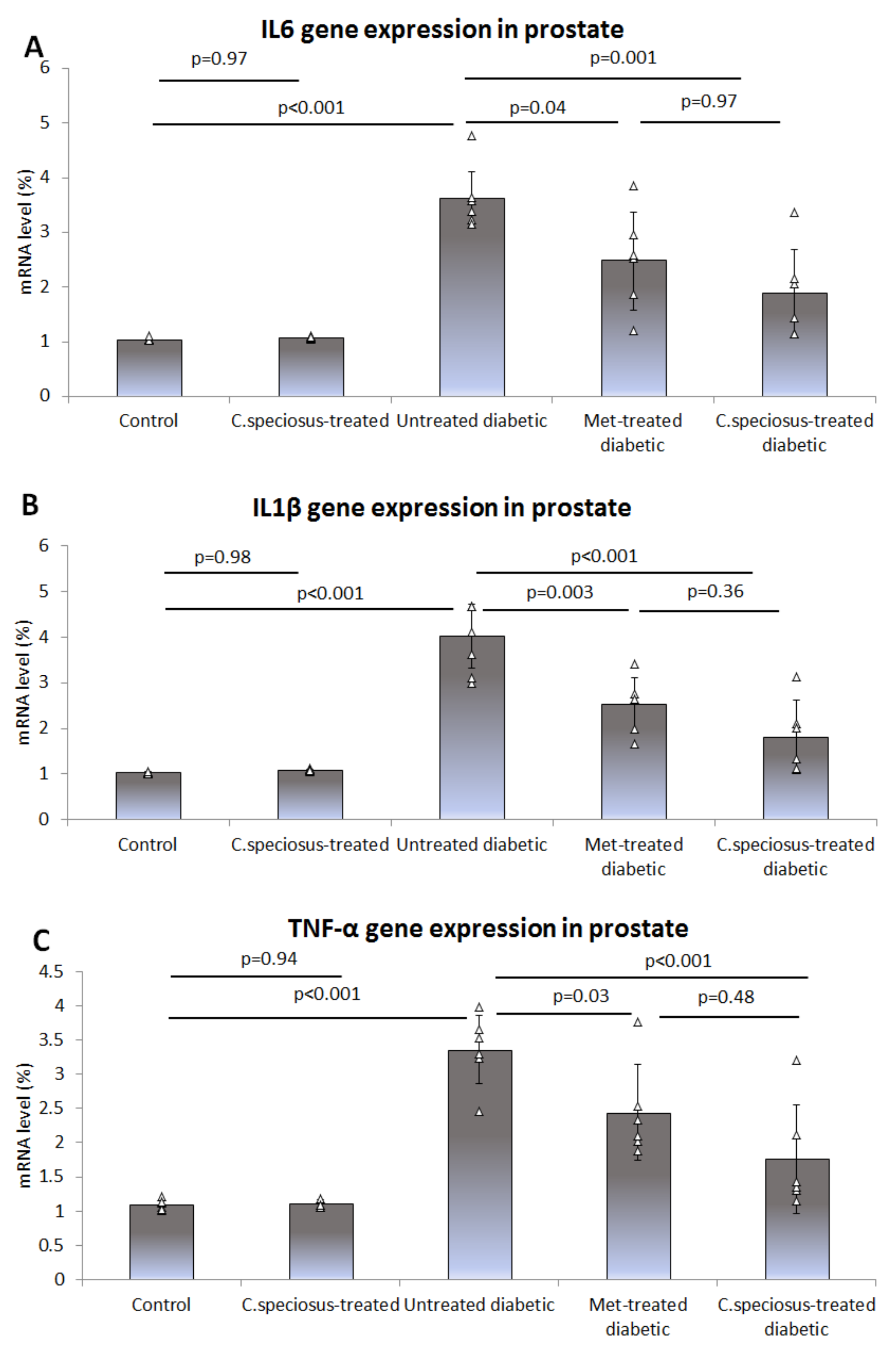
3.6. Effect of C. speciosus on Gene Expression of Pro-Inflammatory Cytokines
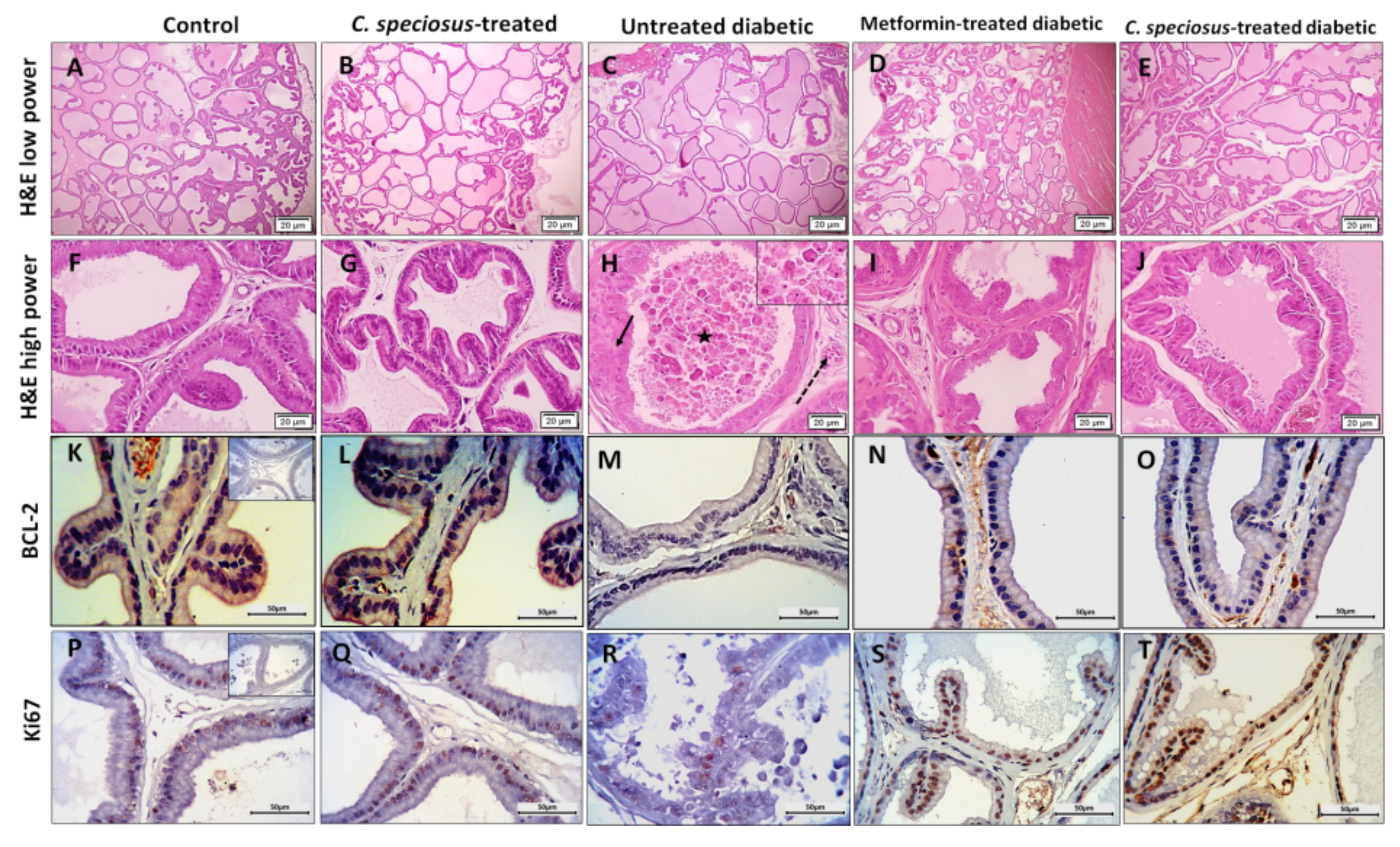
3.7. Effect of C. speciosus on Histopathological Structure of the Ventral Prostate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, M.M. Gestational diabetes in the Arab gulf countries: Sitting on a land-mine. Int. J. Environ. Res. Public Health 2020, 17, 9270. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.G.; Costacou, T.; Orchard, T.J. Risk factor modeling for cardiovascular disease in type 1 diabetes in the pittsburgh epidemiology of diabetes complications (EDC) study: A comparison with the diabetes control and complications trial/epidemiology of diabetes interventions and complications study (DCCT/EDIC). Diabetes 2019, 68, 409–419. [Google Scholar]
- Rato, L.; Oliveira, P.F.; Sousa, M.; Silva, B.M.; Alves, M.G. Role of reactive oxygen species in diabetes-induced male reproductive dysfunction. In Oxidants, Antioxidants and Impact of the Oxidative Status in Male Reproduction; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–147. [Google Scholar]
- Bais, N.; Choudhary, G. Recent updates on natural compounds in treatment of diabetes mellitus: A comprehensive Approach. J. Drug Deliv. Ther. 2019, 9, 1019–1024. [Google Scholar]
- Bansal, D.; Bhansali, A.; Kapil, G.; Undela, K.; Tiwari, P. Type 2 diabetes and risk of prostate cancer: A meta-analysis of observational studies. Prostate Cancer Prostatic Dis. 2013, 16, 151–158. [Google Scholar] [CrossRef]
- Gavillán-Suárez, J.; Aguilar-Perez, A.; Rivera-Ortiz, N.; Rodríguez-Tirado, K.; Figueroa-Cuilan, W.; Morales-Santiago, L.; Maldonado-Martínez, G.; Cubano, L.A.; Martínez-Montemayor, M.M. Chemical profile and in vivo hypoglycemic effects of Syzygium jambos, Costus speciosus and Tapeinochilos ananassae plant extracts used as diabetes adjuvants in Puerto Rico. BMC Complement. Altern. Med. 2015, 15, 244. [Google Scholar] [CrossRef] [Green Version]
- Maji, P.; Ghosh Dhar, D.; Misra, P.; Dhar, P. Costus speciosus (Koen ex. Retz.) Sm.: Current status and future industrial prospects. Ind. Crop. Prod. 2020, 152, 112571. [Google Scholar] [CrossRef]
