Hydroxypropylation of Polyphenol-Rich Alkaline Extracts from Pinus radiata Bark and Their Physicochemical Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of P. radiata Bark
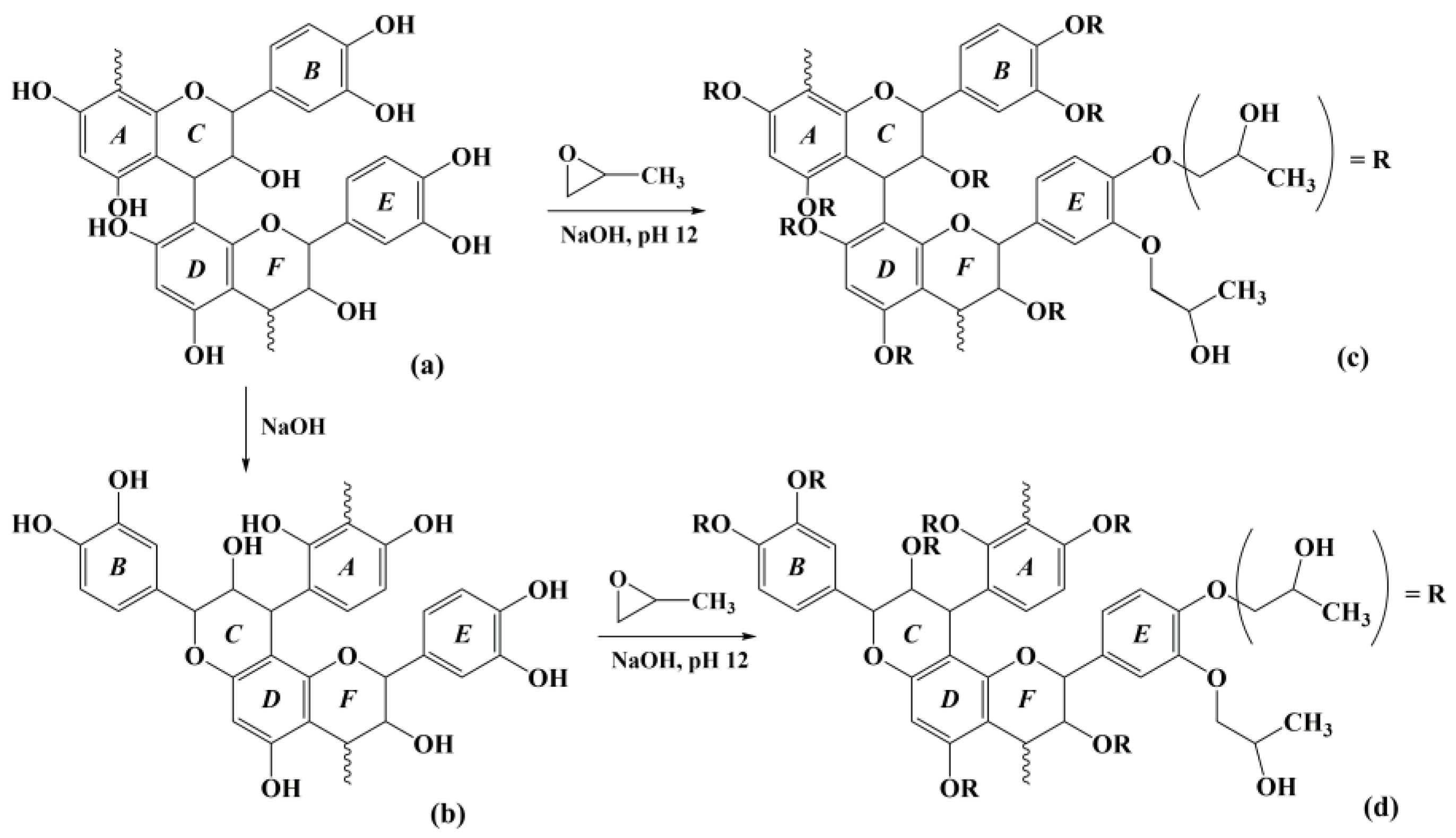
