Chickpea and Lupin Sprouts, Stimulated by Different LED Lights, As Novel Examples of Isoflavones-Rich Functional Food, and Their Impact on Breast and Prostate Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Qualitative Isoflavones Analysis
2.2. Chickpea and Lupin Seeds—Isoflavones Content and Cytotoxicity
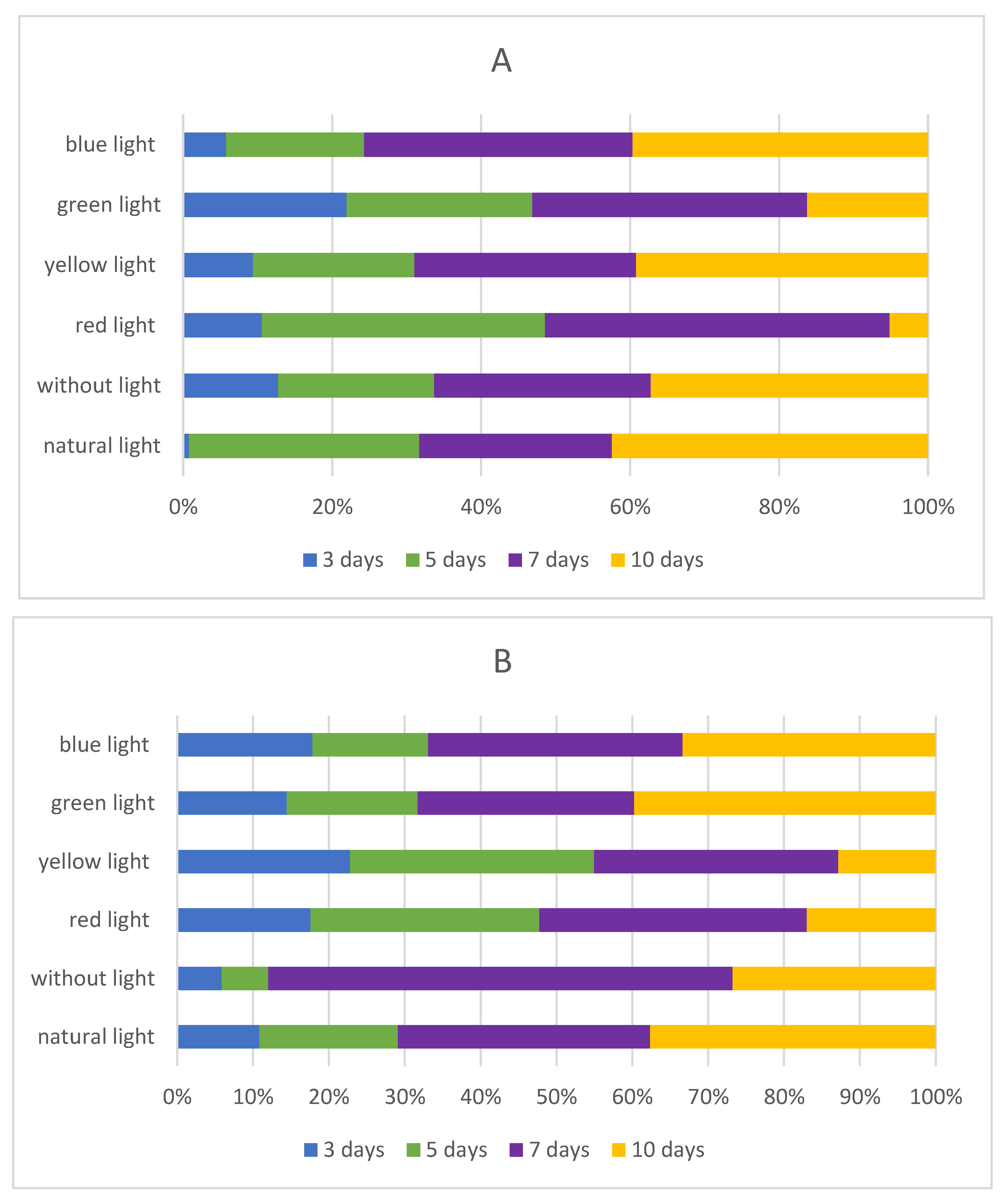
2.3. Influence of Light Quality on the Isoflavones Content in Sprouts
2.3.1. Influence of Darkness on the Isoflavones Content in Sprouts
2.3.2. Influence of Red LED Light on Isoflavones Content in Chickpea and Lupin Sprouts
2.3.3. Influence of Yellow LED Light on Isoflavones Content in Chickpea and Lupin Sprouts
2.3.4. Influence of Green LED Light on Isoflavones Content in Chickpea and Lupin Sprouts
2.3.5. Influence of Blue LED Light on Isoflavones Content in Chickpea and Lupin Sprouts
2.4. Cytotoxic Activity of Chickpea and Lupin Sprouts Harvested in Different Light Quality
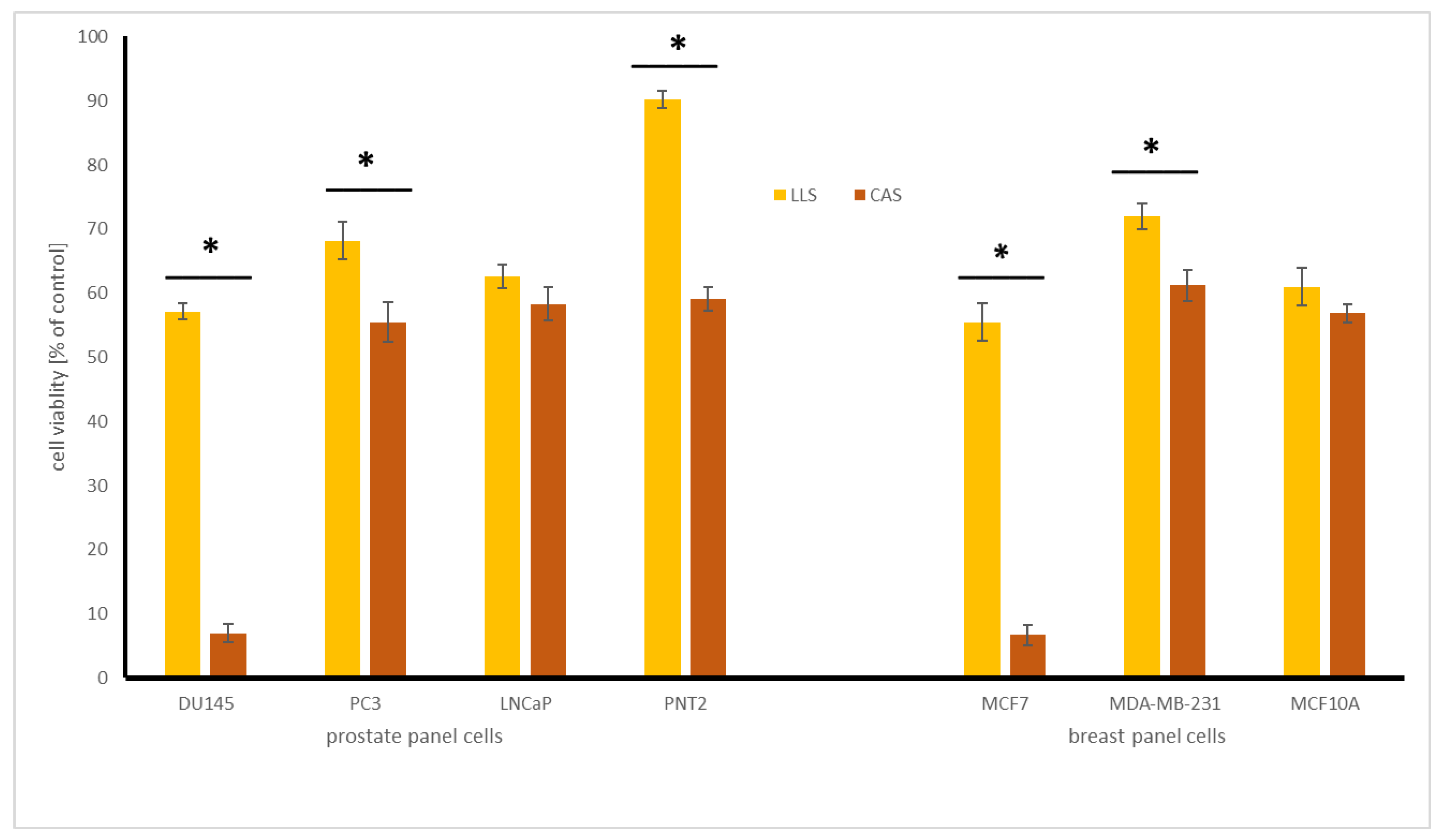
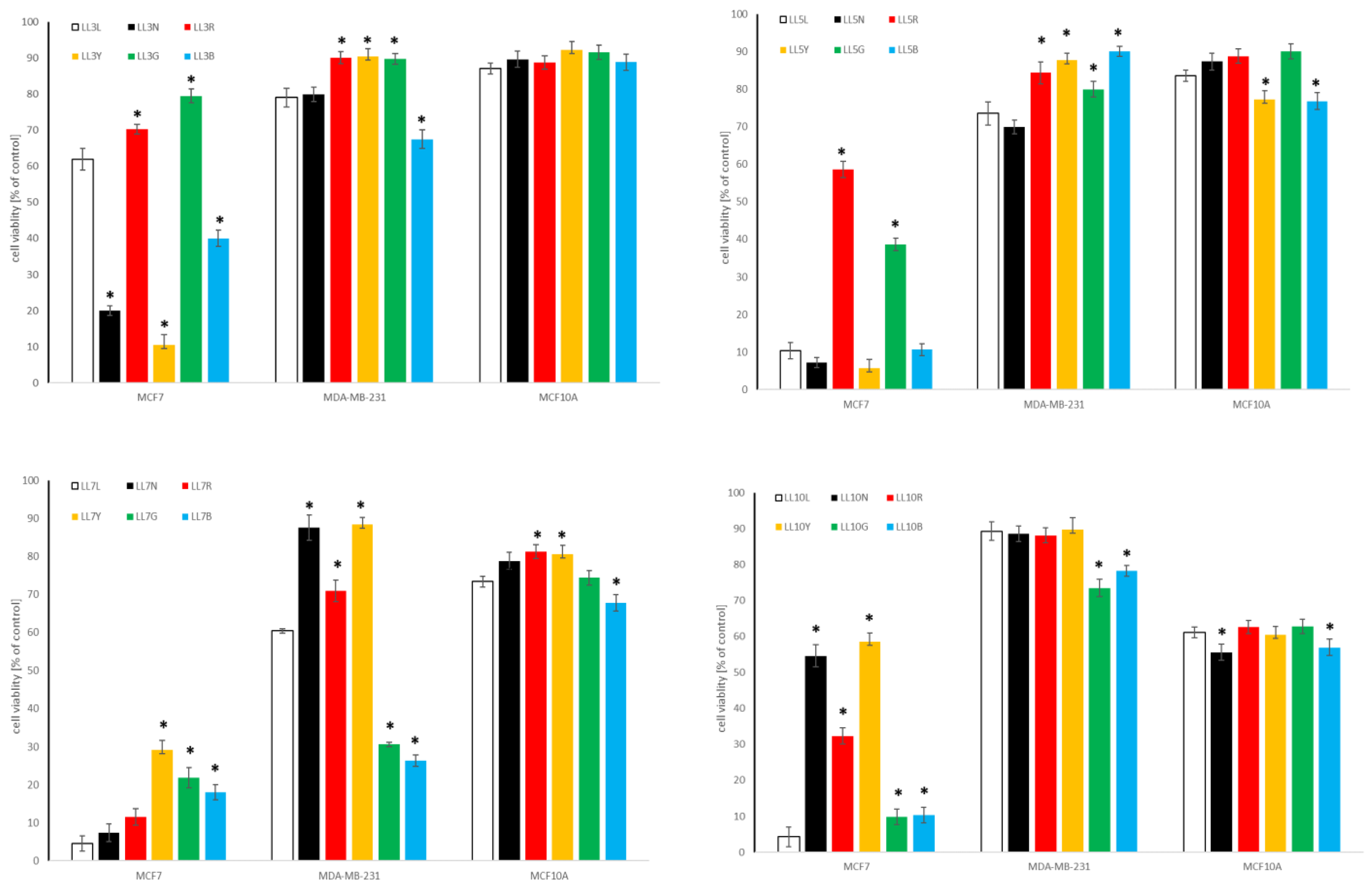
2.4.1. Influence of Chickpea and Lupin Sprouts on Breast Cells’ Viability
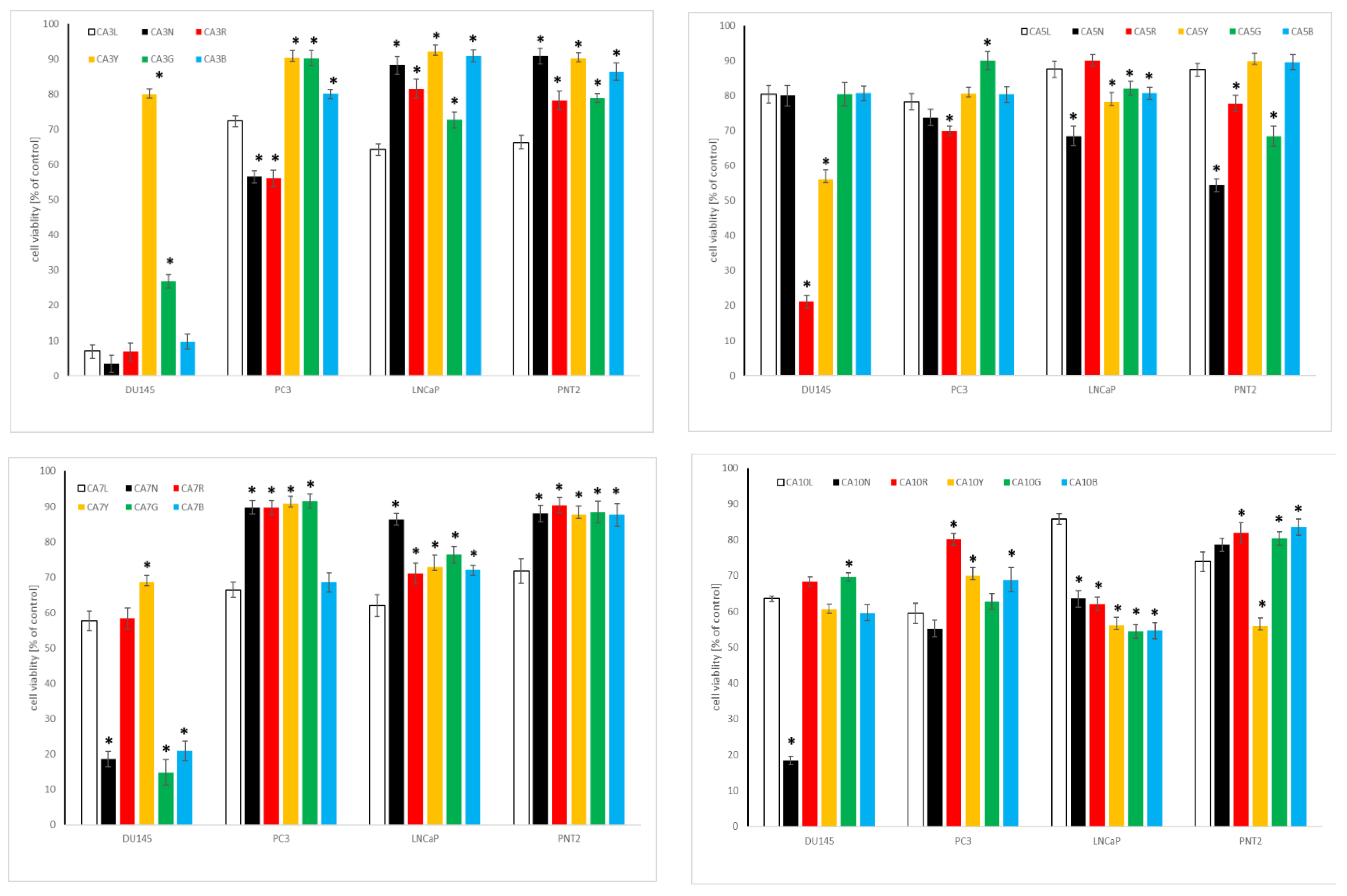
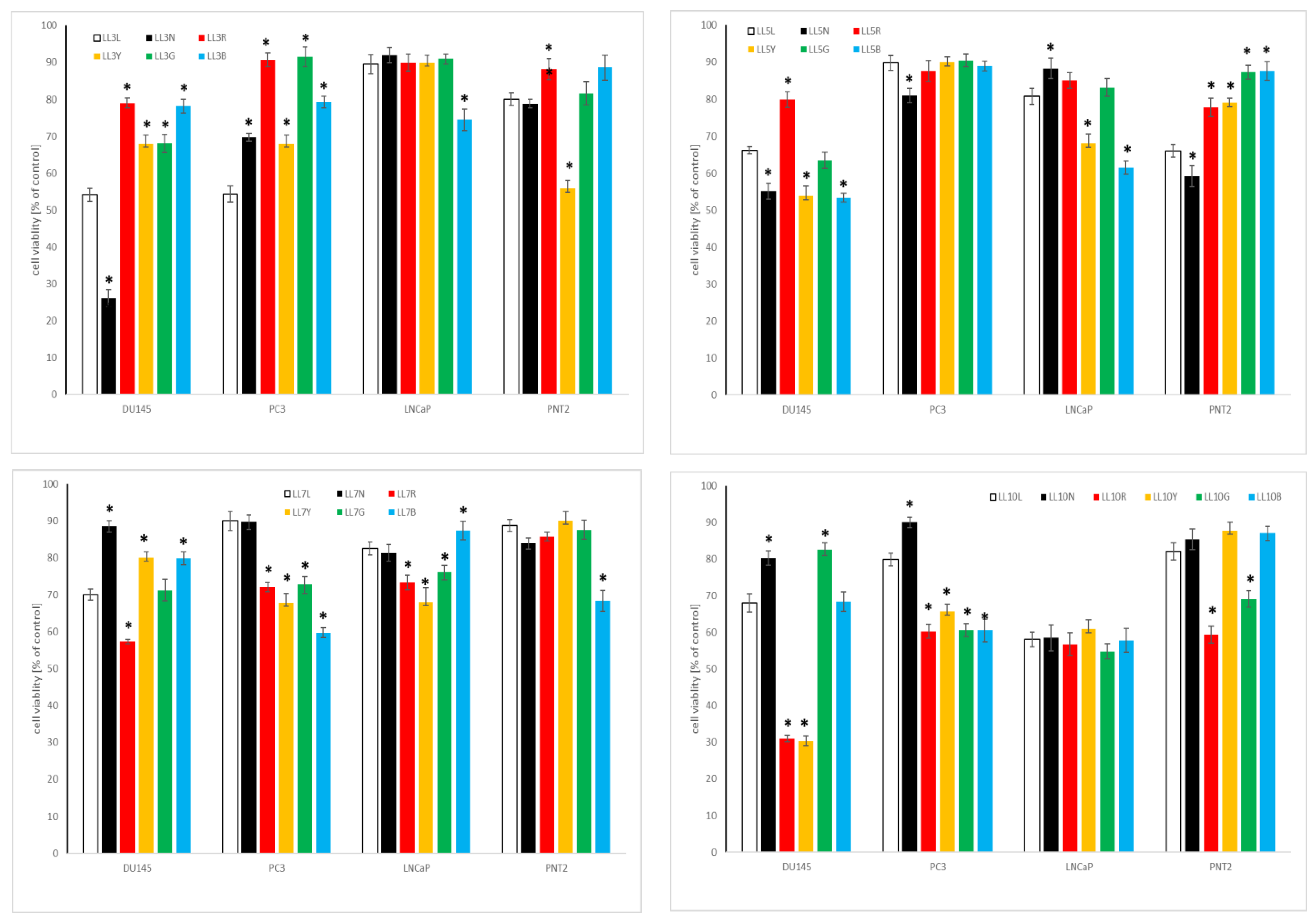
2.4.2. Influence of Chickpea and Lupin Sprouts on Prostate Cells’ Viability
2.5. Chemometric Analysis
3. Materials and Methods
3.1. Plant Materials and Growth Conditions in LED Chambers
3.2. Reagents
3.3. Extract Preparation
3.4. Isoflavones Analysis
3.5. Cell Cultures
3.6. Cytotoxic and Viability Assay
3.7. Statistical Analysis
Chemometric Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Miyahira, R.F.; Lopes, J.D.; Antunes, A.E.C. The use of sprouts to improve the nutritional value of food products: A brief review. Plant Foods Hum. Nutr. 2021, 76, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Cid-Gallegos, M.S.; Sánchez-Chino, X.M.; Juárez Chairez, M.F.; Álvarez González, I.; Madrigal-Bujaidar, E.; Jiménez-Martínez, C. Anticarcinogenic activity of phenolic compounds from sprouted legumes. Food Rev. Int. 2020, 1–16. [Google Scholar] [CrossRef]
- Galanty, A.; Niepsuj, M.; Grudzińska, M.; Zagrodzki, P.; Podolak, I.; Paśko, P. In the search for novel, isoflavone-rich functional foods—comparative studies of four clover species sprouts and their chemopreventive potential for breast and prostate cancer. Pharmaceuticals 2022, 15, 806. [Google Scholar] [CrossRef] [PubMed]
- Paśko, P.; Tyszka-Czochara, M.; Galanty, A.; Gdula-Argasińska, J.; Żmudzki, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S. Comparative study of predominant phytochemical compounds and proapoptotic potential of broccoli sprouts and florets. Plant Foods Hum. Nutr. 2018, 73, 95–100. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A critical review on phytochemical profile and health promoting effects of mung bean (Vigna radiata). Food Sci. Hum. Wellness 2018, 7, 11–33. [Google Scholar] [CrossRef]