- Ahmad Emami, S.; Sahebkar, A.; Javadi, B. Paresthesia: A review of its definition, etiology and treatments in view of the traditional medicine. Curr. Pharm. Des. 2016, 22, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Huguet-Casquero, A.; Moreno-Sastre, M.; López-Méndez, T.B.; Gainza, E.; Pedraz, J.L. Encapsulation of oleuropein in nanostructured lipid carriers: Biocompatibility and antioxidant efficacy in lung epithelial cells. Pharmaceutics 2020, 12, 429. [Google Scholar] [CrossRef]
- Chahardoli, A.; Karimi, N.; Sadeghi, F.; Fattahi, A. Green approach for synthesis of gold nanoparticles from Nigella arvensis leaf extract and evaluation of their antibacterial, antioxidant, cytotoxicity and catalytic activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, A.; Costa, B.; Ferreira, M.V.; Miguéis, D.; Louros, J.M.S.; Durazzo, A.; Lucarini, M.; Eder, P.; Chaud, M.V.; Morsink, M.; et al. Nanotoxicology and nanosafety: Safety-by-design and testing at a glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. [Google Scholar] [CrossRef]
- Zhu, W.; Wei, Z.; Han, C.; Weng, X. Nanomaterials as promising theranostic tools in nanomedicine and their applications in clinical disease diagnosis and treatment. Nanomaterials 2021, 11, 3346. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.A.; Almaghrabi, O.A.; Afifi, M.E. Molecular mechanisms of anti-hyperglycemic effects of Costus speciosus extract in streptozotocin-induced diabetic rats. Saudi Med. J. 2014, 35, 1501–1506. [Google Scholar]
- Alamoudi, E.F.; Khalil, W.K.; Ghaly, I.S.; Hassan, N.H.; Ahmed, E.S. Nanoparticles from of Costus speciosus extract improves the antidiabetic and antilipidemic effects against STZ-induced diabetes mellitus in albino rats. Int. J. Pharm. Sci. Rev. Res. 2014, 29, 279–288. [Google Scholar]
- Bahshwan, S.M.; Rabah, S.O.A.; Turkistani, A.M. A comparative study of the effect of crude and nanoparticles Costus speciosus on testicular damage associated to experimentally induced type 2 diabetes. Pharmacophore 2019, 10, 99–106. [Google Scholar]
- Shediwah, F.M.H.; Naji, K.M.; Gumaih, H.S.; Alhadi, F.A.; Al-Hammami, A.L.; D'Souza, M.R. Antioxidant and antihyperlipidemic activity of Costus speciosus against atherogenic diet-induced hyperlipidemia in rabbits. J. Integr. Med. 2019, 17, 181–191. [Google Scholar] [CrossRef]
- Shrotriya, S.; Ranpise, N.; Satpute, P.; Vidhate, B. Skin targeting of curcumin solid lipid nanoparticles-engrossed topical gel for the treatment of pigmentation and irritant contact dermatitis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1471–1482. [Google Scholar] [CrossRef] [Green Version]