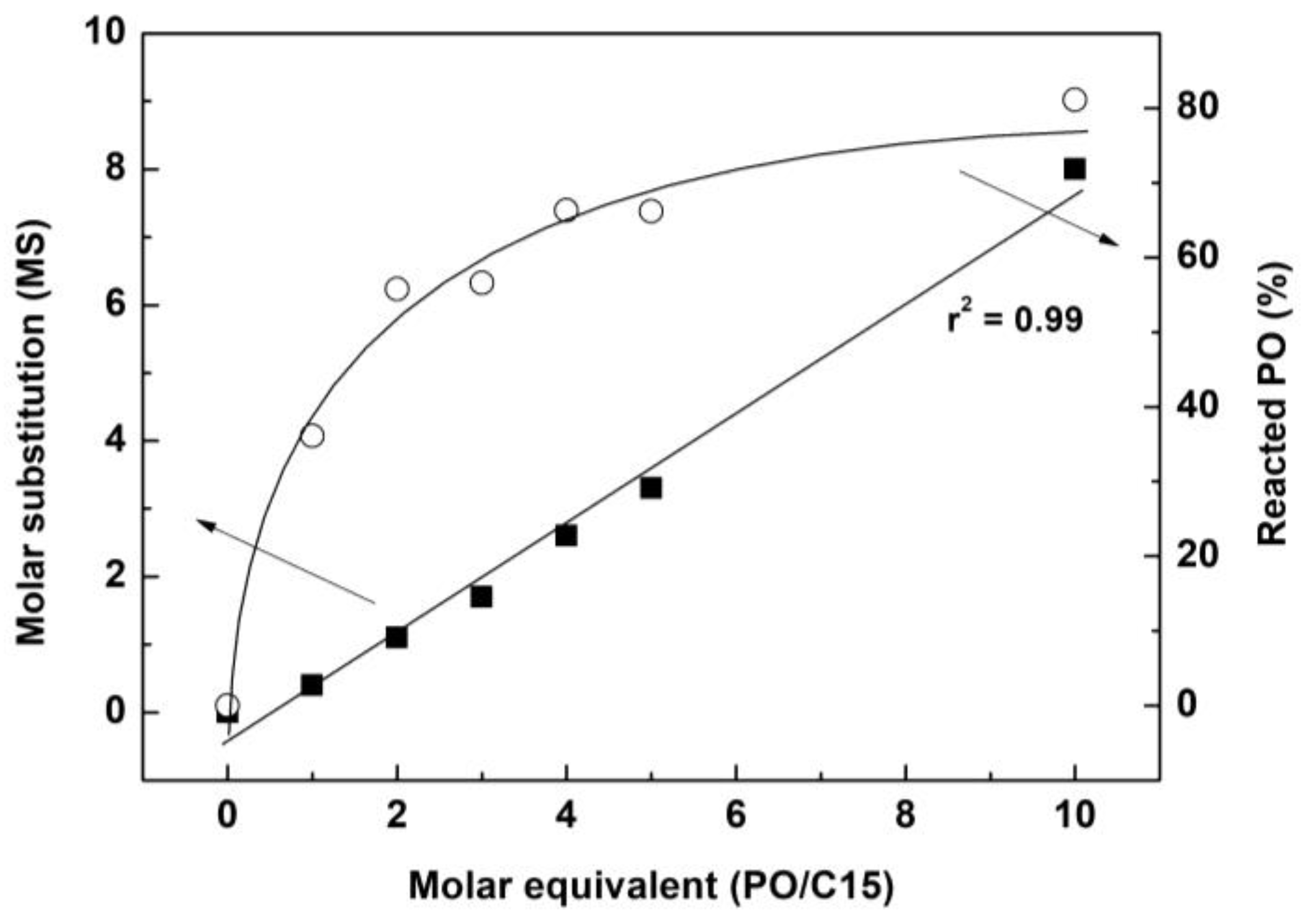
2.2. Hydroxypropylation of Alkaline Extracts (AEs)
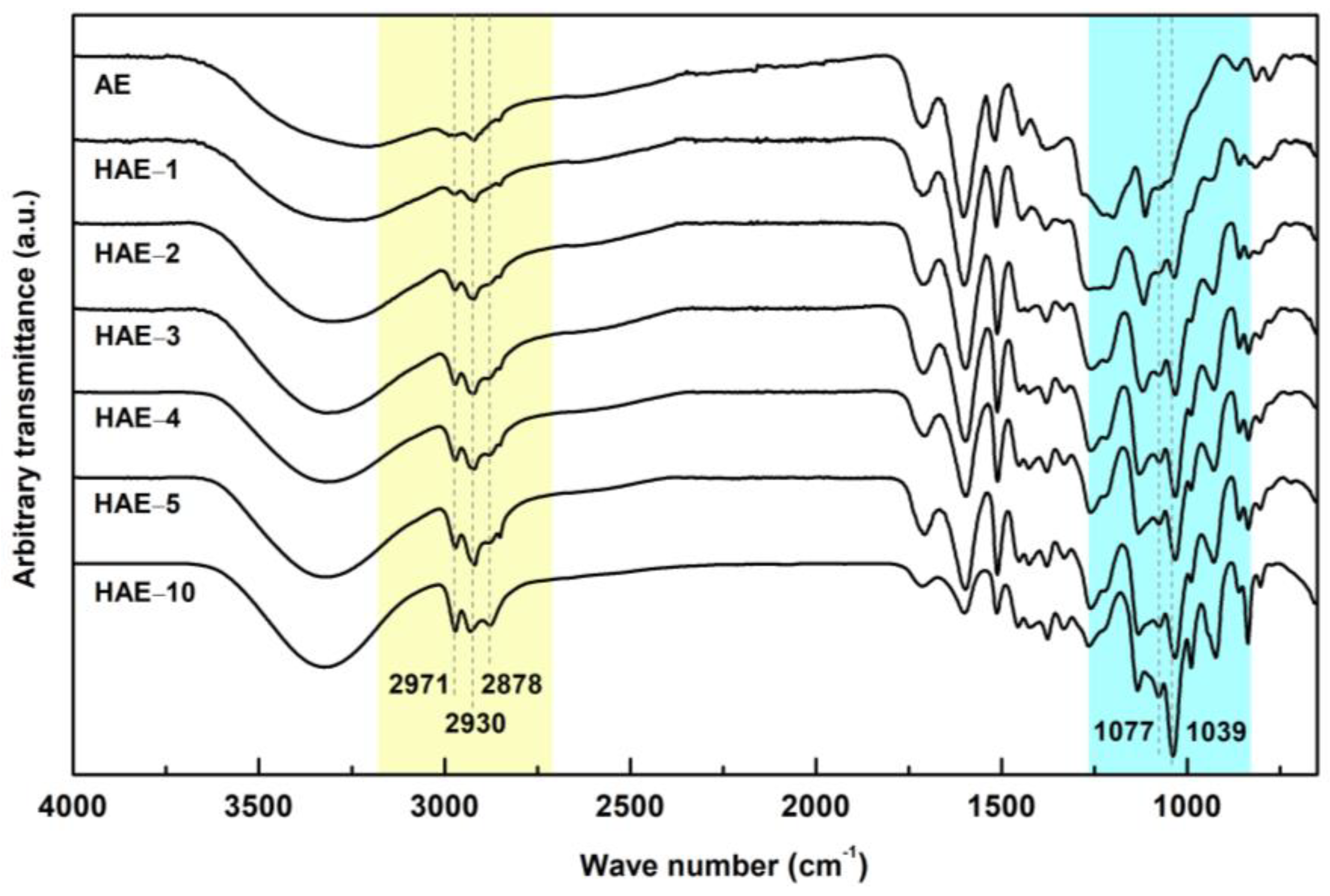
2.3. FT-IR Spectroscopy
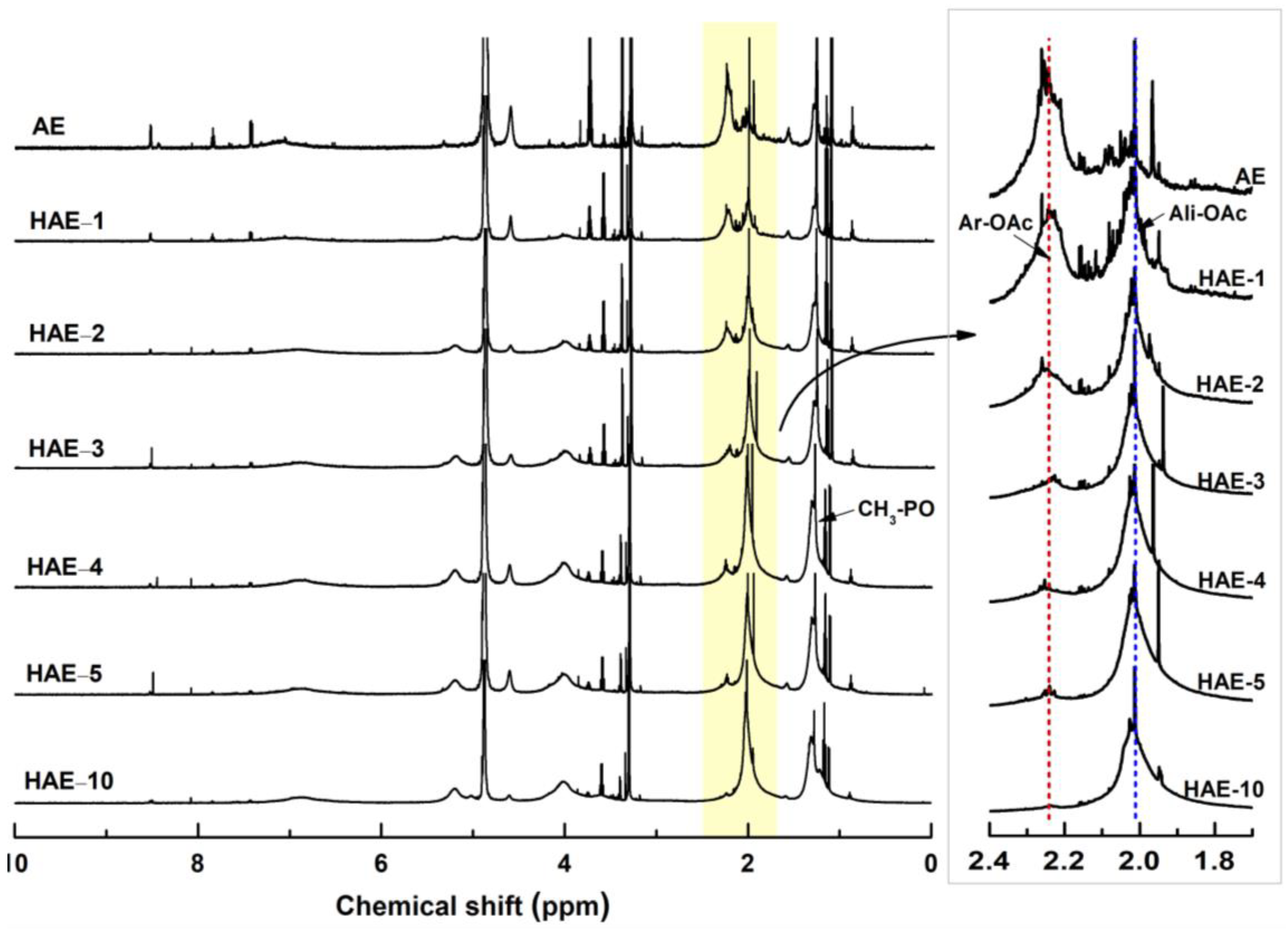