- Busambwa, K.; Sunkara, R.; Diby, N.; Offei-Okyne, R.; Boateng, J.; Verghese, M. Cytotoxic and apoptotic effects of sprouted and non-sprouted lentil, green and yellow split-peas. Int. J. Cancer Res. 2016, 12, 51–60. [Google Scholar] [CrossRef]
- Farag, M.A.; El-Din, M.G.S.; Selim, M.A.F.; Owis, A.I.; Abouzid, S.F. Mass spectrometry-based metabolites profiling of nutrients and anti-nutrients in major legume sprouts. Food Biosci. 2021, 39, 100800. [Google Scholar] [CrossRef]
- Zhang, K.; Li, H.; Zhang, T.; Wang, S.; Liu, L.; Dong, X.; Cong, L.; Song, H.; Wang, A.; Yang, G.; et al. Comprehensive transcriptomic and metabolomic profiling reveals the differences between alfalfa sprouts germinated with or without light exposure. Front. Plant Sci. 2022, 2790. [Google Scholar] [CrossRef]
- Paśko, P.; Galanty, A.; Żmudzki, P.; Gdula-Argasińska, J.; Zagrodzki, P. Influence of different light conditions and time of sprouting on harmful and beneficial aspects of rutabaga sprouts in comparison to their roots and seeds. J. Sci. Food Agric. 2019, 99, 302–308. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Peng, L.X.; Zou, L.; Su, Y.M.; Fan, Y.; Zhao, G. Effects of light on growth, levels of anthocyanin, concentration of metabolites in Fagopyrum tataricum sprout cultures. Int. J. Food Sci. Technol. 2015, 50, 1382–1389. [Google Scholar] [CrossRef]
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W.; Wang, Q. Effects of light quality on main health-promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Folta, K.M.; Carvalho, S.D. Photoreceptors and control of horticultural plant traits. HortScience 2015, 50, 1274–1280. [Google Scholar] [CrossRef]
- Heijde, M.; Ulm, R. UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 2012, 17, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Blankenship, R.E. Expanding the solar spectrum used by photosynthesis. Trends Plant Sci. 2011, 16, 427–431. [Google Scholar] [CrossRef]
- Samuolienė, G.; Urbonavičiūtė, A.; Brazaitytė, A.; Šabajevienė, G.; Sakalauskaitė, J.; Duchovskis, P. The impact of LED illumination on antioxidant properties of sprouted seeds. Open Life Sci. 2011, 6, 68–74. [Google Scholar] [CrossRef]
- Aisyah, S.; Gruppen, H.; Madzora, B.; Vincken, J.P. Modulation of isoflavonoid composition of Rhizopus oryzae elicited soybean (Glycine max) seedlings by light and wounding. J. Agric. Food Chem. 2013, 61, 8657–8667. [Google Scholar] [CrossRef]
- Gao, Y.; Yao, Y.; Zhu, Y.; Ren, G. Isoflavone content and composition in chickpea (Cicer arietinum L.) sprouts germinated under different conditions. J. Agric. Food Chem. 2015, 63, 2701–2707. [Google Scholar] [CrossRef]
- HaiRong, M.; HuaBo, W.; Zhen, C.; Yi, Y.; ZhengHua, W.; Madina, H.; Xu, C.; Haji Akber, A. The estrogenic activity of isoflavones extracted from chickpea Cicer arietinum L. sprouts in vitro. Phytother. Res. 2013, 27, 1237–1242. [Google Scholar] [CrossRef]
- Dulce-María, D.A.; Adrián, C.R.; Cuauhtémoc, R.M.; Ada-Keila, M.N.; Jorge, M.C.; Erika, A.S.; Edith-Oliva, C.R. Isoflavones from black chickpea (Cicer arietinum L.) sprouts with antioxidant and antiproliferative activity. Saudi J. Biol. Sci. 2021, 28, 1141–1146. [Google Scholar] [CrossRef]