- Khan, D.H.; Bashir, S.; Khan, M.I.; Figueiredo, P.; Santos, H.A.; Peltonen, L. Formulation optimization and in vitro characterization of rifampicin and ceftriaxone dual drug loaded niosomes with high energy probe sonication technique. J. Drug Deliv. Sci. Technol. 2020, 58, 101763. [Google Scholar] [CrossRef]
- Girgis, S.; Shoman, T.; Kassem, S.; El-Din, A.E.; Abdel-Aziz, K. Potential protective effect of Costus speciosus or its nanoparticles on streptozotocin-induced genotoxicity and histopathological alterations in rats. J. Nutr. Food Sci. 2015, 5, S3-002. [Google Scholar]
- Al-Hariri, M.T. Comparison the rate of diabetes mellitus induction using streptozotocin dissolved in different solvents in male rats. J. Comp. Clin. Pathol. Res. ISSN 2012, 2252, 0422. [Google Scholar] [CrossRef]
- Gurudeeban, S.; Kaliamurthi, S.; Thirugnanasambandam, R. Positive regulation of Rhizophora mucronata poir extracts on blood glucose and lipid profile in diabetic rats. Herb. Med. 2016, 2. [Google Scholar]
- Pushparaj, P.; Tan, C.; Tan, B.K. Effects of Averrhoa bilimbi leaf extract on blood glucose and lipids in streptozotocin-diabetic rats. J. Ethnopharmacol. 2000, 72, 69–76. [Google Scholar] [CrossRef]
- Gamal, M.; Moawad, J.; Rashed, L.; Morcos, M.A.; Sharawy, N. Possible involvement of tetrahydrobiopterin in the disturbance of redox homeostasis in sepsis–Induced brain dysfunction. Brain Res. 2018, 1685, 19–28. [Google Scholar] [CrossRef]
- Packer, L. Superoxide Dismutase; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Zanoli, P.; Rivasi, M.; Zavatti, M.; Brusiani, F. Activity of single components of Ferula hermonis on male rat sexual behavior. Int. J. Impot. Res. 2005, 17, 513–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balgoon, M.J.; Al-Zahrani, M.H.; Jaouni, S.A.; Ayuob, N. Combined oral and topical application of pumpkin (Cucurbita pepo L.) Alleviates contact dermatitis associated with depression through downregulation pro-inflammatory cytokines. Front. Pharmacol. 2021, 12, 898. [Google Scholar] [CrossRef] [PubMed]
- Carleton, H.M.; Haynes, F. Histological Technique; Oxford University Press: Oxford, UK, 1926; Volume 2. [Google Scholar]
- Zhou, Q.; Nie, R.; Prins, G.S.; Saunders, P.T.; Katzenellenbogen, B.S.; Hess, R.A. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J. Androl. 2002, 23, 870–881. [Google Scholar]