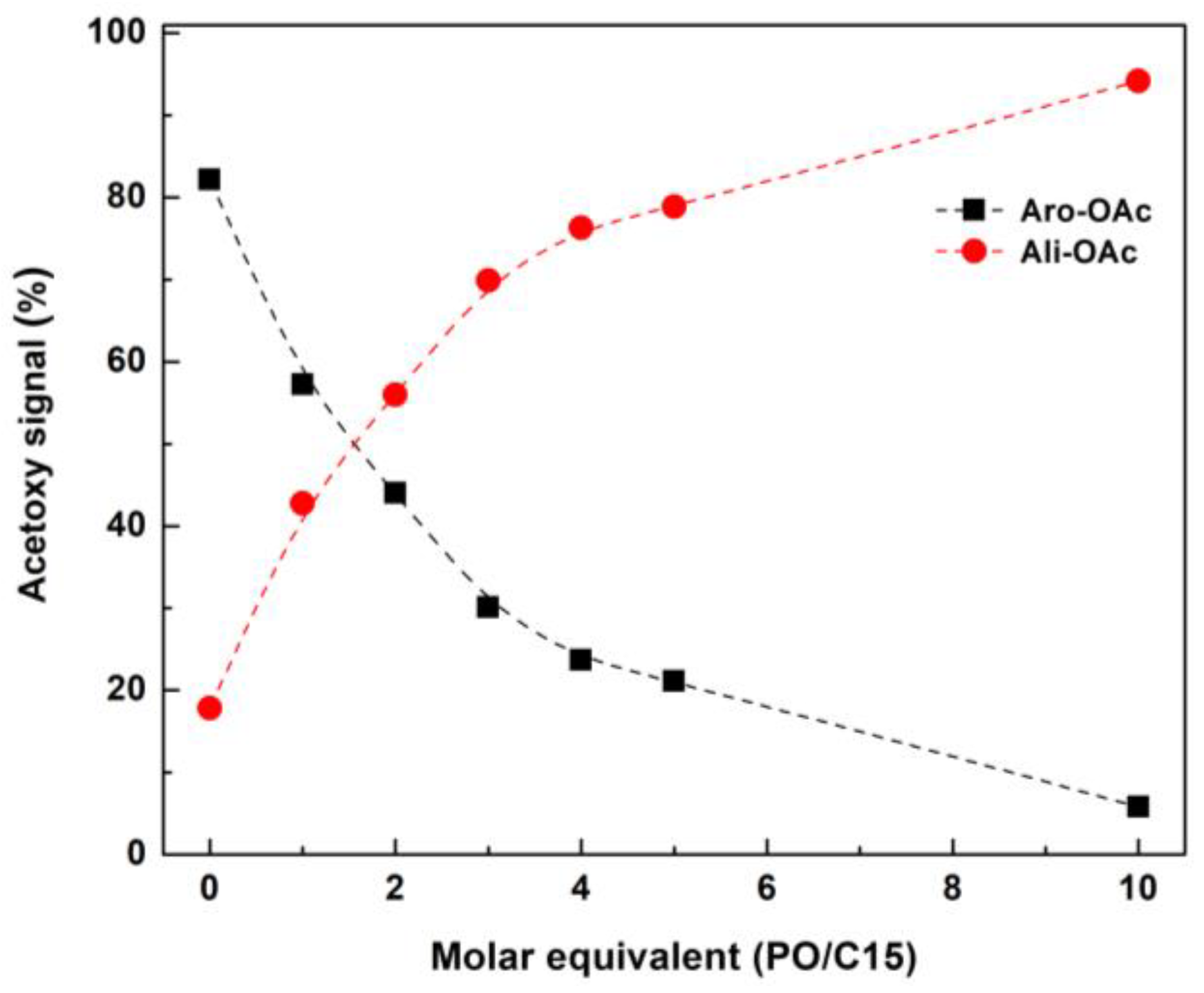
2.4. 1H NMR Spectroscopy
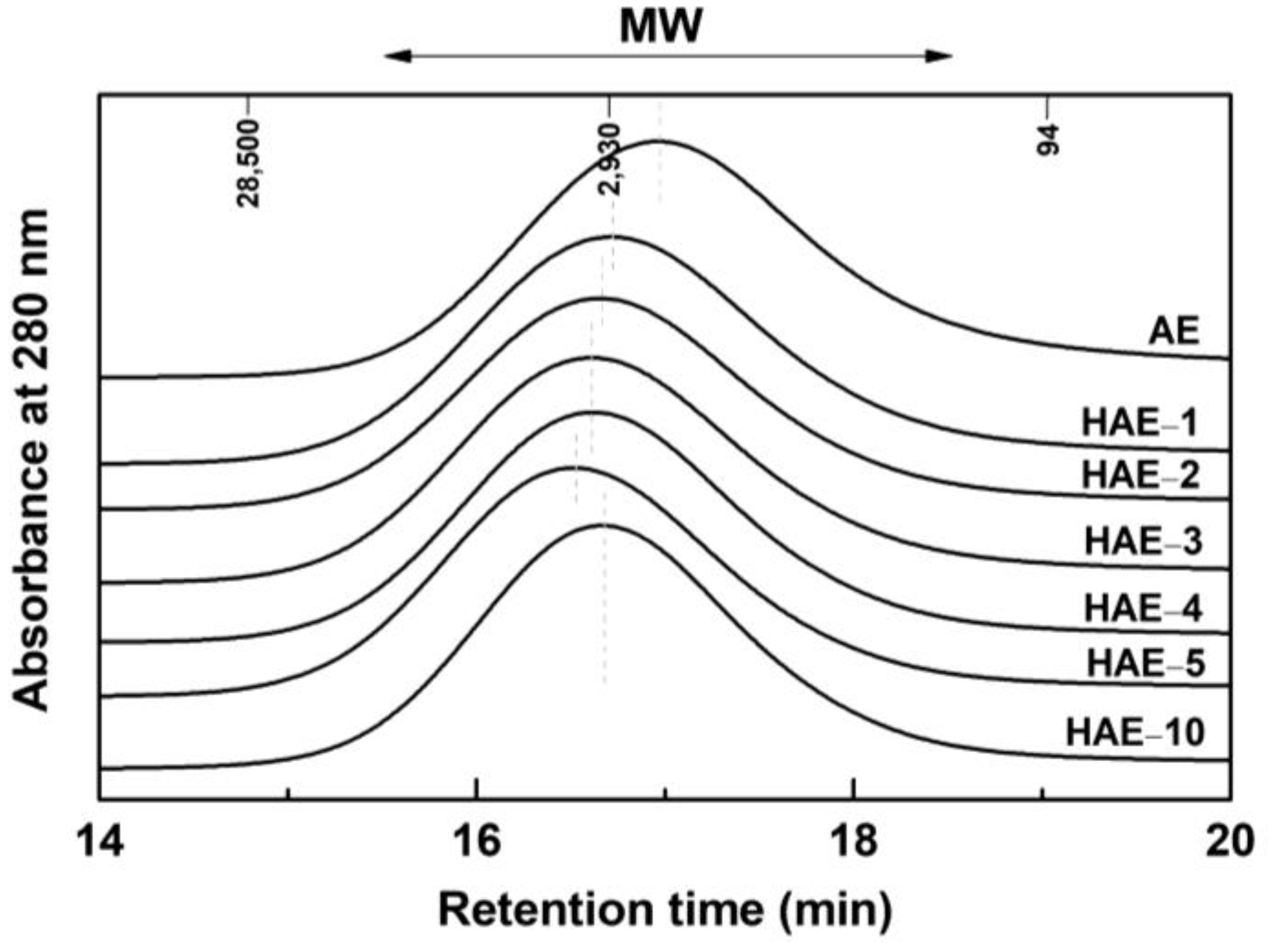
2.5. Molecular Weight Distribution
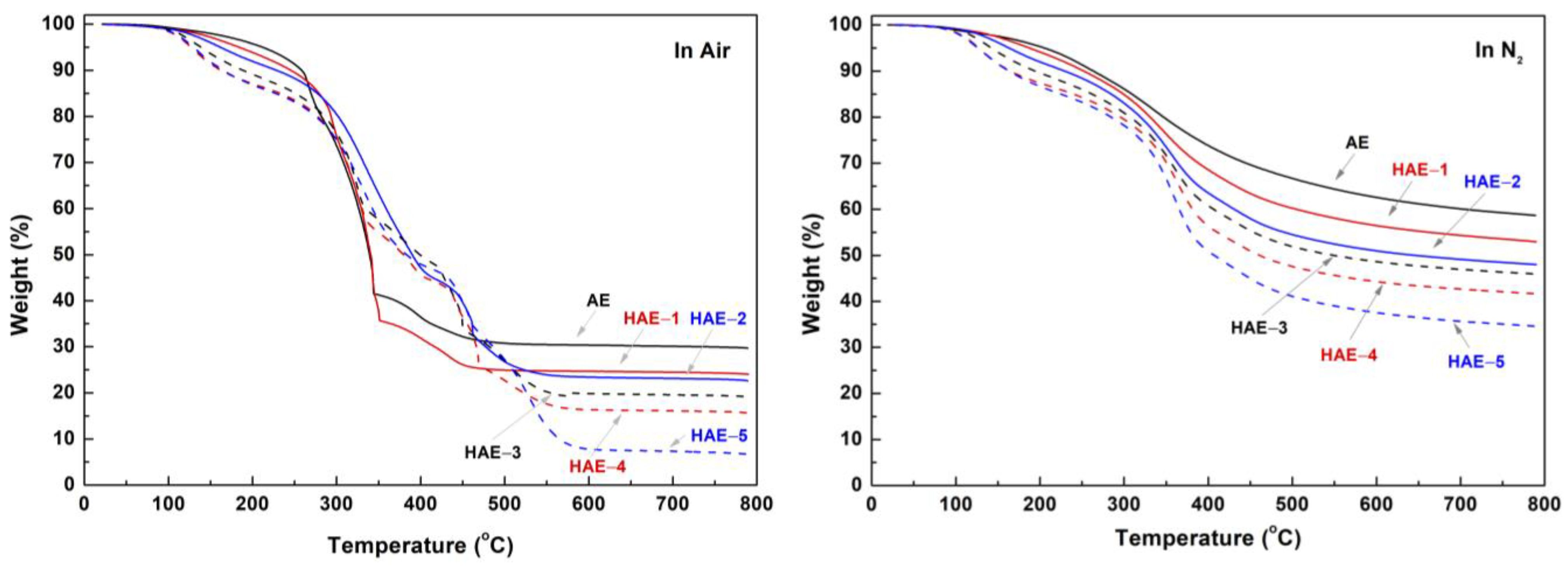