- Duenas, M.; Hernandez, T.; Estrella, I.; Fernandez, D. Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinus angustifolius L.). Food Chem. 2009, 117, 599–607. [Google Scholar] [CrossRef]
- Mazur, W.M.; Duke, J.A.; Wähälä, K.; Rasku, S.; Adlercreutz, H. Isoflavonoids and lignans in legumes: Nutritional and health aspects in humans. J. Nutr. Biochem. 1998, 9, 193–200. [Google Scholar] [CrossRef]
- Khan, M.K.; Karnpanit, W.; Nasar-Abbas, S.M.; Huma, Z.; Jayasena, V. Phytochemical composition and bioactivities of lupin: A review. Int. J. Food Sci. Techno. 2015, 50, 2004–2012. [Google Scholar] [CrossRef]
- Hahlbrock, K.; Scheel, D. Physiology and molecular biology of phenylpropanoid metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 347–369. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, S.H.; Chung, J.I.; Chi, H.Y.; Kim, J.A.; Chung, I.M. Analysis of phenolic compounds and isoflavones in soybean seeds (Glycine max (L.) Merill) and sprouts grown under different conditions. Eur. Food Res. Technol. 2006, 222, 201–208. [Google Scholar] [CrossRef]
- Liu, C.J.; Blount, J.W.; Steele, C.L.; Dixon, R.A. Bottlenecks for metabolic engineering of isoflavone glycoconjugates in Arabidopsis. Proc. Natl. Acad. Sci. USA 2002, 99, 14578–14583. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.R.; Wang, J.; Qi, H.X.; Gao, Y.H.; Pang, L.J.; Yang, Y.; Aisa, H.A. Assessment of the estrogenic activities of chickpea (Cicer arietinum L.) sprout isoflavone extract in ovariectomized rats. Acta Pharmacol. Sin. 2013, 34, 380–386. [Google Scholar] [CrossRef]
- Lim, Y.J.; Jeong, H.Y.; Gil, C.S.; Kwon, S.J.; Na, J.K.; Lee, C.; Eom, S.H. Isoflavone accumulation and the metabolic gene expression in response to persistent UV-B irradiation in soybean sprouts. Food Chem. 2020, 303, 125376. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Kaufman, P.; Nelson, R.L.; Kasperbauer, M.J.; Duke, J.A.; Seymour, E.; Chang, S.C.; Warber, S.; Bolling, S. Isoflavone levels in five soybean (Glycine max) genotypes are altered by phytochrome-mediated light treatments. J. Agric. Food Chem. 2006, 54, 54–58. [Google Scholar] [CrossRef]
- Graham, T.L. Flavonoid and isoflavonoid distribution in developing soybean seedling tissues and in seed and root exudates. Plant Physiol. 1991, 95, 594–603. [Google Scholar] [CrossRef]
- Vidya, N.; Saravanan, K.; Halka, J.; Kowsalya, K.; Preetha, J.S.Y.; Gurusaravanan, P.; Radhakrishnan, R.; Usha Raja Nanthini, A.; Arun, M. An insight into in vitro strategies for bioproduction of isoflavones. Plant Biotechnol. Rep. 2021, 15, 717–740. [Google Scholar] [CrossRef]