- Madsbad, S. Impact of postprandial glucose control on diabetes-related complications: How is the evidence evolving? J. Diabetes Its Complicat. 2016, 30, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Nurdiana, S.; Goh, Y.M.; Ahmad, H.; Dom, S.M.; Syimal'ain Azmi, N.; Noor Mohamad Zin, N.S.; Ebrahimi, M. Changes in pancreatic histology, insulin secretion and oxidative status in diabetic rats following treatment with Ficus deltoidea and vitexin. BCM Complement. Altern. Med. 2017, 17, 290. [Google Scholar] [CrossRef] [Green Version]
- Al-Roujeaie, A.S.; Abuohashish, H.M.; Ahmed, M.M.; Alkhamees, O.A. Effect of rutin on diabetic-induced erectile dysfunction: Possible involvement of testicular biomarkers in male rats. Andrologia 2017, 49, e12737. [Google Scholar] [CrossRef]
- Bavarva, J.H.; Narasimhacharya, A. Antihyperglycemic and hypolipidemic effects of Costus speciosus in alloxan induced diabetic rats. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2008, 22, 620–626. [Google Scholar]
- Eliza, J.; Daisy, P.; Ignacimuthu, S.; Duraipandiyan, V. Antidiabetic and antilipidemic effect of eremanthin from Costus speciosus (Koen.) Sm. in STZ-induced diabetic rats. Chem. Biol. Interact. 2009, 182, 67–72. [Google Scholar] [CrossRef]
- Shanmugam, K.R.; Mallikarjuna, K.; Kesireddy, N.; Reddy, K.S. Neuroprotective effect of ginger on anti-oxidant enzymes in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2011, 49, 893–897. [Google Scholar] [CrossRef]
- Bahshwan, S.M.; Rabah, S.O.A.; Almukadi, H.S.; Bakhshwin, D.M. Can adjuvant supplements of Costus speciosus nanoparticles improve metformin control of hyperglycemia, oxidative stress, and apoptotic changes in Langerhans islets in a rat model of type 2 diabetes. Med. Sci. 2020, 24, 1904–1914. [Google Scholar]
- Revathy, J.; Abdullah, S.S.; Kumar, P.S. Antidiabetic effect of Costus speciosus rhizome extract in alloxan induced albino rats. J. Chem. Biochem. 2014, 2, 13–22. [Google Scholar]
- Nehete, J.; Bhatia, M.; Narkhede, M. In-vitro evaluation of antioxidant activity and phenolic content of Costus speciosus (Koen) JE Sm. Iran. J. Pharm. Res. IJPR 2010, 9, 271. [Google Scholar] [PubMed]