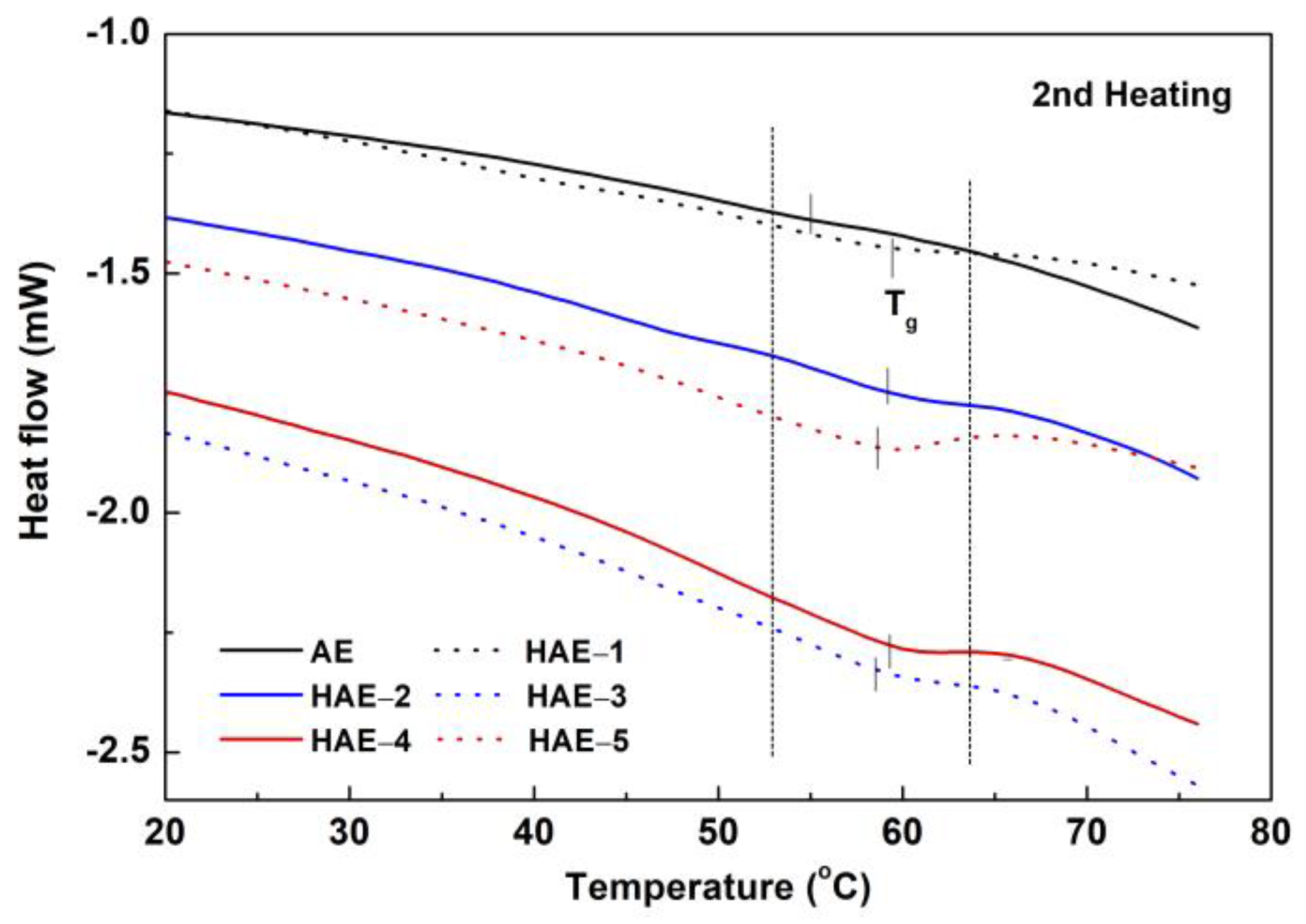
2.6. Thermal Analysis
2.7. Hydroxyl Value
2.8. Solubility in Polyols
3. Materials and Methods
3.1. Materials
3.2. Chemical Composition of P. radiata Bark
3.3. Preparation of Alkaline Extract (AE)
3.4. Hydroxypropylation of AE
3.5. Acetylation of AE and HAEs
3.6. FT-IR
3.7. 1H NMR
3.8. Molecular Weight Analysis
3.9. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
3.10. Hydroxyl Value
3.11. Solubility Test
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Korea Forest Service (KFS). Statistical Yearbook of Forestry; Korea Forest Service: Daejeon, Republic of Korea, 2019.