- Park, C.H.; Park, Y.E.; Yeo, H.J.; Kim, J.K.; Park, S.U. Effects of light-emitting diodes on the accumulation of phenolic compounds and glucosinolates in Brassica juncea sprouts. Horticulturae 2020, 6, 77. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L.; Gómez, P.A.; Pennisi, G.; Crepaldi, A.; Fernández, J.A.; Artés-Hernández, F. Postharvest LED lighting: Effect of red, blue and far red on quality of minimally processed broccoli sprouts. J. Sci. Food Agric. 2021, 101, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Geng, S.; Chakravorty, D.; Guan, Q.; Chen, S.; Assmann, S.M. Metabolomics of red-light-induced stomatal opening in Arabidopsis thaliana: Coupling with abscisic acid and jasmonic acid metabolism. Plant J. 2020. 101, 1331–1348. [CrossRef]
- Zhen, S.; Haidekker, M.; van Iersel, M.W. Far-red light enhances photochemical efficiency in a wavelength-dependent manner. Physiol. Plant. 2019, 167, 21–33. [Google Scholar] [CrossRef]
- Zhen, S.; van Iersel, M.W. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef]
- Kwack, Y.; Kim, K.K.; Hwang, H.; Chun, C. Growth and quality of sprouts of six vegetables cultivated under different light intensity and quality. Hortic. Environ. Biotechnol. 2015, 56, 437–443. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Q.; Qu, M.; Gao, L.; Hou, L. Blue light alleviates ‘red light syndrome’ by regulating chloroplast ultrastructure, photosynthetic traits and nutrient accumulation in cucumber plants. Sci. Hortic. 2019, 257, 108680. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Sakalauskienė, S.; Samuolienė, G.; Jankauskienė, J.; Viršilė, A.; Novičkovas, A.; Sirtautas, R.; Miliauskienė, J.; Vaštakaitė, V.; Dabašinskas, L.; et al. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef]
- Khattak, A.B.; Zeb, A.; Bibi, N. Impact of germination time and type of illumination on carotenoid content, protein solubility and in vitro protein digestibility of chickpea (Cicer arietinum L.) sprouts. Food Chem. 2008, 109, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Alrifai, O.; Mats, L.; Liu, R.; Hao, X.; Marcone, M.F.; Tsao, R. Effect of combined light-emitting diodes on the accumulation of glucosinolates in Brassica microgreens. Food Prod. Process. Nutr. 2021, 3, 30. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Castillejo, N.; Martínez-Zamora, L. UV and visible spectrum led lighting as abiotic elicitors of bioactive compounds in sprouts, microgreens, and baby leaves—A comprehensive review including their mode of action. Foods 2022, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Wheeler, R.M.; Sager, J.C.; Gains, G.D.; Naikane, J.H. Evaluation of lettuce growth using supplemental green light with red and blue light-emitting diodes in a controlled environment—A review of research at Kennedy Space Center. Acta Hortic. 2005, 711, 111–120. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Kim, W.W.; Park, C.H.; Cho, D.H. Effect of artificial LED light and far infrared irradiation on phenolic compound, isoflavones and antioxidant capacity in soybean (Glycine max L.) sprout. Foods 2018, 7, 174. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem. 2010, 119, 1485–1490. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations—Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Grever, M.R.; Schepartz, S.A.; Chabner, B.A. The National Cancer Institute: Cancer drug discovery and development program. Semin. Oncol. 1992, 19, 6–622. [Google Scholar]