- Pai Kotebagilu, N.; Palvai, V.R.; Urooj, A. Protective effect of selected medicinal plants against hydrogen peroxide induced oxidative damage on biological substrates. Int. J. Med. Chem. 2014, 2014, 861084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayan, R.; Joseph, S.; Mathew, B. Costus speciosus rhizome extract mediated synthesis of silver and gold nanoparticles and their biological and catalytic properties. Inorg. Nano Met. Chem. 2019, 49, 249–259. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Synthesis of silver nanoparticles using Sacha inchi (Plukenetia volubilis L.) leaf extracts. Saudi J. Biol. Sci. 2014, 21, 605–609. [Google Scholar] [CrossRef] [Green Version]
- Soliman, G.A.; Saeedan, A.S.; Abdel-Rahman, R.F.; Ogaly, H.A.; Abd-Elsalam, R.M.; Abdel-Kader, M.S. Olive leaves extract attenuates type II diabetes mellitus-induced testicular damage in rats: Molecular and biochemical study. Saudi Pharm. J. 2019, 27, 326–340. [Google Scholar] [CrossRef]
- Al-Shathly, M.R.; Ali, S.S.; Ayuob, N.N. Zingiber officinale preserves testicular structure and the expression of androgen receptors and proliferating cell nuclear antigen in diabetic rats. Andrologia 2020, 52, e13528. [Google Scholar] [CrossRef]
- Ballester, J.; Muñoz, M.C.; Domínguez, J.; Rigau, T.; Guinovart, J.J.; Rodríguez-Gil, J.E. Insulin-dependent diabetes affects testicular function by FSH-and LH-linked mechanisms. J. Androl. 2004, 25, 706–719. [Google Scholar] [CrossRef]
- Schoeller, E.L.; Albanna, G.; Frolova, A.I.; Moley, K.H. Insulin rescues impaired spermatogenesis via the hypothalamic-pituitary-gonadal axis in Akita diabetic mice and restores male fertility. Diabetes 2012, 61, 1869–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, F.; Yassin, A.A.; Haider., A.; Gooren, L. Effects of testosterone on the lower urinary tract go beyond the prostate: New insights, new treatment options. Arab. J. Urol. 2011, 9, 147–152. [Google Scholar] [CrossRef]
- Melloul, D.; Marshak, S.; Cerasi, E. Regulation of insulin gene transcription. Diabetologia 2002, 45, 309–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.S.; Kang, S.H.; Kim, M.J.; Kim, S.K.; Kim, Y.L.; Park, W.K.; Park, S.W.; Cho, Y.W. Low serum testosterone concentrations in hospitalized men with poorly controlled type 2 diabetes. Endocrinol. Metab. 2014, 29, 574–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sari, I.P.; Nurrochmad, A.; Setiawan, I.M.; Hertiani, T.; Paramita, A.D.; Annisa, A.Y. Effects of Costus speciosus ethanolic extract on male rats: The action mechanism and the ability to impregnate. Pak. J. Pharm. Sci. 2018, 31, 997–1001. [Google Scholar] [PubMed]