- Lee, M.; Jeong, S.H.; Mun, S.P. Conditions for the extraction of polyphenols from radiata pine (Pinus radiata) bark for bio-foam preparation. J. Korean Wood Sci. Technol. 2020, 48, 861–868. [Google Scholar] [CrossRef]
- Mun, S.P.; Ku, C.S. Characterization of low molecular weight polyphenols from pine (Pinus radiata) bark. Food Sci. Biotechnol. 2006, 15, 424–430. [Google Scholar]
- Ku, C.S.; Mun, S.P. Characterization of proanthocyanidin in hot water extract isolated from Pinus radiata bark. Wood Sci. Technol. 2007, 41, 235–247. [Google Scholar] [CrossRef]
- Ku, C.S.; Jang, J.P.; Mun, S.P. Exploitation of polyphenol-rich pine barks for potent antioxidant activity. J. Wood Sci. 2007, 53, 524–528. [Google Scholar] [CrossRef]
- Ku, C.S.; Mun, S.P. Antioxidant properties of monomeric, oligomeric, and polymeric fractions in hot water extract from Pinus radiata bark. Wood Sci. Technol. 2008, 42, 47–60. [Google Scholar] [CrossRef]
- Ku, C.S.; Mun, S.P.; Jang, J.P. Effects of water extraction temperatures on the yield, molecular weight, and antioxidant activity of proanthocyanidins extracted from Pinus radiata bark. For. Products J. 2011, 61, 321–325. [Google Scholar] [CrossRef]
- Ku, C.S.; Sathishkumar, M.; Mun, S.P. Binding affinity of proanthocyanidin from waste Pinus radiata bark onto proline-rich bovine achilles tendon collagen type I. Chemosphere 2007, 67, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.S.; Mun, S.P. Stabilization of extracellular matrix by Pinus radiata bark extracts with different molecular weight distribution against enzymatic degradation and radicals. Wood Sci. Technol. 2008, 42, 427–436. [Google Scholar] [CrossRef]
- Venkatesan, T.; Choi, Y.W.; Mun, S.P.; Kim, Y.K. Pinus radiata bark extract induces caspase-independent apoptosis-like cell death in MCF-7 human breast cancer cells. Cell Biol. Toxicol. 2016, 32, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Song, C.H.; Mun, S.P. Proanthocyanidin-rich Pinus radiata bark extract inhibits mast cell-mediated anaphylaxis-like reactions. Phytother. Res. 2018, 32, 290–297. [Google Scholar] [CrossRef]
- Mun, J.S.; Kim, H.C.; Mun, S.P. Potential of neutral extract prepared by treating Pinus radiata bark with NaHCO3 as a dyestuff. J. Korean Wood Sci. Technol. 2021, 49, 134–141. [Google Scholar] [CrossRef]
- Mun, J.S.; Kim, H.C.; Mun, S.P. Black color expression of silk and cotton fabrics using neutral extract from Pinus radiata bark and various iron mordants. Fibers Polym. 2022, 23, 1008–1017. [Google Scholar] [CrossRef]
- Zhou, X.; Pizzi, A.; Saugeta, A.; Nicollin, A.; Li, X.; Celzard, A.; Rodec, K.; Pasch, H. Lightweight tannin foam/composites sandwich panels and the coldset tannin. Ind. Crops Prod. 2013, 43, 255–260. [Google Scholar] [CrossRef]
- Tondi, G.; Pizzi, A.; Olives, R. Natural tannin-based rigid foams as insulation in wood construction. Maderas Cienc. Tecnol. 2008, 10, 219–227. [Google Scholar] [CrossRef]
- Basso, M.C.; Giovando, S.; Pizzi, A.; Lagel, M.C.; Celzard, A. Alkaline tannin rigid foams. J. Renew. Mater. 2014, 2, 182–185. [Google Scholar] [CrossRef]
- Steynberg, J.P.; Desmond, A.Y.; Burger, J.F.W.; Ferreira, D.; Roux, D.G. Phlobatannins via facile ring isomerizations of profisetinidin and prorobinetinidin condensed tannin units. J. Chem. Soc. Chem. Commun. 1986, 1013–1015. [Google Scholar] [CrossRef]
- Sealy-Fisher, V.J.; Pizzi, A. Increased pine tannins extraction and wood adhesives development by phlobaphenes minimization. Holz Roh-Werkstoff. 1992, 50, 212–220. [Google Scholar] [CrossRef]
- García, D.E.; Glasser, W.G.; Pizzi, A.; Osorio-Madrazo, A.; Laborie, M.-P. Hydroxypropyl tannin derivatives from Pinus pinaster (Ait.) bark. Ind. Crops Prod. 2013, 49, 730–739. [Google Scholar] [CrossRef]
- García, D.E.; Glasser, W.G.; Pizzi, A.; Osorio-Madrazo, A.; Laborie, M.-P.G. Synthesis and physicochemical properties of hydroxypropyl tannins from maritime pine bark (Pinus pinaster Ait.). Holzforschung 2014, 68, 411–418. [Google Scholar] [CrossRef]
- Kofujita, H.; Ettyu, K.; Ota, M. Characterization of the major components in bark from five Japanese tree species for chemical utilization. Wood Sci. Technol. 1999, 33, 223–228. [Google Scholar] [CrossRef]
- García, D.E.; Fuentealba, C.A.; Salazar, J.P.; Pérez, M.A.; Escobar, D.; Pizzi, A. Mild hydroxypropylation of polyflavonoids obtained under pilot-plant scale. Ind. Crops Prod. 2016, 87, 350–362. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy—A Guide for Students of Organic Chemistry, 2nd ed.; Hardcourt Brace College Publishers: Orlando, FL, USA, 1996; pp. 36, 49–50. [Google Scholar]
- TAPPI T-204 cm-97, Solvent extractives of wood and pulp, TAPPI Test Methods. 2007. Available online: https://www.tappi.org/content/sarg/t204.pdf (accessed on 15 March 2021).