- Khoja, K.K.; Howes, M.J.R.; Hider, R.; Sharp, P.A.; Farrell, I.W.; Latunde-Dada, G.O. Cytotoxicity of fenugreek sprout and seed extracts and their bioactive constituents on MCF-7 breast cancer cells. Nutrients 2022, 14, 784. [Google Scholar] [CrossRef]
- Paśko, P.; Galanty, A.; Zagrodzki, P.; Żmudzki, P.; Bieniek, U.; Prochownik, E.; Domínguez-Álvarez, E.; Bierła, K.; Łobiński, R.; Szpunar, J.; et al. Varied effect of fortification of kale sprouts with novel organic selenium compounds on the synthesis of sulphur and phenolic compounds in relation to cytotoxic, antioxidant and anti-inflammatory activity. Microchem. J. 2022, 179, 107509. [Google Scholar] [CrossRef]
- Frias, J.; Gulewicz, P.; Martinez-Villaluenga, C.; Pilarski, R.; Blazquez, E.; Jimenez, B.; Gulewicz, K.; Vidal-Valverde, C. Influence of germination with different selenium solutions on nutritional value and cytotoxicity of lupin seeds. J. Agric. Food Chem. 2009, 57, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Tay, K.C.; Tan, L.T.H.; Chan, C.K.; Hong, S.L.; Chan, K.G.; Yap, W.H.; Pusparajah, P.; Lee, L.-H.; Goh, B.H. Formononetin: A review of its anticancer potentials and mechanisms. Front. Pharmacol. 2019, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, A.; Javeed, M.; Shah, M.A.; Hussain, G.; Shafiq, N.; Sarfraz, I.; Riaz, A.; Sadiqa, A.; Zara, R.; Zafar, S.; et al. Biochanin A: A novel bioactive multifunctional compound from nature. Sci. Total Environ. 2020, 722, 137907. [Google Scholar] [CrossRef]
- Grudzińska, M.; Paśko, P.; Wróbel-Biedrawa, D.; Podolak, I.; Galanty, A. Antimelanoma potential of Cladonia mitis acetone extracts–comparative in vitro studies in relation to usnic acid content. Chem. Biodivers. 2022, 19, e202200408. [Google Scholar] [CrossRef]
- Boulesteix, A.L.; Strimmer, K. Partial Least Squares: A versatile tool for the analysis of high-dimensional genomic data. Brief. Bioinform. 2007, 8, 32–44. [Google Scholar] [CrossRef]
- Trygg, J.; Holmes, E.; Lundstedt, T. Chemometrics in metabonomics. J. Proteome Res. 2007, 6, 469–479. [Google Scholar] [CrossRef]
- Zagrodzki, P.; Krzyczkowska-Sendrakowska, M.; Nicol, F.; Wietecha-Posłuszny, R.; Milewicz, T.; Kryczyk-Kozioł, J.; Chaykivska, Z.; Jach, R. Selenium status parameters in patients with polycystic ovary syndrome. J. Trace Elem. Med. Biol. 2017, 44, 241–246. [Google Scholar] [CrossRef]
- Paśko, P.; Prochownik, E.; Krośniak, M.; Tyszka-Czochara, M.; Francik, R.; Marcinkowska, M.; Sikora, J.; Malinowski, M.; Zagrodzki, P. Animals in iodine deficiency or sulfadimethoxine models of thyroid damage are differently affected by the consumption of Brassica sprouts. Biol. Trace Elem. Res. 2020, 193, 204–213. [Google Scholar] [CrossRef]