- Das, K.; Kalita, P.P.; Sarma, M.P.; Talukdar, N.; Kakoti, P. Extraction, estimation and comparison of proteins and carbohydrates from different parts of Costus speciosus and a brief study on its phytochemicals content. Int. J. Basic Appl. Biol. 2014, 2, 81–85. [Google Scholar]
- El-Akabawy, G.; El-Kholy, W. Neuroprotective effect of ginger in the brain of streptozotocin-induced diabetic rats. Ann. Anat. Anat. Anz. 2014, 196, 119–128. [Google Scholar] [CrossRef]
- Edrees, H.M.; Elbehiry, A.; Elmosaad, Y.M. Hypoglycemic and anti-inflammatory effect of gold nanoparticles in streptozotocin-induced type 1 diabetes in experimental rats. Nanotechnology 2017, 3, 4. [Google Scholar]
- Selim, S.; Al Jaouni, S. Anti-inflammatory, antioxidant and antiangiogenic activities of diosgenin isolated from traditional medicinal plant, Costus speciosus (Koen ex. Retz.) Sm. Nat. Prod. Res. 2016, 30, 1830–1833. [Google Scholar] [CrossRef]
- Bakhsh, Z.A.; Al-Khatib, T.A.; Al-Muhayawi, S.M.; ElAssouli, S.M.; Elfiky, I.A.; Mourad, S.A. Evaluating the therapeutic efficacy, tolerability, and safety of an aqueous extract of Costus speciosus rhizome in acute pharyngitis and acute tonsillitis: A pilot study. Saudi Med. J. 2015, 36, 997. [Google Scholar] [CrossRef]
- Kamel, E.O.; Abd-Elrhman, A.-S.A.-H. The effect of diabetes mellitus on the rat ventral prostate and the possible protective role of Ginkgo biloba extracts. Al Azhar Assiut. Med. J. 2018, 16, 300. [Google Scholar] [CrossRef]
- Eid, B.G.; Mosli, H.; Hasan, A.; El-Bassossy, H.M. Ginger ingredients alleviate diabetic prostatic complications: Effect on oxidative stress and fibrosis. Evid. Based Complement. Altern. Med. 2017, 2017, 6090269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perlman, H.; Zhang, X.; Chen, M.W.; Walsh, K.; Buttyan, R. An elevated bax/bcl-2 ratio corresponds with the onset of prostate epithelial cell apoptosis. Cell Death Differ. 1999, 6, 48–54. [Google Scholar] [CrossRef]
- Banerjee, P.P.; Banerjee, S.; Brown, T.R. Bcl-2 protein expression correlates with cell survival and androgen independence in rat prostatic lobes. Endocrinology 2002, 143, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Popoola, B.; Ashefor, O.; Akanni, O.; Adaramoye, O. Biochemical, hormonal and histological changes in prostate of Wistar rats following long term streptozotocin-induced diabetes mellitus. Niger. J. Physiol. Sci. 2017, 32, 75–84. [Google Scholar]