- TAPPI T-211 om-02, Ash in wood, pulp, paper, and paperboard, TAPPI Test Methods. 2002. Available online: https://www.tappi.org/content/sarg/t211.pdf (accessed on 15 March 2021).
- TAPPI T-212 om-02, One percent sodium hydroxide solubility of wood and pulp, TAPPI Test Methods. 2002. Available online: https://tappi.micronexx.com/CD/TESTMETHODS/T212.pdf (accessed on 15 March 2021).
- TAPPI T-222 om-02, Acid insoluble lignin in wood and pulp, TAPPI Test Methods. 2006. Available online: https://www.tappi.org/content/sarg/t222.pdf (accessed on 15 March 2021).
- TAPPI UM 250. Acid-soluble lignin in wood and pulp. In TAPPI Useful Methods; TAPPI Press: Atlanta, GA, USA, 1991. [Google Scholar]

| Ash (%) | 0.77 |
| Extracts (%) | |
| Cold water Hot water 1% NaOH Alcohol-benzene | 21.42 31.37 68.77 12.69 |
| Lignin | |
| Klason (1) | 71.12 |
| Acid soluble Total | 1.00 72.12 |
| Lignin after 1% NaOH extraction (%, based on bark) | |
| Klason (2) | 13.78 |
| Acid soluble Total | 0.13 13.91 |
| Total polyphenol (%) = 1–2 | 57.34 |
| Sample | Molar Equivalent (PO/C15) | Molar Substitution (MS) | Reacted PO (%) |
|---|---|---|---|
| HAE−1 | 1 | 0.4 | 36.1 |
| HAE−2 | 2 | 1.1 | 55.8 |
| HAE−3 | 3 | 1.7 | 56.6 |
| HAE−4 | 4 | 2.6 | 66.3 |
| HAE−5 | 5 | 3.3 | 66.2 |
| HAE−10 | 10 | 8.0 | 81.1 |
| AE | HAE−1 | HAE−2 | HAE−3 | HAE−4 | HAE−5 | HAE−10 | |
|---|---|---|---|---|---|---|---|
| w | 3120 | 4079 | 4185 | 4376 | 4439 | 4897 | 4000 |
| n | 792 | 1106 | 1309 | 1186 | 1353 | 1422 | 1336 |
| w/n (PDI) | 3.9 | 3.7 | 3.2 | 3.7 | 3.3 | 3.4 | 3.0 |
| Sample | Temperature, °C | ||
|---|---|---|---|
| Tg, 1st Heating | Hardening Temp. after Cooling | Tg, 2nd Heating | |
| AE | 49.42 | 29.92 | 54.10 |
| HAE−1 | 54.89 | 38.83 | 59.56 |
| HAE−2 | 50.68 | 41.72 | 59.57 |
| HAE−3 | 50.51 | 42.46 | 58.34 |
| HAE−4 | 53.52 | 43.66 | 59.01 |
| HAE−5 | 55.97 | 43.99 | 58.82 |
| AE | HAE−1 | HAE−2 | HAE−3 | HAE−4 | HAE−5 | HAE−10 | |
|---|---|---|---|---|---|---|---|
| Hydroxyl value (mg KOH/g) | 307 ± 2 | 340 ± 10 | 375 ± 5 | 389 ± 6 | 450 ± 8 | 475 ± 10 | 785 ± 15 |
| GPC Configuration | LC-20AD (Pump) + SPD-M20A (Detector) + CTO-20A (Column Oven), Shimadzu, Japan |
|---|---|
| Column | PLgel 10 µm MIXED-B (300 × 7.5 mm, Agilent, Santa Clara, CA, USA) |
| Flow rate | 0.5 mL/min |
| Sample injection volume | 10 μL |
| Eluent | THF |
| Column oven temperature | 30 °C |
| Detector | UV (254 nm: polystyrene standards, 280 nm: samples and phenol) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mun, S.P. Hydroxypropylation of Polyphenol-Rich Alkaline Extracts from Pinus radiata Bark and Their Physicochemical Properties. Molecules 2022, 27, 9002. https://doi.org/10.3390/molecules27249002
Mun SP. Hydroxypropylation of Polyphenol-Rich Alkaline Extracts from Pinus radiata Bark and Their Physicochemical Properties. Molecules. 2022; 27(24):9002. https://doi.org/10.3390/molecules27249002
Chicago/Turabian StyleMun, Sung Phil. 2022. "Hydroxypropylation of Polyphenol-Rich Alkaline Extracts from Pinus radiata Bark and Their Physicochemical Properties" Molecules 27, no. 24: 9002. https://doi.org/10.3390/molecules27249002
APA StyleMun, S. P. (2022). Hydroxypropylation of Polyphenol-Rich Alkaline Extracts from Pinus radiata Bark and Their Physicochemical Properties. Molecules, 27(24), 9002. https://doi.org/10.3390/molecules27249002