- Paśko, P.; Krośniak, M.; Prochownik, E.; Tyszka-Czochara, M.; Fołta, M.; Francik, R.; Sikora, J.; Malinowski, M.; Zagrodzki, P. Effect of broccoli sprouts on thyroid function, haematological, biochemical, and immunological parameters in rats with thyroid imbalance. Biomed. Pharmacother. 2018, 97, 82–90. [Google Scholar] [CrossRef]


| Time of Sprouting | Type of Light | Biochanin A | Daidzein | Formononetin | Genistein | Glycitein | Ononin | Sum of Isoflavones |
|---|---|---|---|---|---|---|---|---|
| CA3 | Natural | 0.39 ± 0.16 | 0.64 ± 0.13 | Tr | 8.51 ± 0.21 | 4.90 ± 0.50 | Tr | 14.44 |
| CA5 | 49.0 ± 5.14 | 1.91 ± 0.16 | 79.7 ± 6.2 | 1.05 ± 0.18 | 2.42 ± 0.12 | 443.3 ± 18.3 | 577.4 | |
| CA7 | 154.5 ± 11.4 | 2.14 ± 0.21 | 267.6 ± 21.9 | 0.91 ± 0.10 | 5.73 ± 0.70 | 51.9 ± 2.8 | 482.8 | |
| CA10 | 309.8 ± 29.8 | 3.38 ± 0.21 | 369.6 ± 23.5 | 3.02 ± 0.26 | 8.32 ± 0.36 | 97.2 ± 13.6 | 791.3 | |
| CA3 | Without light | 67.10 ± 4.2 | 0.53 ± 0.15 | 96.8 ± 8.7 | Tr | 1.64 ± 0.17 | 25.4 ± 0.7 | 191.5 |
| CA5 | 100.3 ± 5.0 | 1.11 ± 0.06 | 177.9 ± 7.0 | 0.36 ± 0.09 | 2.99 ± 0.10 | 31.9 ± 0.7 | 314.6 | |
| CA7 | 152.6 ± 4.6 | 2.02 ± 0.13 | 211.2 ± 15.7 | 0.86 ± 0.11 | 5.00 ± 0.35 | 66.4 ± 3.7 | 438.1 | |
| CA10 | 144.3 ± 7.9 | 1.57 ± 0.09 | 165.7 ± 14.9 | 1.00 ± 0.15 | 4.52 ± 0.16 | 242.5 ± 8.5 | 559.6 | |
| CA3 | Red | 61.6 ± 13.4 | 0.34 ± 0.07 | 82.4 ± 10.4 | 0.16 ± 0.04 | 0.93 ± 0.20 | 30.2 ± 4.9 | 175.6 |
| CA5 | 67.16 ± 4.1 | 0.58 ± 0.10 | 109.2 ± 9.5 | 0.81 ± 0.05 | 1.51 ± 0.11 | 454.0 ± 35.8 | 633.3 | |
| CA7 | 166.9 ± 13.0 | 1.79 ± 0.12 | 182.6 ± 11.4 | 1.76 ± 0.04 | 4.46 ± 0.22 | 413.5 ± 16.7 | 771.0 | |
| CA10 | 44.2 ± 11.9 | 0.99 ± 0.06 | 31.9 ± 2.9 | Tr | 2.65 ± 0.17 | 6.88 ± 0.60 | 86.6 | |
| CA3 | Yellow | 21.98 ± 1.84 | Tr | 36.2 ± 2.8 | 0.19 ± 0.12 | 0.28 ± 0.01 | 169.0 ± 5.5 | 227.7 |
| CA5 | 110.4 ± 8.4 | 0.56 ± 0.12 | 109.6 ± 18.2 | 0.56 ± 0.04 | 1.74 ± 0.10 | 305.2 ± 7.0 | 528.1 | |
| CA7 | 124.8 ± 18.1 | 1.04 ± 0.14 | 136.4 ± 14.7 | 1.15 ± 0.11 | 2.61 ± 0.11 | 458.5 ± 27.0 | 724.5 | |
| CA10 | 293.9 ± 26.1 | 1.57 ± 0.10 | 227.0 ± 12.8 | 1.67 ± 0.15 | 4.11 ± 0.31 | 425.7 ± 6.6 | 953.9 | |
| CA3 | Green | 19.6 ± 1.6 | Tr | 108.0 ± 7.9 | Tr | 1.95 ± 0.12 | 24.6 ± 0.9 | 154.2 |
| CA5 | 78.5 ± 7.2 | 0.77 ± 0.09 | 22.1 ± 2.1 | Tr | 0.43 ± 0.02 | 73.1 ± 9.3 | 174.9 | |
| CA7 | 171.1 ± 6.9 | 0.62 ± 0.11 | 54.7 ± 6.5 | 0.26 ± 0.02 | 4.50 ± 0.23 | 28.4 ± 7.9 | 259.6 | |
| CA10 | 54.7 ± 5.5 | 0.32 ± 0.08 | 46.7 ± 1.4 | 0.15 ± 0.01 | 0.87 ± 0.07 | 11.8 ± 0.8 | 114.5 | |
| CA3 | Blue | 25.2 ± 3.5 | 0.33 ± 0.09 | 40.8 ± 3.2 | Tr | 0.67 ± 0.02 | 14.6 ± 1.1 | 81.6 |
| CA5 | 98.9 ± 6.6 | 0.76 ± 0.13 | 133.5 ± 12.4 | Tr | 1.89 ± 0.10 | 28.6 ± 1.3 | 263.7 | |
| CA7 | 182.6 ± 13.8 | 1.26 ± 0.14 | 272.3 ± 15.1 | Tr | 2.76 ± 0.23 | 54.1 ± 3.3 | 513.0 | |
| CA10 | 257.2 ± 14.8 | 0.71 ± 0.16 | 275.1 ± 13.2 | Tr | 6.52 ± 0.65 | 23.9 ± 0.9 | 563.4 |
| Time of Sprouting | Type of Light | Biochanin A | Genistein | Genistin | Glycitein | Sum of Isoflavones |