- Kaneto, H.; Kawamori, D.; Matsuoka, T.-A.; Kajimoto, Y.; Yamasaki, Y. Oxidative stress and pancreatic β-cell dysfunction. Am. J. Ther. 2005, 12, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Lutgendorff, F.; Trulsson, L.M.; van Minnen, L.P.; Rijkers, G.T.; Timmerman, H.M.; Franzén, L.E.; Gooszen, H.G.; Akkermans, L.M.; Söderholm, J.D.; Sandström, P.A. Probiotics enhance pancreatic glutathione biosynthesis and reduce oxidative stress in experimental acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1111–G1121. [Google Scholar] [CrossRef] [Green Version]
- Gobbo, M.G.; Ribeiro, D.L.; Taboga, S.R.; de Almeida, E.A.; Góes, R.M. Oxidative stress markers and apoptosis in the prostate of diabetic rats and the influence of vitamin C treatment. J. Cell. Biochem. 2012, 113, 2223–2233. [Google Scholar] [CrossRef]
- Zup, S.L.; Forger, N.G. Testosterone regulates BCL-2 immunoreactivity in a sexually dimorphic motor pool of adult rats. Brain Res. 2002, 950, 312–316. [Google Scholar] [CrossRef]


| Component | Amount |
|---|---|
| Oleic acid | 500 mg |
| Compritol 888 ATO | 150 mg |
| Precirol ATO5 | 350 mg |
| Dried plant extract | 60 mg |
| PluronicF-68 1% | 200 mg |
| Tween®80 1% | 200 mg |
| Characterization | Value |
|---|---|
| Particle size (nm) | 568.4 ± 0.53 |
| Polydispersity index | 0.498 ± 0.06 |
| Zetapotential mV | −39.00 ± 2.30 |
| Encapsulation efficiency % | 89.00 ± 1.90 |
| Loading capacity % | 4.37 ± 0.05 |
| Control | C. speciosus-Treated | Untreated Diabetic | Metformin-Treated Diabetic | C. speciosus-Treated Diabetic | |
|---|---|---|---|---|---|
| Body weight (g) at the start of the experiment | 209.13 ± 5.63 | 208.33 ± 5.91 p = 0.92 | 211.50 ± 6.97 p1 = 0.94 | 210.96 ± 7.32 p1 = 0.84 | 214.04 ± 6.45 p1 = 0.89 p2 = 0.87 |
| Body weight (g) at the end of the experiment | 314.83 ± 43.24 | 304.83 ± 23.35 p = 0.95 | 261.33 ± 20.78 p1 = 0.01 | 296.33 ± 12.39 p1 = 0.24 | 299.17 ± 13.93 p1 = 0.16 p2 = 0.94 |
| Prostate weight (g) | 1.18 ± 0.07 | 1.17.00 ± 0.37 p = 0.89 | 0.78 ± 0.15 p1 = 0.02 | 0.96 ± 0.13 p1 = 0.84 | 0.90 ± 0.19 p1 = 0.65 p2 = 0.76 |
| BGL level (mg/dL) at the start of the experiment | 79.57 ± 7.81 | 67.50 ± 30.00 p = 0.95 | 81.24 ± 12.53 p1 = 0.93 | 84.17 ± 13.58 p1 = 0.89 | 79.00 ± 11.49 p1 = 0.91 p2 = 0.88 |