|---|---|---|---|---|---|---|
| LL3 | Natural | 0.21 ± 0.02 | Tr | 21.80 ± 0.44 | 3.03 ± 010 | 25.0 |
| LL5 | 0.59 ± 0.11 | Tr | 30.82 ± 0.74 | 10.95 ± 1.28 | 42.4 | |
| LL7 | 1.09 ± 0.13 | Tr | 66.85 ± 2.30 | 9.20 ± 0.69 | 77.1 | |
| LL10 | 1.91 ± 0.16 | Tr | 67.42 ± 2.06 | 18.04 ± 0.78 | 87.4 | |
| LL3 | Without light | 0.14 ± 0.02 | Tr | 17.60 ± 0.43 | 1.81 ± 0.07 | 19.5 |
| LL5 | 1.24 ± 0.13 | Tr | 11.93 ± 0.64 | 7.23 ± 0.15 | 20.4 | |
| LL7 | 8.40 ± 0.48 | 17.67 ± 2.21 | 147.2 ± 3.6 | 31.31 ± 1.04 | 204.6 | |
| LL10 | 1.13 ± 0.11 | 42.43 ± 1.48 | 18.60 ± 0.54 | 27.37 ± 1.64 | 89.5 | |
| LL3 | Red | 0.79 ± 0.14 | 18.00 ± 0.94 | 42.60 ± 1.42 | 13.75 ± 0.17 | 75.1 |
| LL5 | 2.43 ± 0.31 | 6.42 ± 0.65 | 107.1 ± 6.1 | 12.65 ± 0.36 | 128.6 | |
| LL7 | 4.00 ± 0.24 | 10.41 ± 0.30 | 119.9 ± 2.6 | 16.20 ± 0.56 | 150.5 | |
| LL10 | 1.00 ± 0.33 | 19.35 ± 3.00 | 34.27 ± 1.00 | 17.97 ± 0.74 | 72.6 | |
| LL3 | Yellow | 2.41 ± 0.20 | 6.72 ± 1.95 | 56.90 ± 3.92 | 9.78 ± 0.45 | 75.8 |
| LL5 | 3.63 ± 0.20 | 6.60 ± 0.20 | 89.19 ± 1.75 | 7.54 ± 0.22 | 107.0 | |
| LL7 | 2.69 ± 0.20 | 13.38 ± 1.04 | 79.14 ± 1.32 | 11.90 ± 0.43 | 107.1 | |
| LL10 | 1.38 ± 0.18 | 12.95 ± 2.60 | 15.60 ± 0.53 | 12.72 ± 0.41 | 42.6 | |
| LL3 | Green | 0.45 ± 0.08 | 5.54 ± 0.74 | 29.31 ± 0.74 | 10.42 ± 0.20 | 45.7 |
| LL5 | 0.44 ± 0.20 | 6.05 ± 0.12 | 37.54 ± 1.90 | 10.62 ± 0.35 | 54.6 | |
| LL7 | 2.83 ± 0.20 | 13.37 ± 0.23 | 63.36 ± 2.15 | 10.75 ± 0.60 | 90.3 | |
| LL10 | 3.10 ± 0.40 | 46.22 ± 1.65 | 33.86 ± 1.21 | 42.69 ± 1.21 | 125.9 | |
| LL3 | Blue | 4.11 ± 0.19 | 4.29 ± 0.08 | 21.80 ± 1.00 | 12.29 ± 1.05 | 42.5 |
| LL5 | 0.87 ± 0.17 | 9.10 ± 0.78 | 22.81 ± 1.12 | 3.42 ± 0.28 | 36.2 | |
| LL7 | 3.14 ± 0.26 | 12.36 ± 0.78 | 50.05 ± 4.40 | 14.27 ± 2.56 | 79.8 | |
| LL10 | 4.09 ± 0.28 | 31.66 ± 0.33 | 19.50 ± 2.60 | 24.11 ± 1.18 | 79.4 |
| Samples | DU145 | MCF7 | Samples | DU145 | MDA-MB-231 | MCF7 |
|---|---|---|---|---|---|---|
| CA3L | >Cmax | >Cmax | LL3L | >Cmax | >Cmax | >Cmax |
| CA5L | >Cmax | 418.6 | LL5L | >Cmax | >Cmax | 198.2 |
| CA7L | >Cmax | 214.5 | LL7L | >Cmax | >Cmax | 111.6 |
| CA10L | 102.1 | 35.0 | LL10L | >Cmax | >Cmax | 77.2 |
| CA3N | >Cmax | 148.6 | LL3N | 360.0 | >Cmax | 202.9 |
| CA5N | 205.6 | 42.1 | LL5N | >Cmax | >Cmax | 152.8 |
| CA7N | 173.5 | 133.4 | LL7N | >Cmax | >Cmax | 174.6 |
| CA10N | 117.4 | 51.6 | LL10N | >Cmax | >Cmax | >Cmax |
| CA3R | 302.1 | 484.9 | LL3R | >Cmax | >Cmax | >Cmax |
| CA5R | >Cmax | 183.6 | LL5R | >Cmax | >Cmax | >Cmax |
| CA7R | >Cmax | 178.6 | LL7R | >Cmax | >Cmax | 201.9 |
| CA10R | >Cmax | 166.2 | LL10R | 375.5 | >Cmax | 370.5 |
| CA3Y | >Cmax | 317.9 | LL3Y | >Cmax | >Cmax | 187.1 |
| CA5Y | >Cmax | >Cmax | LL5Y | >Cmax | >Cmax | 154.8 |
| CA7Y | >Cmax | 93.8 | LL7Y | >Cmax | >Cmax | 399.6 |
| CA10Y | 343.4 | 70.6 | LL10Y | 360.4 | >Cmax | >Cmax |
| CA3G | >Cmax | >Cmax | LL3G | >Cmax | >Cmax | >Cmax |
| CA5G | 179.8 | 193.0 | LL5G | >Cmax | >Cmax | 419.9 |