| BGL level (mg/dL) at the end of the experiment | 80.90 ± 5.39 | 88.45 ± 10.26 p = 0.98 | 354.07 ± 80.39 p1 < 0.001 | 259.85 ± 39.37 p1 = 0.01 | 268.90 ± 45.26 p1 = 0.03 p2 = 0.91 |
| Insulin Level in serum (µIU/mL) | 5.23 ± 0.40 | 5.30 ± 0.37 p = 0.92 | 2.28 ± 0.77 p1 < 0.001 | 3.56 ± 0.39 p1 = 0.003 | 3.42 ± 0.57 p1 = 0.01 p2 = 0.89 |
| Testosterone (ng/mL) | 28.69 ± 1.41 | 29.07 ± 2.64 p = 0.92 | 18.75 ± 2,11 p1 < 0.001 | 24.19 ± 3.76 p1 = 0.02 | 23.64 ± 2.88 p1 = 0.04 p2 = 0.93 |
| MDA in serum (nmoL/mL) | 1.27 ± 0.13 | 1.34 ±0.16 p = 0.93 | 2.20 ± 0.73 p1 = 0.004 | 1.47 ± 0.43 p1 = 0.04 | 1.37 ±0.18 p1 = 0.01 p2 = 0.93 |
| SOD in serum(µ/mL) | 19.32 ± 1.97 | 18.73 ± 3.42 p = 0.92 | 10.52 ± 3.00 p1 < 0.001 | 16.46 ± 3.18 p1 = 0.01 | 17.52 ± 2.35 p1 = 0.002 p2 = 0.89 |
| GPX in serum(µ/mL) | 54.49 ± 6.94 | 58.94 ± 9.89 p = 0.88 | 35.01 ± 5.87 p1 = 0.003 | 50.94 ± 5.41 p1 = 0.02 | 52.09 ± 10.92 p1 = 0.01 p2 = 0.91 |
| CAT in serum (µ/L) | 0.44 ± 0.08 | 0.47 ± 0.07 p = 0.95 | 0.16 ± 0.06 p1 < 0.001 | 0.36 ± 0.08 p1 = 0.001 | 0.39 ± 0.08 p1 < 0.001 p2 = 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhshwin, D.; Faddladdeen, K.A.J.; Ali, S.S.; Alsaggaf, S.M.; Ayuob, N.N. Nanoparticles of Costus speciosus Ameliorate Diabetes-Induced Structural Changes in Rat Prostate through Mediating the Pro-Inflammatory Cytokines IL 6, IL1β and TNF-α. Molecules 2022, 27, 1027. https://doi.org/10.3390/molecules27031027
Bakhshwin D, Faddladdeen KAJ, Ali SS, Alsaggaf SM, Ayuob NN. Nanoparticles of Costus speciosus Ameliorate Diabetes-Induced Structural Changes in Rat Prostate through Mediating the Pro-Inflammatory Cytokines IL 6, IL1β and TNF-α. Molecules. 2022; 27(3):1027. https://doi.org/10.3390/molecules27031027
Chicago/Turabian StyleBakhshwin, Duaa, Khadija Abdul Jalil Faddladdeen, Soad Shaker Ali, Samar Mohammed Alsaggaf, and Nasra Naeim Ayuob. 2022. "Nanoparticles of Costus speciosus Ameliorate Diabetes-Induced Structural Changes in Rat Prostate through Mediating the Pro-Inflammatory Cytokines IL 6, IL1β and TNF-α" Molecules 27, no. 3: 1027. https://doi.org/10.3390/molecules27031027
APA StyleBakhshwin, D., Faddladdeen, K. A. J., Ali, S. S., Alsaggaf, S. M., & Ayuob, N. N. (2022). Nanoparticles of Costus speciosus Ameliorate Diabetes-Induced Structural Changes in Rat Prostate through Mediating the Pro-Inflammatory Cytokines IL 6, IL1β and TNF-α. Molecules, 27(3), 1027. https://doi.org/10.3390/molecules27031027