| CA7G | >Cmax | 171.4 | LL7G | >Cmax | 320.6 | 292.0 |
| CA10G | 288.3 | 137.8 | LL10G | >Cmax | >Cmax | 142.8 |
| CA3B | >Cmax | 301.8 | LL3B | >Cmax | >Cmax | 424.1 |
| CA5B | 352.0 | 332.2 | LL5B | >Cmax | >Cmax | 164.2 |
| CA7B | >Cmax | 67.2 | LL7B | >Cmax | 307.5 | 282.2 |
| CA10B | 427.5 | 46.8 | LL10B | >Cmax | >Cmax | 145.9 |
| CAS | 154.1 | 120.5 | LLS | >Cmax | >Cmax | >Cmax |
| Pairs of Correlated Parameters | Correlation Weights | |
|---|---|---|
| Chickpea sprouts | ||
| Time (7d) | PC3 | 0.295 |
| Time (7d) | PNT2 | 0.235 |
| Time (3d) | MCF7 | 0.205 |
| Time (10d) | Formononetin | 0.195 |
| Time (10d) | Daidzein | 0.168 |
| PNT2 | PC3 | 0.146 |
| Time (10d) | Glycitein | 0.137 |
| Time (3d) | Genistein | 0.110 |
| Formononetin | Du145 | −0.109 |
| Glycitein | MCF7 | −0.114 |
| Daidzein | Du145 | −0.124 |
| Daidzein | MCF7 | −0.142 |
| Genistein | PC3 | −0.144 |
| Time (3d) | Glycitein | −0.148 |
| Formononetin | MCF7 | −0.168 |
| Time (3d) | Daidzein | −0.186 |
| Time (10d) | MCF7 | −0.190 |
| Time (3d) | Formononetin | −0.210 |
| Time (7d) | Genistein | −0.277 |
| Lupin sprouts | ||
| LNCaP | MCF10A | 0.237 |
| Time (3d) | MCF10A | 0.215 |
| Time (3d) | LNCaP | 0.197 |
| Genistein | DU145 | 0.185 |
| Biochanin A | DU145 | 0.180 |
| Time (7d) | Genistein | 0.170 |
| Time (10d) | Genistein | 0.159 |
| Time (7d) | Biochanin A | 0.152 |
| Time (7d) | DU145 | 0.143 |
| Glycitein | DU145 | 0.107 |
| Time (10d) | Biochanin A | −0.100 |
| Time (10d) | MCF10A | −0.124 |
| Time (10d) | Genistein | −0.174 |
| Time (3d) | Glycitein | −0.191 |
| Time (3d) | Genistein | −0.192 |
| Glycitein | LNCaP | −0.205 |
| Genistein | LNCaP | −0.214 |
| Glycitein | MCF10A | −0.222 |
| Genistein | MCF10A | −0.239 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galanty, A.; Zagrodzki, P.; Miret, M.; Paśko, P. Chickpea and Lupin Sprouts, Stimulated by Different LED Lights, As Novel Examples of Isoflavones-Rich Functional Food, and Their Impact on Breast and Prostate Cells. Molecules 2022, 27, 9030. https://doi.org/10.3390/molecules27249030
Galanty A, Zagrodzki P, Miret M, Paśko P. Chickpea and Lupin Sprouts, Stimulated by Different LED Lights, As Novel Examples of Isoflavones-Rich Functional Food, and Their Impact on Breast and Prostate Cells. Molecules. 2022; 27(24):9030. https://doi.org/10.3390/molecules27249030
Chicago/Turabian StyleGalanty, Agnieszka, Paweł Zagrodzki, Marina Miret, and Paweł Paśko. 2022. "Chickpea and Lupin Sprouts, Stimulated by Different LED Lights, As Novel Examples of Isoflavones-Rich Functional Food, and Their Impact on Breast and Prostate Cells" Molecules 27, no. 24: 9030. https://doi.org/10.3390/molecules27249030
APA StyleGalanty, A., Zagrodzki, P., Miret, M., & Paśko, P. (2022). Chickpea and Lupin Sprouts, Stimulated by Different LED Lights, As Novel Examples of Isoflavones-Rich Functional Food, and Their Impact on Breast and Prostate Cells. Molecules, 27(24), 9030. https://doi.org/10.3390/molecules27249030






